The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pesticide Residues Determination
GC-MS/MS Analysis
LC-MS/MS Analysis
2.2.2. Antibiotic Residues Determination
Nitroimidazoles Residues Determination by LC-MS/MS
Extraction Sample
LC-MS/MS Analysis
Nitrofurans Residues Determination by ELISA
Samples Extraction
ELISA Analysis
Chloramphenicol Residues Determination by ELISA
Extraction Sample
ELISA Analysis
2.2.3. Mineral Elements Determination
Water Content
Sugars
Electrical Conductivity (EC)
Color Measurement
Viscosity
Diastase Activity Determination
Hydroxymethylfurfural Concentration
2.2.4. Statistical Analysis
3. Results and Discussion
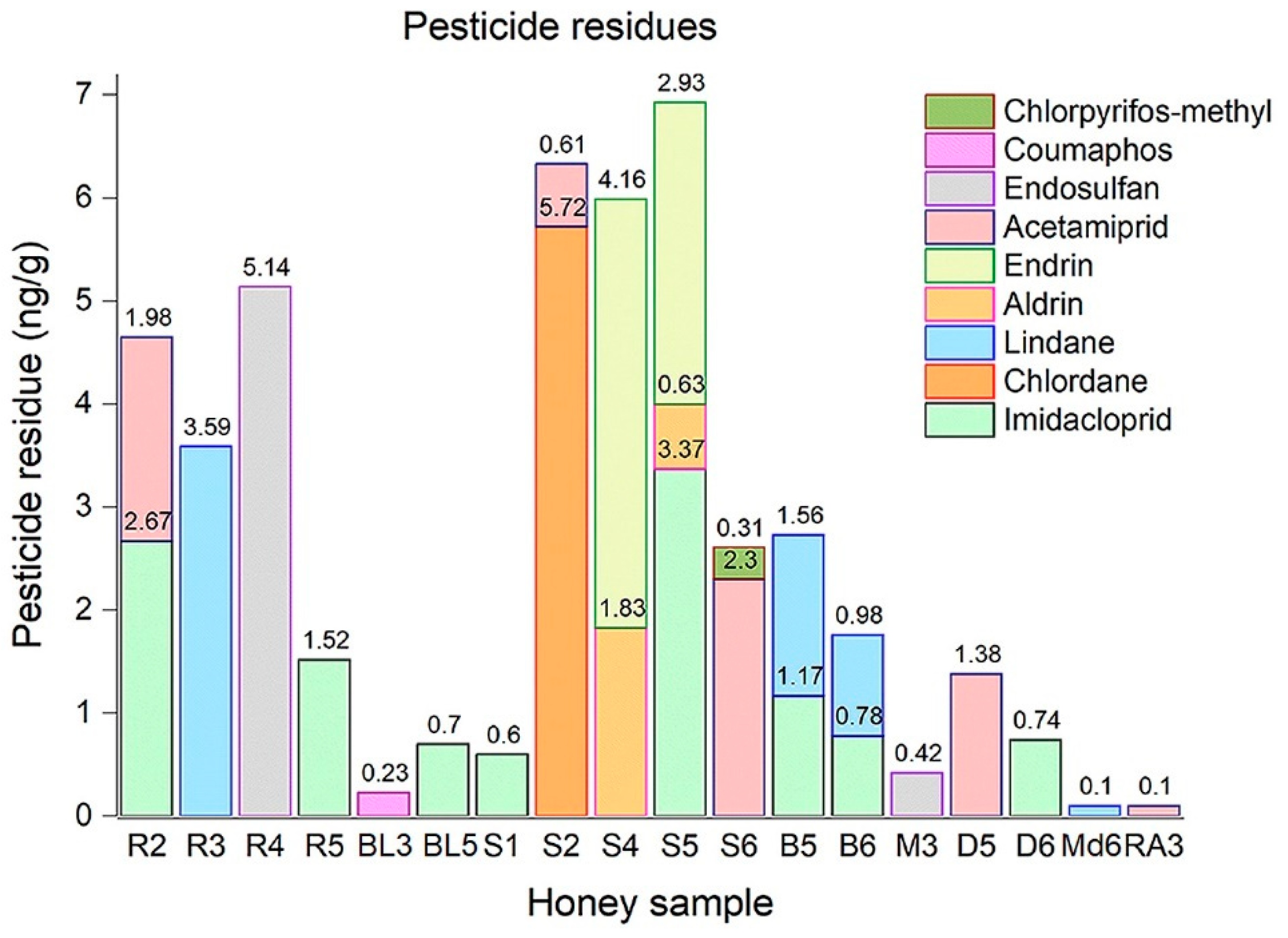
3.1. Pesticide Residues
3.2. Antibiotic Residues
3.3. Mineral Elements
3.4. Water Content
3.5. Sugars
3.6. Electrical Conductivity
3.7. Color
3.8. Viscosity
3.9. Diastase Activity
3.10. HMF Concentration
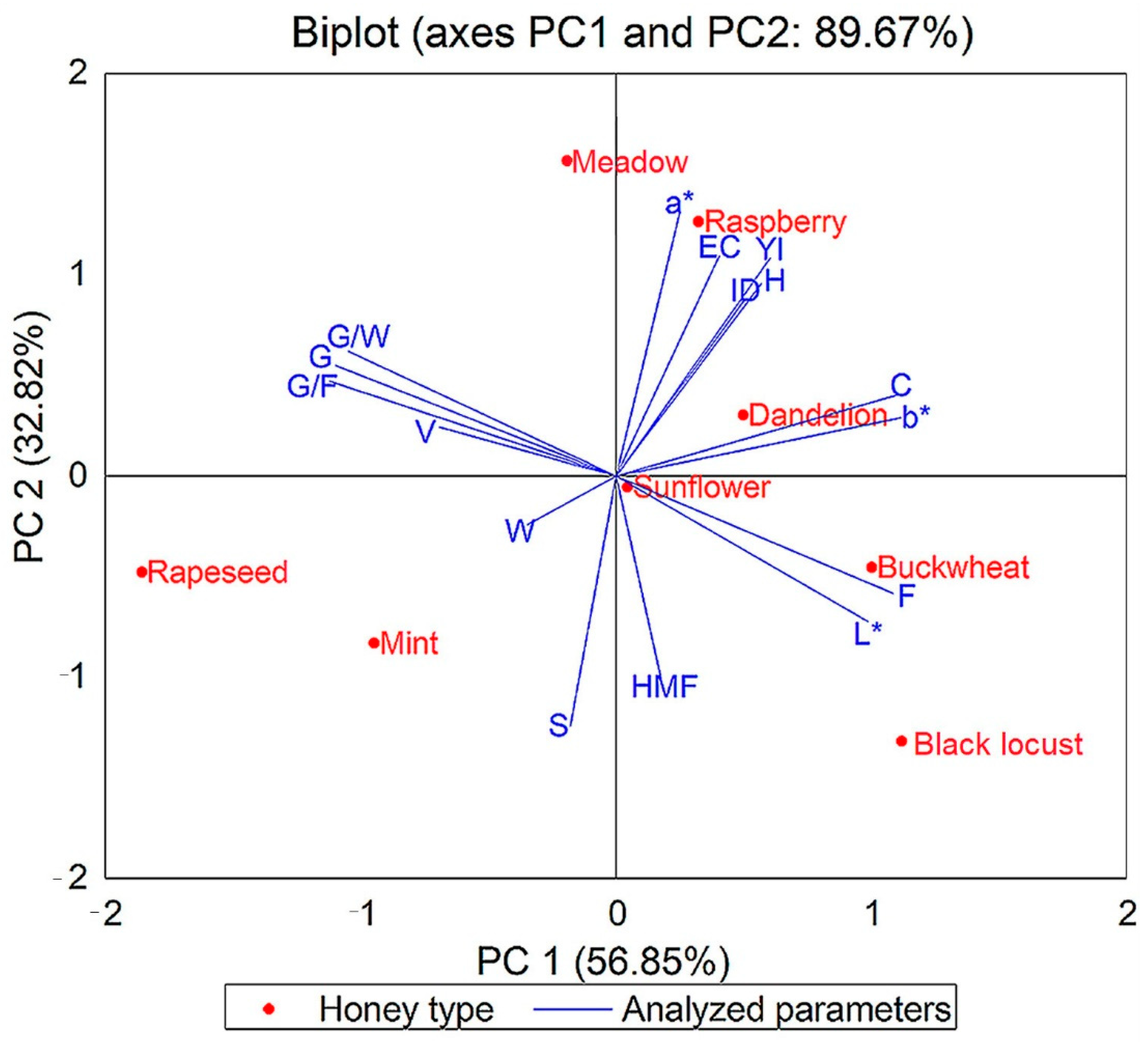
3.11. Statistical Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Commun. 2002, L.10, 47–52. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32001L0110 (accessed on 8 May 2021).
- Otero, M.C.B.; Bernolo, L. Honey as Functional Food and Prospects in Natural Honey Production. In Functional Foods and Nutraceuticals; Springer: Cham, Switzerland, 2020; pp. 197–210. [Google Scholar]
- Nisbet, C.; Kazak, F.; Ardalı, Y. Determination of quality criteria that allow differentiation between honey adulterated with sugar and pure honey. Biol. Trace Elem. Res. 2018, 186, 288–293. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2018, 38, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. J. Planar Chromatogr. Mod. TLC 2020, 33, 489–499. [Google Scholar] [CrossRef]
- Scripca, L.; Amariei, S. Research on Honey Crystalization. Rev. Chim. 2018, 69, 2953–2957. [Google Scholar] [CrossRef]
- Crăciun, M.E.; Pârvulescu, O.C.; Donise, A.C.; Dobre, T.; Stanciu, D.R. Characterization and classification of Romanian acacia honey based on its physicochemical parameters and chemometrics. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lanjwani, M.F.; Channa, F.A. Minerals content in different types of local and branded honey in Sindh, Pakistan. Heliyon 2019, 5, e02042. [Google Scholar] [CrossRef]
- Altunatmaz, S.S.; Tarhan, D.; Aksu, F.; Ozsobaci, N.P.; Or, M.E.; Barutcu, U.B. Levels of chromium, copper, iron, magnesium, manganese, selenium, zinc, cadmium, lead and aluminium of honey varieties produced in Turkey. Food Sci. Technol. 2018, 39, 392–397. [Google Scholar] [CrossRef]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of mineral content and heavy metal of honey samples from south and east region of Turkey by using ICP-MS. Int. J. Anal. Chem. 2017. [Google Scholar] [CrossRef]
- Altundag, H.; Albayrak, S.; Dundar, M.S.; Tuzen, M.; Soylak, M. Investigation of the influence of selected soil and plant properties from Sakarya, Turkey, on the bioavailability of trace elements by applying an in vitro digestion model. Biol. Trace. Elem. Res. 2015, 168, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 1–14. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Košice (eastern Slovakia). Environ. Sci. Pollut. Res. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Nega, A.; Mulugeta, E.; Abebaw, A. Physicochemical Analysis and Determination of the Levels of Some Heavy Metals in Honey Samples Collected from Three District Area of East Gojjam Zone of Amhara Region, Ethiopia. J. Agri. Sci. Food Res. 2020, 11, 279. [Google Scholar]
- Lasić, D.; Bubalo, D.; Bošnir, J.; Šabarić, J.; Konjačić, M.; Dražić, M.; Racz, A. Influence of the botanical and geographical origin on the mineral composition of honey. Agric. Conspec. Sci. 2018, 83, 335–343. [Google Scholar]
- Nkansah, M.A.; Shamsu–Deen, M.; Opoku, F. Phytocompounds, heavy metal and mineral contents in honey samples from selected markets in the kumasi metropolis. Emerg. Sci. J. 2018, 2, 287–294. [Google Scholar] [CrossRef]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.D.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Darko, G.; Addai Tabi, J.; Adjaloo, M.K.; Borquaye, L.S. Pesticide residues in honey from the major honey producing forest belts in Ghana. J. Environ. Public Health 2017. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, W.; Xie, J.; Wang, W.; Lu, T.; Dong, Q.; Yang, H. Monitoring and risk assessment of pesticide residue in plant-soil-groundwater systxem about medlar planting in Golmud. Environ. Sci. Pollut. Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef] [PubMed]
- Agbohessi, P.T.; Imorou Toko, I.; Ouédraogo, A.; Jauniaux, T.; Mandiki, S.N.M.; Kestemont, P. Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa). Sci. Total Environ. 2015, 506–507, 567–584. [Google Scholar]
- Agbohessi, P.T.; Toko, I.I.; Atchou, V.; Tonato, R.; Mandiki, S.N.M.; Kestemont, P. Pesticides used in cotton production affect reproductive development, endocrine regulation, liver status and offspring fitness in African catfish Clarias gariepinus (Burchell, 1822). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 167, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Watermann, B.T.; Albanis, T.A.; Galassi, S. Effects of anti-androgens cyproterone acetate, linuron, vinclozolin, and p,p′-DDE on the reproductive organs of the copepod Acartia tonsa. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 1111–1120. [Google Scholar] [CrossRef]
- Febvey, O.; Schüz, J.; Bailey, H.D. Risk of central nervous system tumors in children related to parental occupational pesticide exposures in three European case-control studies. J. Occup. Environ. Med. 2016, 58, 1046–1052. [Google Scholar] [CrossRef]
- Sturza, J.; Silver, M.K.; Xu, L. Prenatal exposure to multiple pesticides is associated with auditory brainstem response at 9 months in a cohort study of Chinese infants. Environ. Int. 2016, 92–93, 478–485. [Google Scholar] [CrossRef]
- Ghorab, A.; Rodríguez-Flores, M.S.; Nakib, R.; Escuredo, O.; Haderbache, L.; Bekdouche, F.; Seijo, M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria). Foods 2021, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Li, X.; Ouyang, G.; Cui, J.; Zhang, L.; Yu, A.; Cui, Y. Morphology-maintaining synthesis of copper hydroxy phosphate@ metal–organic framework composite for extraction and determination of trace mercury in rice. Food Chem. 2021, 343, 128508. [Google Scholar] [CrossRef]
- Mancuso, T.; Croce, L.; Vercelli, M. Total brood removal and other biotechniques for the sustainable control of Varroa mites in honey bee colonies: Economic impact in beekeeping farm case studies in northwestern Italy. Sustainability 2020, 12, 2302. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Suarez-Varela, M.M.; Picó, Y. Influence of pesticide use in fruit orchards during blooming on honeybee mortality in 4 experimental apiaries. Sci. Total Environ. 2016, 541, 33–41. [Google Scholar] [CrossRef]
- Kumar, A.; Gill, J.P.S.; Bedi, J.S.; Kumar, A. Pesticide residues in Indian raw honeys, an indicator of environmental pollution. Environ. Sci. Pollut. Res. 2018, 25, 34005–34016. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Codling, G.; Giesy, J.P. Human dietary intake and hazard characterization for residues of neonicotinoides and organophosphorus pesticides in Egyptian honey. Toxicol. Environ. Chem. 2017, 99, 1397–1408. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Pywell, R.F. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Wright, G.A. Bees prefer foods containing neonicotinoid pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef]
- Badawy, M.E.; Ismail, A.M.; Ibrahim, A.I. Quantitative analysis of acetamiprid and imidacloprid residues in tomato fruits under greenhouse conditions. J. Environ. Sci. Health Part B 2019, 54, 898–905. [Google Scholar] [CrossRef]
- Souza Tette, P.A.; Rocha Guidi, L.; de Abreu Glória, M.B.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 839/2008 of 31 July 2008 Amending Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards ANNEXES II, III and IV on Maximum Residue Levels of Pesticides in or on Certain Products. 2008. Available online: https://op.europa.eu/en/publication-detail/-/publication/615762a0-2719-49fa-b46b-46c33a6a940d/language-en (accessed on 8 May 2021).
- Commission Regulation (EC) No 149/2008 of 29 January 2008 Amending Regulation (EC) No 396/2005 of the European Parliament and of the Council by establishing Annexes II, III and IV Setting Maximum Residue Levels for Products Covered by Annex I Thereto. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:058:0001:0398:en:PDF (accessed on 8 May 2021).
- Commission Directive 2007/11/EC of 21 February 2007. Amending certain Annexes to Council Directives 86/362/EEC, 86/363/EEC and 90/642/EEC as regards maximum residue levels of acetamiprid, thiacloprid, amazosulfuron, methoxyfenozide, S-metholachlor, milbemectin and tribenuron. Off. J. Eur. Com. 2007, L63, 26–37.
- Commission Regulation (EU) No. 491/2014 of 5 May 2014. Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for ametoctradin, azoxystrobin, cycloxydim, cyfluthrin, dinotefuran, fenbuconazole, fenvalerate, fludioxonil, fluopyram, flutriafol, fluxapyroxad, glufosinateammonium, imidacloprid, indoxacarb, MCPA, methoxyfenozide, penthiopyrad, spinetoram and trifloxystrobin in or on certain products. Off. J. Eur. Com. 2014, L146, 1–91.
- Commission Implementing Regulation (EU) No. 485/2013 of 24 May 2013. Amending Implementing Regulation (EU) No 540/2011, as regards the conditions of approval of the active substances clothianidin, thiamethoxam and imidacloprid, and prohibiting the use and sale of seeds treated with plant protection products containing those active substances. Off. J. Eur. Com. 2013, L139, 12–26.
- Commission Regulation (EU) 2020/1085 of 23 July 2020 Amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Chlorpyrifos and Chlorpyrifos-Methyl in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R1085R%2801%29 (accessed on 8 May 2021).
- Commission Regulation (EU) 2017/978 of 9 June 2017 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Fluopyram; Hexachlorocyclohexane (HCH), Alpha-Isomer; Hexachlorocyclohexane (HCH), Beta-Isomer; Hexachlorocyclohexane (HCH), Sum of Isomers, Except the Gamma Isomer; Lindane (Hexachlorocyclohexane (HCH), Gamma-Isomer); Nicotine and Profenofos in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32017R0978 (accessed on 8 May 2021).
- Commission Regulation (EU) 2017/623 of 30 March 2017 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Acequinocyl, Amitraz, Coumaphos, Diflufenican, Flumequine, Metribuzin, Permethrin, Pyraclostrobin and Streptomycin in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0623 (accessed on 8 May 2021).
- Commission Regulation (EU) No 310/2011 of 28 March 2011 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Aldicarb, Bromopropylate, CHLORFENVINPHOS, ENDOSULFAN, EPTC, Ethion, Fenthion, Fomesafen, Methabenzthiazuron, Methidathion, Simazine, Tetradifon and Triforine in or on Certain Products. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0310 (accessed on 8 May 2021).
- Bonerba, E.; Panseri, S.; Arioli, F.; Nobile, M.; Terio, V.; Di Cesare, F.; Tartillo, G.; Chiesa, L.M. Determination of antibiotic residues in honey in relation to different potential sources and relevance for food inspection. Food Chem. 2021, 334, 127575. [Google Scholar] [CrossRef]
- Al-Mashhadany, D.A. Detection of antibiotic residues among raw beef in Erbil city (Iraq) and impact of temperature on antibiotic remains. Ital. J. Food Saf. 2019, 8, 7897. [Google Scholar] [CrossRef] [PubMed]
- Mensah, S.E.; Koudande, O.D.; Sanders, P.; Laurentie, M.; Mensah, G.A.; Abiola, F.A. Antimicrobial residues in foods of animal origin in Africa: Public health risks. Rev. Sci. Tech. 2014, 33, 987–996. [Google Scholar] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S. Antibiotic residues and mycotoxins in raw milk in Punjab (India): A rising concern for food safety. J. Food Sci. Technol. 2019, 56, 5146–5151. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. A hybrid material composed of reduced graphene oxide and porous carbon prepared by carbonization of a zeolitic imidazolate framework for voltammetric determination of chloramphenicol. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Paramasivam, M.; Sasikala, M.; Tamilam, T.V.; Sumithra, A. Review on antibiotic residues in animal products and its impact on environments and human health. J. Entomol. Zool. Stud. 2017, 5, 1446–1451. [Google Scholar]
- Gaudin, V.; Hedou, C.; Soumet, C.; Verdon, E. Evaluation and validation of biochip multi-array technology for the screening of six families of antibiotics in honey according to the European guideline for the validation of screening methods for residues of veterinary medicines. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1699–1711. [Google Scholar] [CrossRef]
- Commission regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. 2009, L15, 72.
- Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R0470 (accessed on 8 May 2021).
- CRL Guidance Paper (7dec2007). Available online: https://www.rivm.nl/bibliotheek/digitaaldepot/crlguidance2007.pdf (accessed on 8 May 2021).
- Nemati Niko, Z.; Jahed Khaniki, G.; Alikord, M.; MolaeeAghaee, E. ELISA and Copan Based Evaluation and Analysis of Antibiotic Residues in Cattle Milk in Qazvin, Iran. Infect. Epidemiol. Microbiol. 2020, 6, 219–227. [Google Scholar]
- Liu, Y.C.; Jiang, W.; Chen, Y.J.; Zeng, P.; Zhang, M.; Wang, Q. Simultaneous detection of four nitrofuran metabolites in honey using high-throughput suspension array technology. Anal. Methods 2015, 7, 4097–4103. [Google Scholar] [CrossRef]
- Guo, X.C.; Xia, Z.Y.; Wang, H.H.; Kang, W.Y.; Lin, L.M.; Cao, W.Q.; Zhang, H.W.; Zhou, W.H. Molecularly imprinted solid phase extraction method for simultaneous determination of seven nitroimidazoles from honey by HPLC-MS/MS. Talanta 2017, 166, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Xu, D. Simultaneous detection of four nitrofuran metabolites in honey by using a visualized microarray screen assay. Food Chem. 2017, 221, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2019/1871 of 7 November 2019 on Reference Points for Action for Non-Allowed Pharmacologically Active Substances Present in Food of Animal Origin and Repealing Decision 2005/34/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2019.289.01.0041.01.ENG&toc=OJ%3AL%3A2019%3A289%3ATOC (accessed on 8 May 2021).
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Apic. Res. 2018, 57, 97–112. [Google Scholar] [CrossRef]
- Scripcă, L.A.; Norocel, L.; Amariei, S. Comparison of Physicochemical, Microbiological Properties and Bioactive Compounds Content of Grassland Honey and other Floral Origin Honeys. Molecules 2019, 24, 2932. [Google Scholar] [CrossRef]
- Paradis, D.; Bérail, G.; Bonmatin, J.-M.; Belzunces, L.P. Sensitive analytical methods for 22 relevant insecticides of 3 chemical families in honey by GC-MS/MS and LC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 621–633. [Google Scholar] [CrossRef]
- Gaweł, M.; Kiljanek, T.; Niewiadowska, A.; Semeniuk, S.; Goliszek, M.; Burek, O.; Posyniak, A. Determination of neonicotinoids and 199 other pesticide residues in honey by liquid and gas chromatography coupled with tandem mass spectrometry. Food Chem. 2019, 282, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Sante/11813/2017. Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 15 March 2021).
- Kanda, M.; Sasamoto, T.; Takeba, K.; Hayashi, H.; Kusano, T.; Matsushima, Y.; Nakajima, T.; Kanai, S.; Takano, I. Rapid Determination of Nitroimidazole Residues in Honey by Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2012, 95, 923–931. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Li, X.; Wang, Y.; Li, H.; Fu, Q.; Shan, Y.; Liu, T.; Xia, X. Multi-class method for the determination of nitroimidazoles, nitrofurans, and chloramphenicol in chicken muscle and egg by dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2017, 217, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, J.; Lv, Z.; Zhu, X.; Xue, X.; Wu, L.; Cao, W. Simultaneous determination of nitroimidazoles and quinolones in honey by modified QuEChERS and LC-MS/MS analysis. Int. J. Anal. Chem. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ke, Y.; Wang, Y.; Wang, C.; Ye, D.; Hu, X.; Xia, X. Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2018, 23, 3350. [Google Scholar] [CrossRef] [PubMed]
- Jester, E.L.; Abraham, A.; Wang, Y.; El Said, K.R.; Plakas, S.M. Performance evaluation of commercial ELISA kits for screening of furazolidone and furaltadone residues in fish. Food Chem. 2014, 145, 593–598. [Google Scholar] [CrossRef]
- Wang, J.; Chang, X.; Zuo, X.; Liu, H.; Ma, L.; Li, H.; Tao, X. A multiplex immunochromatographic assay employing colored latex beads for simultaneously quantitative detection of four nitrofuran metabolites in animal-derived food. Food Anal. Methods 2019, 12, 503–516. [Google Scholar] [CrossRef]
- Biernacki, B. ELISA validation and determination of cut-off level for chloramphenicol residues in honey. Bull. Vet. Inst. Pulawy 2015, 59, 353–356. [Google Scholar] [CrossRef]
- Yanovych, D.; Berendsen, B.; Zasadna, Z.; Rydchuk, M.; Czymai, T. A study of the origin of chloramphenicol isomers in honey. Drug Test. Anal. 2018, 10, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, G.G.; Huth, T.; Harms, D. Screening of stereoisomeric chloramphenicol residues in honey by ELISA and CHARM® II test–the potential risk of systematically false-compliant (false negative) results. Food Addit. Contam. Part A 2020, 37, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Amariei, S.; Gutt, G.; Oroian, M. Study on toxic metal levels in Food supplements. Rev. Chim. 2017, 68, 1396–1400. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R. Honey quality and international regulatory standards: Review by the international honey commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P. Honey authenticity. Mitt. Aus Leb. Hyg. 2002, 93, 232–254. [Google Scholar]
- Amariei, S.; Norocel, L.; Scripcă, L.A. An innovative method for preventing honey crystallization. Innov. Food Sci. Emerg. Technol. 2020, 66, 102481. [Google Scholar] [CrossRef]
- Lullah-Deh, J.A.; Khan, M.E.; Eneji, I.S. Physicochemical Characteristics of Honey Samples from Mambilla Plateau, Nigeria. J. Biomater. 2018, 2, 7. [Google Scholar] [CrossRef]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical properties and pollen profile of Oak honeydew and Evergreen Oak honeydew honeys from Spain: A comparative study. Foods 2019, 8, 126. [Google Scholar] [CrossRef]
- Samira, N. The effect of heat treatment on the quality of Algerian honey. Researcher 2016, 8, 1–6. [Google Scholar]
- Taha, A.A.; Balabel, N.M.; Elshishtawy, H.M. Physicochemical Characterization and Antimicrobial Activity of Sidr Honey Produced by Dwarf Honey Bees (Apis florea F.). J. Plant Prot. Pathol. 2019, 10, 621–628. [Google Scholar] [CrossRef]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Achanta, K.; Aryana, K.J.; Boeneke, C.A. Fat free plain set yogurts fortified with various minerals. LWT-Food Sci. Technol. 2007, 40, 424–429. [Google Scholar] [CrossRef]
- Couto, D.S.; Cabral, L.M.C.; Matta, V.M.D.; Deliza, R.; Freitas, D.D.G.C. Concentration of pineapple juice by reverse osmosis: Physicochemical characteristics and consumer acceptance. Food Sci. Technol. 2011, 31, 905–910. [Google Scholar] [CrossRef]
- Valdovinos-Flores, C.; Alcantar-Rosales, V.M.; Gaspar-Ramírez, O.; Saldaña-Loza, L.M.; Dorantes-Ugalde, J.A. Agricultural pesticide residues in honey and wax combs from Southeastern, Central and Northeastern Mexico. J. Apic. Res. 2017, 56, 667–679. [Google Scholar] [CrossRef]
- Bommuraj, V.; Chen, Y.; Klein, H.; Sperling, R.; Barel, S.; Shimshoni, J.A. Pesticide and trace element residues in honey and beeswax combs from Israel in association with human risk assessment and honey adulteration. Food Chem. 2019, 299, 125123. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, H.A.; Yadav, G.S.; Kumari, B. Validation and monitoring of pesticide residues in honey using QuEChERS and gas chromatographic analysis. J. Apic. Res. 2015, 54, 260–266. [Google Scholar] [CrossRef]
- Pravcová, K.; Macháčková, N.; Česlová, L. Determination of selected pesticides in honey and mead by HPLC. Sci. Pap. Univ. Pardubic. Ser. Afac. Chem. Technol. 2020, 26. [Google Scholar] [CrossRef]
- Deng, M.; Yu, T.; Luo, H.; Zhu, T.; Huang, X.; Luo, L. Direct detection of multiple pesticides in honey by neutral desorption-extractive electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 111–118. [Google Scholar] [CrossRef]
- Rafique, N.; Nazir, S.; Akram, S.; Ahad, K.; Gohar, A.; Abbasi, S.T.; Rafique, K. Screening of multiclass pesticide residues in honey by SPE-GC/MSD: A pilot study. Environ. Monit. Assess. 2018, 190, 1–11. [Google Scholar] [CrossRef]
- Song, S.; Zhang, C.; Chen, Z.; He, F.; Wei, J.; Tan, H.; Li, X. Simultaneous determination of neonicotinoid insecticides and insect growth regulators residues in honey using LC–MS/MS with anion exchanger-disposable pipette extraction. J. Chromatogr. A 2018, 1557, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Development of a gas chromatography-tandem mass spectrometry procedure for determination of pesticide residues in honey and honeybee samples. J. Chromatogr. Sep. Tech. 2015, 6, 002. [Google Scholar]
- Medici, S.K.; Blando, M.; Sarlo, E.; Maggi, M.; Espinosa, J.P.; Ruffinengo, S.; Bianchi, B.; Eguaras, M.; Recavarren, M. Pesticide residues used for pest control in honeybee colonies located in agroindustrial areas of Argentina. Int. J. Pest Manag. 2020, 66, 163–172. [Google Scholar] [CrossRef]
- Panseri, S.; Bonerba, E.; Nobile, M.; Di Cesare, F.; Mosconi, G.; Cecati, F.; Arioli, F.; Tantillo, G.; Chiesa, L. Pesticides and Environmental Contaminants in Organic Honeys According to Their Different Productive Areas toward Food Safety Protection. Foods 2020, 9, 1863. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Petcu, C.D.; Ciobotaru-Pîrvu, E.; Ghimpețeanu, O.M.; Goran, G.V.; Predescu, C.N.; Oprea, O.D. Study regarding the honey contamination degree assessed in a specialized production unit. Sci. Pap. Ser. D Anim. Sci. Int. Sess. Sci. Commun. Fac. Anim. Sci. 2020, 63, 442–449. [Google Scholar]
- Chiesa, L.M.; Panseri, S.; Nobile, M.; Ceriani, F.; Arioli, F. Distribution of POPs, pesticides and antibiotic residues in organic honeys from different production areas. Food Addit. Contam. Part A 2018, 35, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Morariu, I.D.; Avasilcăi, L.; Vieriu, M.; Cioancă, O.; Hâncianu, M. Immunochemical assay of chloramphenicol in honey. Farmacia 2019, 67, 235–239. [Google Scholar] [CrossRef]
- Jia, Q.; Yu, S.; Cheng, N.; Wu, L.; Jia, J.; Xue, X.; Cao, W. Stability of nitrofuran residues during honey processing and nitrofuran removal by macroporous adsorption resins. Food Chem. 2014, 162, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, J.; Zhao, W.; Zhang, W.; Wang, L.; Zhou, J.; Li, Y. Development and validation of a multiclass method for the quantification of veterinary drug residues in honey and royal jelly by liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 221, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Gunes, N.; Cibik, R.; Gunes, M.E.; Aydin, L. Erythromycin residue in honey from the Southern Marmara region of Turkey. Food Addit. Contam. Part A 2008, 25, 1313–1317. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Moosavy, M.H.; Norian, R.; Kazemi, S.; Asadi Nadari, M.R.; Mardani, K. Detection of oxytetracycline residues in honey samples using ELISA and HPLC methods. Pharm. Sci. 2014, 19, 145–151. [Google Scholar]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A.; Hannan, M.A. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014, 18, 618–625. [Google Scholar] [CrossRef]
- De Andrade, C.K.; dos Anjos, V.E.; Felsner, M.L.; Torres, Y.R.; Quináia, S.P. Direct determination of Cd, Pb and Cr in honey by slurry sampling electrothermal atomic absorption spectrometry. Food Chem. 2014, 146, 166–173. [Google Scholar] [CrossRef]
- Camina, M.; Pellerano, J.G.R.; J Marchevsky, E. Geographical and botanical classification of honeys and apicultural products by chemometric methods. A review. Curr. Anal. Chem. 2012, 8, 408–425. [Google Scholar] [CrossRef]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental analysis of Hungarian honey samples and bee products by MP-AES method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Jovetić, M.; Trifković, J.; Stanković, D.; Manojlović, D.; Milojković-Opsenica, D. Mineral content as a tool for the assessment of honey authenticity. J. Aoac Int. 2017, 100, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical properties of eleven monofloral honey samples produced in Morocco. Arab J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy metals determination in honey samples using inductively coupled plasma-optical emission spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Uršulin-Trstenjak, N.; Levanić, D.; Primorac, L.; Bošnir, J.; Vahčić, N.; Šarić, G. Mineral profile of Croatian honey and differences due to its geographical origin. Czech J. Food Sci. 2015, 33, 156–164. [Google Scholar] [CrossRef]
- Dżugan, M.; Zaguła, G.; Wesołowska, M.; Sowa, P.; Puchalski, C. Levels of toxic and essential metals in varietal honeys from Podkarpacie. J. Elem. 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Nowak, L.; Dzieżyc, H.; Piotrowski, M. Content of bioelements and toxic metals in honey of various botanical origin from Lower Silesia. J. Elem. 2011, 16, 437–444. [Google Scholar]
- Nayik, G.A.; Suhag, Y.; Majid, I.; Nanda, V. Discrimination of high altitude Indian honey by chemometric approach according to their antioxidant properties and macro minerals. J. Saudi Soc. Agric. Sci. 2018, 17, 200–207. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Passos, T.M.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.; Lichtenberg-Kraag, B. Long-term changes in naturally produced honey depending on processing and temperature. J. Apic. Res. 2018, 57, 615–626. [Google Scholar] [CrossRef]
- Ramli, A.S.; Basrawi, F.; Yusof, M.H.B.; Oumer, A.N.; Johari, N.A.; Muhamad, A.; Mamat, M.R.; Habib, K.; Ibrahim, T.K. Experimental analysis on a novel low-temperature vacuum drying with induced nucleation technique for dewatering stingless bees honey. Dry. Technol. 2019, 37, 149–155. [Google Scholar] [CrossRef]
- Silici, S. Effects of crystallization on antioxidant property of honey. J. Apitherapy 2018, 3, 24–30. [Google Scholar]
- Codex Alimentarius Commission. Draft Revised Standard for Honey (at Step 10 of the Codex Procedure); Codex Alimentarius Commission, FAO: Rome, Italy, 2001; Volume 25, pp. 19–26. [Google Scholar]
- Abdulkhaliq, A.; Swaileh, K.M. Physico-chemical properties of multi-floral honey from the West Bank, Palestine. Int. J. Food Prop. 2017, 20, 447–454. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- Küçük, M.; Kolayli, S.; Karaoǧlu, Ş.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Al Marghitas, L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar]
- Baloš, M.Ž.; Jakšić, S.; Popov, N.; Mihaljev, Ž.; Pelić, D.L. Comparative study of water content in honey produced in different years. Arch. Vet. Med. 2019, 12, 43–53. [Google Scholar] [CrossRef]
- Özenirler, Ç. Dandelion Honey: A New Monofloral Honey Record for Turkey. Uludağ Arıcılık Derg. 2018, 18, 87–93. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Żak, N. Commodity Characteristics of Rare Honey Varieties. Towar. Probl. Jakości 2018, 4, 86–93. [Google Scholar]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Skałecki, P.; Litwińczuk, A. Characterisation of viscosity, colour, 5-hydroxymethylfurfural content and diastase activity in raw rape honey (Brassica napus) at different temperatures. J. Food Sci. Technol. 2016, 53, 2092–2098. [Google Scholar] [CrossRef]
- Saeed, M.A.; Jayashankar, M. Physico-chemical characteristics of some Indian and Yemeni Honey. J. Bioenergy Food Sci. 2020, 7, 2832019. [Google Scholar] [CrossRef]
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P.; Stojanov, I.M.; Jakšić, S.M. Sugar profile of different floral origin honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Zielińska, S.; Wesołowska, M.; Bilek, M.; Kaniuczak, J.; Dżugan, M. The saccharide profile of Polish honeys depending on their botanical origin. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 387–390. [Google Scholar]
- Beşir, D.A.Ğ. Investigation of some properties of chestnut honey produced in black sea region of Turkey. Batman Üniversitesi Yaşam Bilimleri Derg. 2017, 7, 118–123. [Google Scholar]
- Taleuzzaman, M.; Kala, C.; Gilani, S.J. Validation, Chemical Composition, and Stability of Honey from Indian Himalayas. In Therapeutic Applications of Honey and Its Phytochemicals; Springer: Singapore, 2020; pp. 81–100. [Google Scholar]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Comparison of the physicochemical characteristics of sidr (Ziziphus spp.) honey produced by Apis florea F. and Apis mellifera L. J. Apic. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Mohammed, S.A.; Ansari, M.J.; Adgaba, N. Comparison of physicochemical properties and effects of heating regimes on stored Apis mellifera and Apis florea honey. Saudi J. Biol. Sci. 2019, 26, 845–848. [Google Scholar] [CrossRef]
- Wiratmoko, M.D.E.; Pribadi, A. Physicochemical characteristics of west Sumatera’s forest honey. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 415, p. 012015. [Google Scholar]
- Marc, L.; Mărghitaş, L.A.; Bobiş, O.; Bonta, V.; Mihai, C. Preliminary study on the authenticity of Acacia, Multifloral and Linden honey from Cluj County. Bull. Usamv Anim. Sci. Biotechnol. 2012, 69, 129–135. [Google Scholar]
- Mădaş, N.; Mărghitaş, L.A.; Dezmirean, D.; Bonta, V.; Bobiş, O. Botanical origin authentication of Black locust (Robinia pseudoacacia) honey, by means of sugar spectrum determnination. Agricultura 2012, 3, 95–100. [Google Scholar]
- Yayinie, M.; Atlabachew, M.; Tesfaye, A.; Hilluf, W.; Reta, C. Quality authentication and geographical origin classification of honey of Amhara region, Ethiopia based on physicochemical parameters. Arab. J. Chem. 2021, 102987. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, R.; Barbaro, E.; Zangrando, R.; Rizzoli, A.; Malagnini, V.; Gambaro, A.; Capodaglio, G. Carbohydrate determination in honey samples by ion chromatography–mass spectrometry (HPAEC-MS). Anal. Bioanal. Chem. 2020, 412, 5217–5227. [Google Scholar] [CrossRef]
- Juan-Borrás, M.; Domenech, E.; Hellebrandova, M.; Escriche, I. Effect of 324 country origin on physicochemical, sugar and volatile composition of acacia, 325 sunflower and tilia honeys. Food Res. Int. 2014, 60, 86–94. [Google Scholar] [CrossRef]
- Sakač, M.B.; Jovanov, P.T.; Marić, A.Z.; Pezo, L.L.; Kevrešan, Ž.S.; Novaković, A.R.; Nedeljković, N.M. Physicochemical properties and mineral content of honey samples from Vojvodina (Republic of Serbia). Food Chem. 2019, 276, 15–21. [Google Scholar] [CrossRef]
- Salonen, A.; Hiltunen, J.; Julkunen-Tiitto, R. Composition of unique unifloral honeys from the boreal coniferous forest zone: Fireweed and raspberry honey. J. Apiproduct Apimedical Sci. 2011, 3, 128–136. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Spirić, D.; Ćirić, J.; Đorđević, V.; Nikolić, D.; Janković, S.; Nikolić, A.; Petrović, Z.; Katanić, N.; Teodorović, V. Toxic and essential element concentrations in different honey types. Int. J. Environ. Anal. Chem. 2019, 99, 474–485. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bouddine, T.; Bakour, M.; Ousaaid, D.; Lyoussi, B. Physicochemical properties, mineral content, antioxidant activities, and microbiological quality of Bupleurum spinosum Gouan honey from the middle atlas in Morocco. J. Food Qual. 2020, 2020. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Ceylan, D.A.; Uslu, N.; Gül, A.; Özcan, M.M.; Özcan, M.M. Effect of honey types on physico-chemical properties, electrical conductivity and mineral contents of honeys. J. Agroaliment. Process. Technol. 2019, 25, 31–35. [Google Scholar]
- Conti, M.E.; Canepari, S.; Finoia, M.G.; Mele, G.; Astolfi, M.L. Characterization of Italian multifloral honeys on the basis of their mineral content and some typical quality parameters. J. Food Compos. Anal. 2018, 74, 102–113. [Google Scholar] [CrossRef]
- Escuredo, O.; Rodríguez-Flores, M.S.; Meno, L.; Seijo, M.C. Prediction of Physicochemical Properties in Honeys with Portable Near-Infrared (microNIR) Spectroscopy Combined with Multivariate Data Processing. Foods 2021, 10, 317. [Google Scholar] [CrossRef]
- Gizaw, A.; Tassew, A.; Begna, D. The Status of Honey Quality Produced in Gedebano Gutazer Wolene, Central Ethhiopia. J. Food Sci. Eng. 2020, 10, 110–119. [Google Scholar]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Pontis, J.A.; Costa, L.A.M.A.D.; Silva, S.J.R.D.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Popov-Raljić, J.; Arsić, N.; Zlatković, B.; Basarin, B.; Mladenović, M.; Laličić-Petronijević, J.; Ivkov, M.; Popov, V. Evaluation of color, mineral substances and sensory uniqueness of meadow and acacia honey from Serbia. Rom. Biotechnol. Lett. 2015, 20, 10784–10799. [Google Scholar]
- Kaczmarek, A.; Muzolf-Panek, M.G.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Bayram, N.E.; Kara, H.H.; Can, A.M.; Bozkurt, F.; Akman, P.K.; Vardar, S.U.; Çebi, N.; Yılmaz, M.T.; Sagdıc, O.; Dertli, E. Characterization of physicochemical and antioxidant properties of Bayburt honey from the North-east part of Turkey. J. Apic. Res. 2021, 60, 46–56. [Google Scholar] [CrossRef]
- Belay, A.; Haki, G.D.; Birringer, M.; Borck, H.; Lee, Y.C.; Cho, C.W.; Kim, K.T.; Bayissa, B.; Baye, K.; Melaku, S. Sugar profile and physicochemical properties of Ethiopian monofloral honey. Int. J. Food Prop. 2017, 20, 2855–2866. [Google Scholar] [CrossRef]
- Saxena, S.; Panicker, L.; Gautam, S. Rheology of Indian honey: Effect of temperature and gamma radiation. Int. J. Food Sci. 2014, 2014, 935129. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Honey moisture reduction and its quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.T.; Rosa, I.G.; Conti-Silva, A.C. Honey from Tiúba stingless bees (Melipona fasciculata) produced in different ecosystems: Physical and sensory studies. J. Sci. Food Agric. 2020, 100, 3748–3754. [Google Scholar] [CrossRef]
- Gairola, A.; Tiwari, P.; Tiwari, J.K. Physico-chemical properties of Apis cerana-indica F. honey from Uttarkashi district of Uttarakhand, India. J. Glob. Biosci. 2013, 2, 20–25. [Google Scholar]
- Bambang, N.; Ikhsan, M.; Sukri, N. Rheological Properties of Honey and its Application on Honey Flow Simulation through Vertical Tube. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 334, p. 012041. [Google Scholar]
- Leme, L.M.; Montenegro, H.R.; dos Santos, L.D.R.; Sereia, M.J.; Valderrama, P.; Março, P.H. Relation between near-infrared spectroscopy and physicochemical parameters for discrimination of honey samples from Jatai weyrauchi and Jatai angustula bees. Food Anal. Methods 2018, 11, 1944–1950. [Google Scholar] [CrossRef]
- Al-Habsi, N.A.; Davis, F.J.; Niranjan, K. Development of novel methods to determine crystalline glucose content of honey based on DSC, HPLC, and viscosity measurements, and their use to examine the setting propensity of honey. J. Food Sci. 2013, 78, E845–E852. [Google Scholar] [CrossRef] [PubMed]
- Tôrres, W.D.L.; Vilvert, J.C.; Carvalho, A.T.; Leite, R.H.D.L.; Santos, F.K.G.D.; Aroucha, E.M.M. Quality of Apis mellifera honey after being used in the feeding of jandaira stingless bees (Melipona subnitida). Acta Sci. Anim. Sci. 2021, 43. [Google Scholar] [CrossRef]
- Mesele, T.L. Review on physico-chemical properties of honey in Eastern Africa. J. Apic. Res. 2021, 60, 33–45. [Google Scholar] [CrossRef]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese honeys from different geographical and botanical origins: A 4-year stability study regarding quality parameters and antioxidant activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef]
- Matović, K.; Ćirić, J.; Kaljević, V.; Nedić, N.; Jevtić, G.; Vasković, N.; Baltić, M.Ž. Physicochemical parameters and microbiological status of honey produced in an urban environment in Serbia. Environ. Sci. Pollut. Res. 2018, 25, 14148–14157. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gonzaga, L.V.; Biluca, F.C.; Schulz, M.; Vitali, L.; Micke, G.A.; Fett, R. Stability of Brazilian Apis mellifera L. honey during prolonged storage: Physicochemical parameters and bioactive compounds. LWT 2020, 129, 109521. [Google Scholar] [CrossRef]
- Chambó, E.D.; Garcia, R.C.; Cunha, F.; de Carvalho, C.A.L.; de Jesus Oliveira, D.; Caldas, M.J.M.; Silva, N.L.S.; Ronqui, L.; Silva, J.; Rosa Santos, P.; et al. Development of Beekeeping: An Analysis Using the Technique of Principal Components. In Insect Science-Diversity, Conservation and Nutrition; IntechOpen: London, UK, 2018; Chapter 4; pp. 61–74. [Google Scholar]
- Kamal, M.M.; Rashid, M.H.U.; Mondal, S.C.; El Taj, H.F.; Jung, C. Physicochemical and microbiological characteristics of honey obtained through sugar feeding of bees. J. Food Sci. Technol. 2019, 56, 2267–2277. [Google Scholar] [CrossRef]
- Khalafi, R.; Goli, S.A.H.; Behjatian, M. Characterization and Classif. of several monofloral Iranian honeys based on physicochemical properties and antioxidant activity. Int. J. Food Prop. 2016, 19, 1065–1079. [Google Scholar] [CrossRef]
- Isopescu, R.D.; Josceanu, A.M.; Minca, I.; Colta, T.; Postelnicescu, P.; Mateescu, C. Characterization of Romanian honey based on physicochemical properties and multivariate analysis. Rev. Chim. Buchar. 2014, 65, 381–385. [Google Scholar]
- Pasias, I.N.; Kiriakou, I.K.; Proestos, C. HMF and diastase activity in honeys: A fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Mouhoubi-Tafinine, Z.; Ouchemoukh, S.; Louaileche, H.; Tamendjari, A. Effect of storage on hydroxymethylfurfural (HMF) and color of some Algerian honey. Int. Food Res. J. 2018, 25, 1044–1050. [Google Scholar]
- Aypak, S.Ü.; Aslıhan, İ.N.C.İ.; Bakirci, S.; Fidan, E.D.; Soysal, M. Comparision of the Antioxidant Activity and Hydroxymethylfurfural (HMF) Levels in Honey Taken from Hives and Markets. Gıda 2019, 44, 86–92. [Google Scholar]
- Rajs, B.B.; Flanjak, I.; Mutić, J.; Vukojević, V.; Đurđić, S.; Primorac, L. Characterization of Croatian Rape (Brassica sp.) Honey by Pollen Spectrum, Physicochemical Characteristics, and Multielement analysis by ICP-OES. J. Aoac Int. 2017, 100, 881–888. [Google Scholar] [CrossRef]
- Dżugan, M.; Sowa, P.; Kwaśniewska, M.; Wesołowska, M.; Czernicka, M. Physicochemical parameters and antioxidant activity of bee honey enriched with herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef]





| Honey Variety | Water Content, % | Glucose, g/100 g | Fructose, g/100 g | Sucrose, g/100 g | G/F | G/W | Conductivity, mS/cm | HMF, mg/kg | Diastase Activity, (°) | Viscosity, Pa·s | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rapeseed | Mean ± SD | 17.63 ± 0.36 | 41.31 ± 0.38 | 29.36 ± 0.53 | 1.80 ± 0.44 | 1.41 ± 0.03 | 2.34 ± 0.05 | 0.16 ± 0.01 | 1.98 ± 0.74 | 15.48 ± 1.13 | 10.51 ± 0.07 |
| Min | 16.7 | 39.79 | 27.39 | 0.56 | 1.32 | 2.18 | 0.13 | 0.24 | 11.36 | 9.79 | |
| Max | 19 | 42.27 | 31.07 | 3.15 | 1.51 | 2.47 | 0.19 | 4.47 | 18.3 | 11.59 | |
| Black locust | Mean ± SD | 17.96 ± 0.55 | 27.03 ± 0.61 | 43.60 ± 0.95 | 1.96 ± 0.49 | 0.61 ± 0.04 | 1.50 ± 0.03 | 0.24 ± 0.02 | 1.99 ± 0.75 | 20.39 ± 1.40 | 4.30 ± 0.02 |
| Min | 16.3 | 25.13 | 40.18 | 0.76 | 0.54 | 1.39 | 0.17 | 0.19 | 16.49 | 3.58 | |
| Max | 19.5 | 29.16 | 47.48 | 3.04 | 0.69 | 1.56 | 0.35 | 4.14 | 24.18 | 5.09 | |
| Sunflower | Mean ± SD | 17.34 ± 0.30 | 34.76 ± 0.05 | 34.51 ± 0.17 | 2.32 ± 0.59 | 0.82 ± 0.18 | 2.00 ± 0.15 | 0.35 ± 0.08 | 2.10 ± 0.12 | 17.71 ± 0.45 | 7.45 ± 0.05 |
| Min | 16.5 | 34.7 | 33.82 | 0.78 | 0.1 | 1.93 | 0.28 | 0.72 | 13.86 | 7.02 | |
| Max | 17.9 | 34.98 | 34.71 | 3.82 | 1.03 | 2.1 | 0.47 | 4.11 | 21.06 | 7.87 | |
| Buckwheat | Mean ± SD | 16.18 ± 0.08 | 28.58 ± 0.09 | 42.28 ± 0.07 | 2.29 ± 0.08 | 0.67 ± 0.03 | 1.76 ± 0.09 | 0.39 ± 0.06 | 2.27 ± 0.05 | 17.16 ± 0.37 | 8.63 ± 0.06 |
| Min | 15.9 | 28.35 | 42.02 | 0.63 | 0.66 | 1.73 | 0.3 | 1.38 | 14.99 | 8.05 | |
| Max | 16.4 | 28.87 | 42.47 | 3.63 | 0.68 | 1.81 | 0.49 | 3.17 | 19.24 | 9.21 | |
| Mint | Mean ± SD | 17.24 ± 0.41 | 35.22 ± 0.06 | 33.35 ± 0.10 | 2.71 ± 0.07 | 1.054 ± 0.02 | 2.04 ± 0.05 | 0.39 ± 0.01 | 1.83 ± 0.11 | 16.85 ± 0.99 | 6.25 ± 0.09 |
| Min | 16.2 | 35.05 | 33.04 | 2.16 | 1.04 | 1.93 | 0.36 | 0.78 | 15.08 | 5.23 | |
| Max | 18.2 | 35.37 | 33.55 | 3.51 | 1.07 | 2.17 | 0.42 | 2.88 | 20.04 | 6.93 | |
| Dandelion | Mean ± SD | 16.76 ± 0.15 | 33.32 ± 0.25 | 36.78 ± 0.45 | 0.67 ± 0.12 | 0.90 ± 0.18 | 1.98 ± 0.09 | 0.34 ± 0.02 | 2.51 ± 0.02 | 17.20 ± 0.24 | 7.37 ± 0.07 |
| Min | 16.4 | 33.22 | 36.69 | 0.19 | 0.9 | 1.94 | 0.27 | 0.96 | 16.83 | 6.89 | |
| Max | 17.1 | 33.45 | 36.83 | 0.99 | 0.91 | 2.04 | 0.43 | 3.94 | 18.14 | 7.89 | |
| Meadow | Mean ± SD | 17.10 ± 0.33 | 37.10 ± 0.12 | 32.15 ± 0.23 | 0.25 ± 0.10 | 1.15 ± 0.09 | 2.17 ± 0.06 | 0.51 ± 0.05 | 0.25 ± 0.01 | 22.68 ± 0.32 | 7.45 ± 0.04 |
| Min | 16.3 | 36.42 | 32.04 | 0.12 | 1.12 | 2.03 | 0.48 | 0.14 | 21.98 | 6.03 | |
| Max | 17.9 | 37.52 | 32.33 | 0.46 | 1.17 | 2.29 | 0.55 | 0.43 | 23.47 | 8.13 | |
| Raspberry | Mean ± SD | 17.36 ± 0.19 | 34.28 ± 0.31 | 35.41 ± 0.26 | 0.68 ± 0.12 | 0.97 ± 0.02 | 1.97 ± 0.32 | 0.51 ± 0.09 | 0.71 ± 0.05 | 21.40 ± 0.30 | 6.69 ± 0.11 |
| Min | 16.8 | 33.64 | 34.92 | 0.32 | 0.94 | 1.88 | 0.46 | 0.18 | 18.25 | 6.06 | |
| Max | 17.9 | 35.61 | 35.77 | 1.02 | 1.00 | 2.06 | 0.56 | 1.26 | 24.28 | 7.04 | |
| Honey Variety | L* | a* | b* | Chroma | Hue angle (°) | Yellow Index | |
|---|---|---|---|---|---|---|---|
| Rapeseed | Mean ± SD | 24.38 ± 1.58 | −0.45 ± 0.03 | 6.32 ± 0.57 | 6.34 ± 0.12 | −1.50 ± 0.08 | 38.97 ± 0.16 |
| Min | 17.61 | −1.25 | 4.56 | 4.56 | −1.54 | 25.91 | |
| Max | 29.02 | −0.16 | 8.17 | 8.26 | −1.41 | 66.27 | |
| Black locust | Mean ± SD | 48.52 ± 0.65 | −1.15 ± 0.07 | 14.90 ± 0.18 | 14.95 ± 0.10 | −1.49 ± 0.02 | 43.97 ± 0.27 |
| Min | 45.96 | −1.69 | 14.18 | 13.3 | −1.52 | 39.37 | |
| Max | 52.39 | −0.83 | 16.34 | 16.36 | −1.48 | 50.48 | |
| Sunflower | Mean ± SD | 37.48 ± 0.58 | 2.22 ± 0.29 | 12.32 ± 0.28 | 12.62 ± 0.23 | 1.38 ± 0.02 | 47.84 ± 0.19 |
| Min | 36.22 | 1.25 | 11.45 | 11.51 | 1.34 | 41.83 | |
| Max | 39.1 | 2.98 | 13.15 | 13.48 | 1.46 | 51.83 | |
| Buckwheat | Mean ± SD | 42.85 ± 0.65 | 0.35 ± 0.08 | 16.10 ± 0.07 | 15.90 ± 0.15 | 1.54 ± 0.05 | 53.03 ± 0.26 |
| Min | 40.7 | 0.21 | 14.17 | 14.17 | 1.54 | 49.73 | |
| Max | 44.85 | 0.51 | 17.37 | 17.37 | 1.55 | 55.33 | |
| Mint | Mean ± SD | 28.43 ± 0.16 | −2.67 ± 0.12 | 5.87 ± 0.22 | 6.29 ± 0.06 | −1.09 ± 0.05 | 28.73 ± 0.21 |
| Min | 26.97 | −3.50 | 3.45 | 4.91 | −1.32 | 17.52 | |
| Max | 29.93 | −1.89 | 7.62 | 7.85 | −0.77 | 36.37 | |
| Dandelion | Mean ± SD | 33.62 ± 0.97 | 1.19 ± 0.25 | 16.07 ± 0.15 | 16.28 ± 0.18 | 1.49 ± 0.06 | 68.49 ± 0.32 |
| Min | 33.06 | 0.87 | 15.25 | 15.31 | 1.47 | 65.89 | |
| Max | 34.49 | 1.56 | 17.13 | 17.15 | 1.52 | 70.95 | |
| Meadow | Mean ± SD | 27.28 ± 0.48 | 5.00 ± 0.29 | 11.85 ± 0.30 | 13.06 ± 0.26 | 1.20 ± 0.10 | 60.42 ± 0.19 |
| Min | 21.56 | 1.07 | 9.67 | 12.07 | 0.92 | 52.04 | |
| Max | 32.14 | 7.23 | 14.09 | 14.61 | 1.49 | 64.07 | |
| Raspberry | Mean ± SD | 27.88 ± 0.13 | 5.11 ± 0.12 | 13.73 ± 0.27 | 14.66 ± 0.12 | 1.21 ± 0.04 | 70.48 ± 0.35 |
| Min | 26.15 | 4.22 | 12.73 | 13.41 | 1.17 | 64.53 | |
| Max | 29.28 | 5.81 | 14.62 | 15.73 | 1.25 | 78.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scripcă, L.A.; Amariei, S. The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey. Foods 2021, 10, 1039. https://doi.org/10.3390/foods10051039
Scripcă LA, Amariei S. The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey. Foods. 2021; 10(5):1039. https://doi.org/10.3390/foods10051039
Chicago/Turabian StyleScripcă, Laura Agripina, and Sonia Amariei. 2021. "The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey" Foods 10, no. 5: 1039. https://doi.org/10.3390/foods10051039
APA StyleScripcă, L. A., & Amariei, S. (2021). The Influence of Chemical Contaminants on the Physicochemical Properties of Unifloral and Multifloral Honey. Foods, 10(5), 1039. https://doi.org/10.3390/foods10051039






