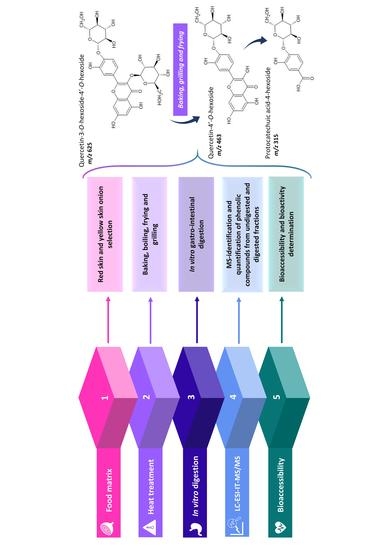
Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Onion Cooking
2.3. Preparation of Phenolic Compounds Methanol Extracts from Raw and Cooked YSO and RSO
2.4. In Vitro Gastro-Intestinal Digestion of Raw and Cooked Onion Samples
2.5. Determination of Total Phenolic Compounds and Antioxidant Activity in Raw and Cooked Onion Samples
2.6. Liquid Chromatography Electrospray Ionization Ion Trap Mass Spectrometry (LC-ESI-IT-MS) Profiling of Phenolic Compounds in Raw and Cooked Onion Samples
2.7. Statistics
3. Results and Discussion
3.1. Total Phenolic Content and Phenolic Profiles of YSO and RSO
3.2. Effect of Cooking Methods on Total and Individual Phenolic Content in YSO and RSO
3.3. Effect of Cooking Methods on Total and Individual Phenolic Content in YSO and RSO as a Function of the Initial Fresh Weight
3.4. Effect of Cooking Methods on the Release and Bioaccessibility of YSO and RSO Phenolic Compounds
3.5. Effect of Cooking and In Vitro Gastro-Intestinal Digestion on the Antioxidant Activity of YSO and RSO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cicco, A. The fruit and vegetable sector in the EU—A statistical overview—Statistics Explained. Eur. Union 2018, 1–14. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Momenan, A.A.; Azizi, F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: A longitudinal follow-up study. J. Hypertens. 2017, 35, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Li, N.; Du, L.; Zhao, R.; Yi, M.; Xu, Q.; Zhou, Y. Allium vegetable consumption and health: An umbrella review of meta-analyses of multiple health outcomes. Food Sci. Nutr. 2019, 7, 2451–2470. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.D.; Kim, S.K. Allium flavonols: Health benefits, molecular targets, and bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Bottazzi, S.; Tagliazucchi, D. Mediterranean diet vegetable foods protect meat lipids from oxidation during in vitro gastro-intestinal digestion. Int. J. Food Sci. Nutr. 2020, 71, 424–439. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species: A comparative study by means of HPLC-DAD-ESI-MS-MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Gennaro, L.; Leonardi, C.; Esposito, F.; Salucci, M.; Maiani, G.; Quaglia, G.; Fogliano, V. Flavonoid and carbohydrate contents in tropea red onions: Effects of homelike peeling and storage. J. Agric. Food Chem. 2002, 50, 1904–1910. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Mesías, M.; Morales, F.J.; Saura-Calixto, S. Evidence for the formation of maillardized insoluble dietary fiber in bread: A specific kind of dietary fiber in thermally processed food. Food Res. Int. 2014, 55, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Nunes, F.M.; Coimbra, M.A. Role of hydroxycinnamates in coffee melanoidin formation. Phytochem. Rev. 2010, 9, 171–185. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int. 2009, 42, 1331–1336. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): Effect on flavonol content and antioxidant status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J.C. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compos. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioactivity and cell metabolism of in vitro digested sweet cherry (Prunus avium) phenolic compounds. Int. J. Food Sci. Nutr. 2019, 70, 335–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Cavalchi, M.; Conte, A.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion. J. Food Eng. 2017, 213, 60–68. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rufián-Henares, J.Á.; Pastoriza, S. Effect of home cooking on the antioxidant capacity of vegetables: Relationship with Maillard reaction indicators. Food Res. Int. 2019, 121, 514–523. [Google Scholar] [CrossRef]
- Harris, S.; Brunton, N.; Tiwari, U.; Cummins, E. Human exposure modelling of quercetin in onions (Allium cepa L.) following thermal processing. Food Chem. 2015, 187, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: A meta-analytic investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavagnaro, P.F.; Galmarini, C.R. Effect of processing and cooking conditions on onion (Allium cepa L.) induced antiplatelet activity and thiosulfinate content. J. Agric. Food Chem. 2012, 60, 8731–8737. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H. Quercetin oxidation paradoxically enhances its antioxidant and cytoprotective properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Eshchenko, A.Y.; Makarova, S.V.; Vitenberg, A.G.; Dobryakov, Y.G.; Utsal, V.A. Identification of the products of oxidation of quercetin by air oxygen at ambient temperature. Molecules 2007, 12, 654–672. [Google Scholar] [CrossRef] [Green Version]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Foods 2017, 32, 195–207. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2016, 27, 201–213. [Google Scholar] [CrossRef] [Green Version]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Digestibility of (poly)phenols and antioxidant activity in raw and cooked cactus cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Gill, C.I.R.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica cladodes impacts Bioaccessibility and Bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef]
- Pellegrini, N.; Miglio, C.; Del Rio, D.; Salvatore, S.; Serafini, M.; Brighenti, F. Effect of domestic cooking methods on the total antioxidant capacity of vegetables. Int. J. Food Sci. Nutr. 2009, 60, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009, 74, 97–103. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Filipa Almeida, A.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of quercetin in humans with a focus on interindividual variations. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| YSO | RSO | |||||||
|---|---|---|---|---|---|---|---|---|
| Baking | Boiling | Frying | Grilling | Baking | Boiling | Frying | Grilling | |
| Cooking temperature (°C) | 180 | 100 | 140 | 110 | 180 | 100 | 140 | 110 |
| Cooking time (min) | 30 | 30 | 8 | 15 | 30 | 30 | 8 | 15 |
| Initial weight (g) | 173 | 129 | 200 | 170 | 133 | 129 | 158 | 155 |
| Final weight (g) | 57 | 49 | 126 | 80 | 48 | 79 | 90 | 88 |
| Weight loss (%) | 67 | 62 | 37 | 53 | 64 | 38 | 43 | 43 |
| Initial/final weight ratio | 3.04 | 2.63 | 1.59 | 2.13 | 2.77 | 1.63 | 1.76 | 1.76 |
| YSO | |||||
|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled |
| Hydroxycinnamic acids | |||||
| Caffeic acid-O-hexoside | 0.41 ± 0.01 a | n.d. | n.d. | n.d. | 0.03 ± 0.00 b |
| Ferulic acid-O-hexoside | 0.43 ± 0.02 a | n.d. | n.d. | n.d. | 0.10 ± 0.01 b |
| Sinapic acid-O-hexoside isomer | 0.11 ± 0.00 c | n.d. | 0.08 ± 0.00 d | 0.25 ± 0.00 b | 0.46 ± 0.00 a |
| Sinapic acid-O-hexoside isomer | 0.66 ± 0.01 c | 0.99 ± 0.01 b | 0.30 ± 0.00 e | 0.58 ± 0.01 d | 1.39 ± 0.03 a |
| Total hydroxycinnamic acids | 1.61 ± 0.03 b | 0.99 ± 0.01 c | 0.38 ± 0.01 e | 0.83 ± 0.01 d | 1.98 ± 0.03 a |
| Flavonols | |||||
| Quercetin | 0.03 ± 0.00 a | n.d. | n.d. | 0.01 ± 0.00 b | 0.03 ± 0.00 a |
| Quercetin-3-O-hexoside | 0.07 ± 0.00 b | 0.06 ± 0.00 b | 0.03 ± 0.00 c | 0.13 ± 0.00 a | n.d. |
| Quercetin-4′-O-hexoside | 4.99 ± 0.29 c | 13.76 ± 0.06 a,b | 1.00 ± 0.05 d | 15.01 ± 0.48 a | 12.07 ± 0.77 b |
| Quercetin-3-O-glucoside | n.d. | 0.19 ± 0.01 a | 0.04 ± 0.00 c | n.d. | 0.12 ± 0.00 b |
| Quercetin-3-O-hexoside-7-O-hexoside | 0.02 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| Quercetin-7-O-hexoside-4′-O-hexoside | 0.04 ± 0.00 b | 0.13 ± 0.00 a | n.d. | n.d. | 0.05 ± 0.00 b |
| Quercetin-3-O-hexoside-4′-O-hexoside | 20.56 ± 1.68 c | 27.67 ± 0.21 a | 15.58 ± 0.98 d | 28.05 ± 0.66 a | 24.26 ± 0.27 b |
| Quercetin-tri-O-hexoside | 0.02 ± 0.00 b | 0.06 ± 0.00 a | 0.02 ± 0.00 b | 0.03 ± 0.00 b | n.d. |
| Isorhamnetin-4′-O-hexoside | 0.06 ± 0.00 d | 0.45 ± 0.00 a | 0.05 ± 0.00 d | 0.19 ± 0.00 b | 0.15 ± 0.00 c |
| Isorhamnetin-3-O-hexoside-4′-O-hexoside | 0.02 ± 0.00 d | 0.21 ± 0.01 a | 0.05 ± 0.00 c | 0.06 ± 0.00 b,c | 0.07 ± 0.00 b |
| Total flavonols | 25.81 ± 1.70 c | 42.53 ± 0.22 a | 16.77 ± 0.98 d | 43.48 ± 0.81 a | 36.75 ± 0.82 b |
| Total phenolic by MS * | 27.42 ± 1.70 c | 43.52 ± 0.22 a | 17.15 ± 0.98 d | 44.31 ± 0.81 a | 38.73 ± 0.82 b |
| Total phenolic by FC ** | 43.21 ± 1.57 d | 168.75 ± 1.03 a | 20.85 ± 0.85 e | 51.71 ± 1.13 c | 79.11 ± 0.31 b |
| RSO | |||||
|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled |
| Hydroxybenzoic acids | |||||
| Protocatechuic acid-O-hexoside | n.d. | 2.99 ± 0.03 b | n.d. | 2.20 ± 0.06 c | 4.56 ± 0.03 a |
| Total hydroxybenzoic acids | n.d. | 2.99 ± 0.03 b | n.d. | 2.20 ± 0.06 c | 4.56 ± 0.03 a |
| Flavan-3-ols | |||||
| (Epi)catechin-3-O-hexoside isomer | 0.04 ± 0.00 d | 0.72 ± 0.01 b | 0.02 ± 0.00 e | 0.20 ± 0.00 c | 0.82 ± 0.02 a |
| (Epi)catechin-3-O-hexoside isomer | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| Total flavan-3-ols | 0.05 ± 0.00 d | 0.72 ± 0.01 b | 0.02 ± 0.00 e | 0.20 ± 0.00 c | 0.82 ± 0.02 a |
| Di-hydro-flavonols | |||||
| Taxifolin-O-hexoside isomer | 0.06 ± 0.00 b | n.d. | 0.04 ± 0.00 b | 0.06 ± 0.00 b | 0.10 ± 0.00 a |
| Taxifolin-O-hexoside isomer | 0.06 ± 0.00 d | n.d. | 0.20 ± 0.00 b | 0.29 ± 0.00 a | 0.16 ± 0.00 c |
| Taxifolin-O-hexoside isomer | 0.05 ± 0.00 b | 0.09 ± 0.00a | n.d. | 0.04 ± 0.00 b | 0.02 ± 0.00 c |
| Taxifolin-O-hexoside isomer | 0.12 ± 0.00 a | 0.06 ± 0.00 c | 0.03 ± 0.00 d | 0.09 ± 0.00 b | 0.05 ± 0.00 c |
| Taxifolin-O-hexoside isomer | n.d. | n.d. | n.d. | 0.07 ± 0.00 | n.d. |
| Total di-hydro-flavonols | 0.29 ± 0.00 c | 0.15 ± 0.00 d | 0.27 ± 0.00 c | 0.55 ± 0.01 a | 0.33 ± 0.00 b |
| Flavonols | |||||
| Quercetin | 0.89 ± 0.05 a | 0.43 ± 0.00 b | 0.06 ± 0.01 c | 0.03 ± 0.01 c | 0.73 ± 0.01 a |
| Quercetin-3-O-hexoside | 0.36 ± 0.01 d | 1.01 ± 0.01 a | 0.29 ± 0.00 d | 0.71 ± 0.01 b | 0.58 ± 0.00 c |
| Quercetin-4′-O-hexoside | 10.46 ± 0.01 d | 12.08 ± 0.05 b,c | 11.47 ± 0.31 c | 12.94 ± 0.46 a,b | 13.49 ± 0.06 a |
| Quercetin-3-O-hexoside-7-O-hexoside | n.d. | n.d. | n.d. | n.d. | 0.15 ± 0.00 |
| Quercetin-7-O-hexoside-4′-O-hexoside | 0.37 ± 0.00 c | 0.65 ± 0.00 a | 0.31 ± 0.00 d | 0.55 ± 0.00 b | 0.38 ± 0.00 c |
| Quercetin-3-O-hexoside-4′-O-hexoside | 20.87 ± 0.14 c | 32.55 ± 0.30 a | 23.02 ± 0.34 c | 36.29 ± 0.37 a | 27.20 ± 0.58 b |
| Quercetin-tri-O-hexoside isomer | 0.15 ± 0.00 a | 0.14 ± 0.00 a | 0.11 ± 0.00 b | n.d. | 0.14 ± 0.00 a |
| Quercetin-tri-O-hexoside isomer | n.d. | 0.03 ± 0.00 a | n.d. | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Kaempferol-7-O-hexoside isomer | 0.26 ± 0.01 b | 0.05 ± 0.00 d | 0.20 ± 0.00 c | 0.20 ± 0.00 c | 0.33 ± 0.00 a |
| Kaempferol-7-O-hexoside isomer | n.d. | 0.07 ± 0.00 a | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.04 ± 0.00 b |
| Kaempferol-3-O-hexoside isomer | n.d. | 0.09 ± 0.00 a | n.d. | 0.02 ± 0.00 b | 0.01 ± 0.00 b |
| Kaempferol-3-O-hexoside-7-O-hexoside isómer | 0.55 ± 0.00 a | 0.19 ± 0.00 b | 0.04 ± 0.00 d | 0.32 ± 0.00 c | n.d. |
| Kaempferol-3-O-hexoside-7-O-hexoside isómer | 0.07 ± 0.00 b | 0.16 ± 0.00 a | 0.05 ± 0.00 b | 0.08 ± 0.00 b | n.d. |
| Kaempferol-hexoside-rhamnoside-rhamnoside | 0.05 ± 0.00 a | 0.04 ± 0.00 a | n.d. | n.d. | n.d. |
| Isorhamnetin-3-O-hexoside isomer | 0.28 ± 0.00 c | 0.98 ± 0.00 b | n.d. | 1.23 ± 0.02 a | 0.81 ± 0.01 b |
| Isorhamnetin-3-O-hexoside isomer | n.d. | 0.21 ± 0.00 a | n.d. | 0.12 ± 0.01 b | 0.13 ± 0.01 b |
| Isorhamnetin-4′-O-hexoside | 2.25 ± 0.04 d | 2.82 ± 0.04 b | n.d. | 3.55 ± 0.07 a | 2.47 ± 0.01 c |
| Isorhamnetin-3-O-hexoside-4′-O-hexoside | 0.52 ± 0.00 e | 2.38 ± 0.03 a | 1.05 ± 0.05 d | 2.20 ± 0.01 b | 1.40 ± 0.01 c |
| Isorhamnetin-O-hexoside-O-pentoside | 0.11 ± 0.00 a | n.d. | n.d. | 0.04 ± 0.00 b | n.d. |
| Myricetin-O-hexoside-O-hexoside isómer | n.d. | 0.11 ± 0.00 a | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.07 ± 0.00 b |
| Myricetin-O-hexoside-O-hexoside isómer | n.d. | n.d. | n.d. | 0.04 ± 0.00 | n.d. |
| Total flavonols | 37.19 ± 0.53 c | 53.98 ± 0.78 a | 36.66 ± 0.88 c | 58.40 ± 0.99 a | 47.95 ± 1.08 b |
| Anthocyanins | |||||
| Cyanidin-3-O-hexoside | 1.00 ± 0.02 a | 0.36 ± 0.01 c | 0.74 ± 0.01 b | n.d. | n.d. |
| Cyanidin-O-hexoside-O-hexoside isómer | 0.09 ± 0.00 b | n.d. | 0.07 ± 0.00 b | 0.07 ± 0.00 b | 0.31 ± 0.02 a |
| Cyanidin-O-hexoside-O-hexoside isómer | 0.31 ± 0.01 b | 0.10 ± 0.00 d | 0.14 ± 0.00 c | 0.07 ± 0.01 d | 2.20 ± 0.02 a |
| Cyanidin-O-malonyl-hexoside isomer | 5.97 ± 0.37 a | n.d. | 3.25 ± 0.03 b | 2.48 ± 0.01 c | 4.93 ± 0.08 a |
| Cyanidin-O-malonyl-hexoside isomer | n.d. | n.d. | n.d. | 0.50 ± 0.01 | n.d. |
| Cyanidin-O-hexoside-O-malonyl-hexoside isómer | 4.60 ± 0.01 a | 1.29 ± 0.07 d | 1.67 ± 0.03 c | 2.82 ± 0.06 b | 3.96 ± 0.08 a |
| Peonidin-3-O-hexoside | 0.21 ± 0.00 a | n.d. | 0.13 ± 0.00 b | n.d. | 0.16 ± 0.00 b |
| Peonidin-O-malonyl-hexoside | 0.37 ± 0.01 a | 0.17 ± 0.00 b | 0.19 ± 0.00 b | 0.39 ± 0.00 a | n.d. |
| Malvidin-O-hexoside-acetaldehyde | 0.02 ± 0.00 a | n.d. | 0.02 ± 0.00 a | n.d. | n.d. |
| Total anthocyanins | 12.57 ± 0.38 a | 1.92 ± 0.07 c | 6.22 ± 0.04 b | 6.33 ± 0.06 b | 11.57 ± 0.12 a |
| Total phenolic by MS * | 50.12 ± 0.65 c | 59.76 ± 0.78 b | 43.15 ± 0.89 d | 67.70 ± 0.99 a | 65.22 ± 1.09 a |
| Total phenolic by FC ** | 65.44 ± 1.88 d | 137.85 ± 1.23 a | 36.79 ± 0.31 e | 81.85 ± 0.54 c | 111.79 ± 1.44 b |
| YSO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled | |||||
| After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | |
| Hydroxycinnamic acids | ||||||||||
| Caffeic acid-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sinapic acid-O-hexoside isómer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sinapic acid-O-hexoside isómer | n.d. | n.d. | 0.76 ± 0.01 | 77.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total hydroxycinnamic acids | n.d. | n.d. | 0.76 ± 0.01 | 77.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavonols | ||||||||||
| Quercetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-hexoside | 0.02 ± 0.00 c | 29.3 | 0.19 ± 0.00 a | 264.1 | n.d. | n.d. | 0.10 ± 0.00 b | 80.3 | 0.09 ± 0.00 b | n.d. |
| Quercetin-4′-O-hexoside | 3.20 ± 0.00 d | 64.0 | 9.17 ± 0.16 a | 66.6 | n.d. | n.d. | 5.67 ± 0.18 b | 37.8 | 4.01 ± 0.02 c | 33.2 |
| Quercetin-3-O-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-hexoside-7-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-7-O-hexoside-4′-O-hexoside | n.d. | n.d. | 0.14 ± 0.00 | 112.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-hexoside-4′-O-hexoside | 11.54 ± 0.13 b | 56.1 | 17.83 ± 1.09 a | 64.4 | 8.76 ± 0.12 c | 56.2 | 16.24 ± 0.18 a | 57.9 | 12.35 ± 0.49 b | 50.9 |
| Quercetin-tri-O-hexoside | n.d. | n.d. | 0.06 ± 0.00 | 86.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin-4′-O-hexoside | 0.02 ± 0.00 c | 38.8 | 0.19 ± 0.00 a | 43.3 | n.d. | n.d. | 0.06 ± 0.00 b | 30.4 | n.d. | n.d. |
| Isorhamnetin-3-O-hexoside-4′-O-hexoside | n.d. | n.d. | 0.19 ± 0.00 a | 92.0 | n.d. | n.d. | 0.04 ± 0.00 b | 74.2 | 0.05 ± 0.00 b | 65.9 |
| Total flavonols | 14.78 ± 0.13 d | 57.2 | 27.77 ± 1.11 a | 65.3 | 8.76 ± 0.12 e | 52.2 | 22.12 ± 0.26 b | 50.9 | 16.50 ± 0.49 c | 44.9 |
| Total phenolic by MS * | 14.78 ± 0.13 d | 53.8 | 28.53 ± 1.11 a | 65.5 | 8.76 ± 0.12 e | 51.0 | 22.12 ± 0.26 b | 49.9 | 16.50 ± 0.49 c | 42.6 |
| Total phenolic by FC ** | 63.38 ± 3.13 c | 146.7 | 207.66 ± 7.08 a | 123.1 | 57.24 ± 2.19 d | 117.8 | 74.76 ± 4.14 c | 179.2 | 111.23 ± 0.43 b | 140.6 |
| RSO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Raw | Baked | Boiled | Fried | Grilled | |||||
| After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | After Digestion | BI (%) | |
| Hydroxybenzoic acids | ||||||||||
| Protocatechuic acid-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total hydroxybenzoic acids | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavan-3-ols | ||||||||||
| (Epi)catechin-3-O-hexoside isomer | n.d. | n.d. | 0.51 ± 0.00 b | 70.0 | n.d. | n.d. | 0.27 ± 0.01 c | 133.4 | 0.71 ± 0.10 a | 86.3 |
| (Epi)catechin-3-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total flavan-3-ols | n.d. | n.d. | 0.51 ± 0.00 b | 70.0 | n.d. | n.d. | 0.27 ± 0.01 c | 133.4 | 0.71 ± 0.10 a | 86.3 |
| Di-hydro-flavonols | ||||||||||
| Taxifolin-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Taxifolin-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Taxifolin-O-hexoside isomer | 0.03 ± 0.00 b | 56.4 | n.d. | n.d. | n.d. | n.d. | 0.08 ± 0.00 a | 217.5 | n.d. | n.d. |
| Taxifolin-O-hexoside isomer | 0.03 ± 0.00 | 24.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Taxifolin-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total di-hydro-flavonols | 0.06 ± 0.00 b | 19.8 | n.d. | n.d. | n.d. | n.d. | 0.08 ± 0.00 a | 217.5 | n.d. | n.d. |
| Flavonols | ||||||||||
| Quercetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-O-hexoside | n.d. | n.d. | 1.28 ± 0.01 a | 126.9 | n.d. | n.d. | 0.89 ± 0.09 b | 126.6 | 0.34 ± 0.01 c | 19.1 |
| Quercetin-4′-O-hexoside | 4.27 ± 0.01 a | 40.8 | 5.59 ± 0.06 b | 46.3 | 1.89 ± 0.03 c | 16.5 | 4.99 ± 0.05 d | 38.5 | 7.07 ± 0.06 e | 52.4 |
| Quercetin-3-O-hexoside-7-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-7-O-hexoside-4′-O-hexoside | 0.22 ± 0.01 b | 59.7 | 0.47 ± 0.01 a | 72.6 | n.d. | n.d. | 0.20 ± 0.02 b | 36.6 | 0.26 ± 0.01 b | 68.0 |
| Quercetin-3-O-hexoside-4′-O-hexoside | 17.73 ± 0.17 d | 84.9 | 35.35 ± 0.36 a | 108.6 | 14.70 ± 0.73 e | 63.8 | 25.08 ± 0.22 c | 69.1 | 31.73 ± 0.52 b | 116.7 |
| Quercetin-tri-O-hexoside isómer | 0.11 ± 0.00 c | 73.0 | 0.23 ± 0.00 a | 165.6 | 0.11 ± 0.00 c | 102.3 | 0.14 ± 0.00 b | n.d. | 0.23 ± 0.00 a | 204.6 |
| Quercetin-tri-O-hexoside isómer | n.d. | n.d. | 0.09 ± 0.00 | 279.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-7-O-hexoside isomer | 0.05 ± 0.00 | 18.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-7-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-O-hexoside-7-O-hexoside isomer | 0.13 ± 0.00 | 23.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-3-O-hexoside-7-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol-hexoside-rhamnoside-rhamnoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin-3-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.55 ± 0.01 b | 44.9 | 0.60 ± 0.00 a | 73.8 |
| Isorhamnetin-3-O-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin-4′-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isorhamnetin-3-O-hexoside-4′-O-hexoside | 0.92 ± 0.01 b | 174.8 | 1.68 ± 0.02 a | 70.5 | 0.29 ± 0.00 d | 27.4 | 0.85 ± 0.02 c | 38.7 | 0.73 ± 0.08 c | 52.4 |
| Isorhamnetin-O-hexoside-O-pentoside | 0.08 ± 0.00 b | 71.6 | n.d. | n.d. | n.d. | n.d. | 0.14 ± 0.00 a | 344.8 | n.d. | n.d. |
| Myricetin-di-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Myricetin-di-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total flavonols | 23.50 ± 0.17 d | 64.7 | 44.68 ± 0.37 a | 83.4 | 16.98 ± 0.73 e | 46.2 | 32.85 ± 0.25 c | 56.3 | 41.02 ± 0.53 b | 83.6 |
| Anthocyanins | ||||||||||
| Cyanidin-3-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.11 ± 0.00 | n.d. |
| Cyanidin-O-hexoside-O-hexoside isómer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cyanidin-O-hexoside-O-hexoside isómer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.24 ± 0.00 | 328.2 | n.d. | n.d. |
| Cyanidin-O-malonyl-hexoside isomer | 0.52 ± 0.00 b | 8.7 | n.d. | n.d. | 0.14 ± 0.00 d | 4.2 | 0.60 ± 0.00 a | 24.0 | 0.45 ± 0.00 c | 9.2 |
| Cyanidin-O-malonyl-hexoside isomer | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cyanidin-O-hexoside-O-malonyl-hexoside isomer | 0.31 ± 0.00 a | 6.7 | n.d. | n.d. | 0.10 ± 0.00 c | 5.8 | 0.28 ± 0.00 b | 9.9 | 0.34 ± 0.01 a | 8.4 |
| Peonidin-3-O-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Peonidin-O-malonyl-hexoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Malvidin-O-hexoside-acetaldehyde | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total anthocyanins | 0.83 ± 0.00 c | 6.6 | n.d. | n.d. | 0.24 ± 0.00 d | 3.8 | 1.11 ± 0.00 a | 17.6 | 0.90 ± 0.00 b | 8.6 |
| Total phenolic by MS * | 24.39 ± 0.17 c | 49.5 | 45.19 ± 0.37 a | 80.2 | 17.22 ± 0.73 d | 39.8 | 34.32 ± 0.25 b | 52.1 | 42.63 ± 0.53 a | 65.3 |
| Total phenolic by FC ** | 65.19 ± 3.26 d | 99.6 | 187.61 ± 4.81 a | 136.1 | 43.34 ± 2.26 e | 117.8 | 102.92 ± 1.74 c | 125.7 | 127.66 ± 3.92 b | 114.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattivelli, A.; Conte, A.; Martini, S.; Tagliazucchi, D. Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods 2021, 10, 1023. https://doi.org/10.3390/foods10051023
Cattivelli A, Conte A, Martini S, Tagliazucchi D. Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods. 2021; 10(5):1023. https://doi.org/10.3390/foods10051023
Chicago/Turabian StyleCattivelli, Alice, Angela Conte, Serena Martini, and Davide Tagliazucchi. 2021. "Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility" Foods 10, no. 5: 1023. https://doi.org/10.3390/foods10051023
APA StyleCattivelli, A., Conte, A., Martini, S., & Tagliazucchi, D. (2021). Influence of Cooking Methods on Onion Phenolic Compounds Bioaccessibility. Foods, 10(5), 1023. https://doi.org/10.3390/foods10051023