Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Extraction
2.3. Characterization of the BSF Powder and the BSFPC
2.3.1. Amino Acid Composition, Total Nitrogen, and Protein Content
2.3.2. Fourier Transform Mid Infrared Spectroscopy (FTIR)
2.4. Techno-Functional Properties of the BSFPC
2.4.1. Protein Solubility
2.4.2. Isoelectric Point (pI) Determination
2.4.3. Water and Oil Binding Capacity
2.4.4. Foaming Capacity (FC) and Foam Stability (FS)
2.4.5. Emulsifying Activity (EA)
2.4.6. Interfacial Tension Analysis
2.5. Premix Membrane Emulsification
2.5.1. Preparation of O/W coarse emulsion
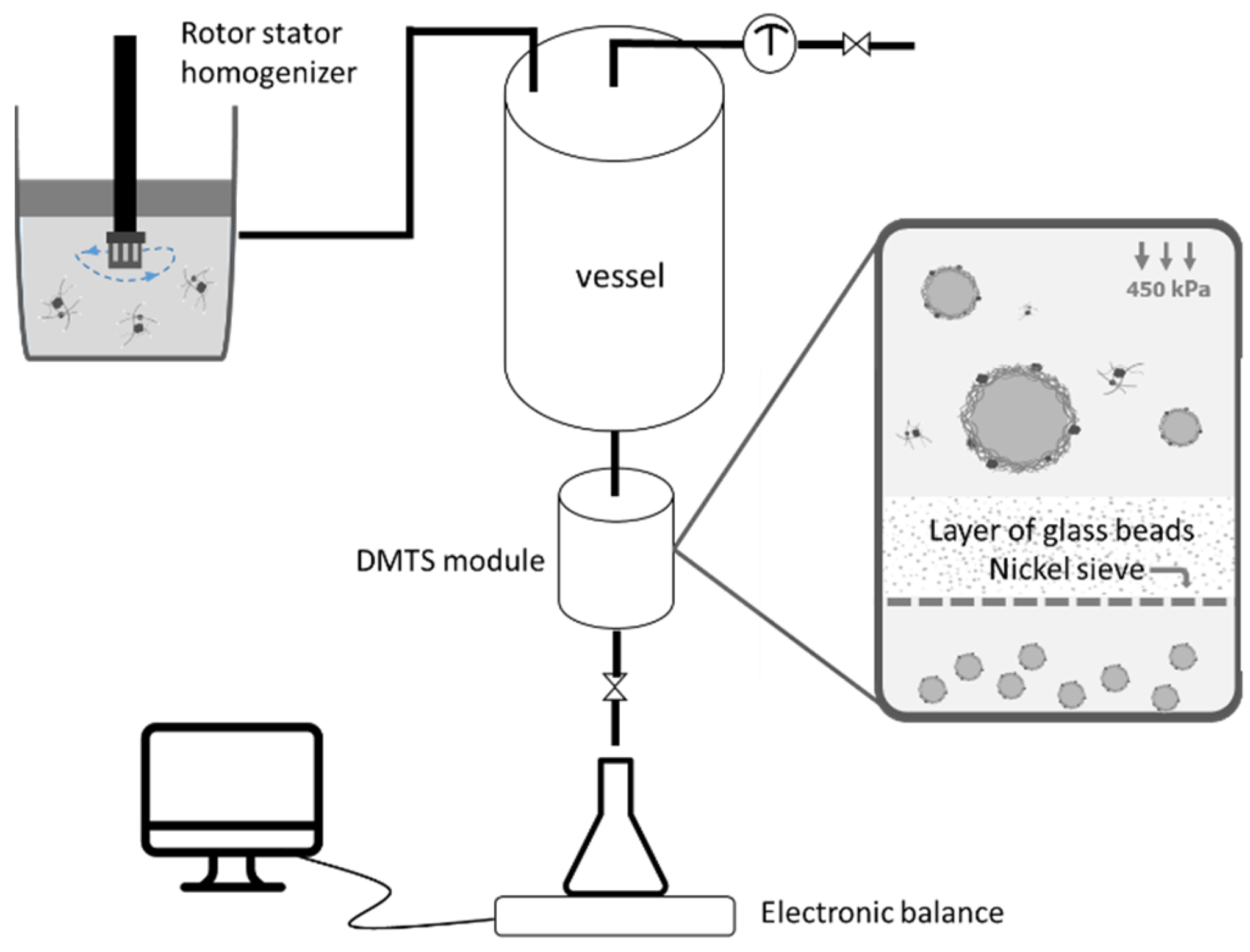
2.5.2. Emulsification with Dynamic Membranes of Tunable Pore Size (DMTS)
2.5.3. Particle Size and Distribution
2.5.4. Zeta Potential
2.5.5. Analysis of Emulsion Stability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of BSF Powder and BSFPC
3.1.1. Amino Acids, Total Nitrogen, and Protein Content
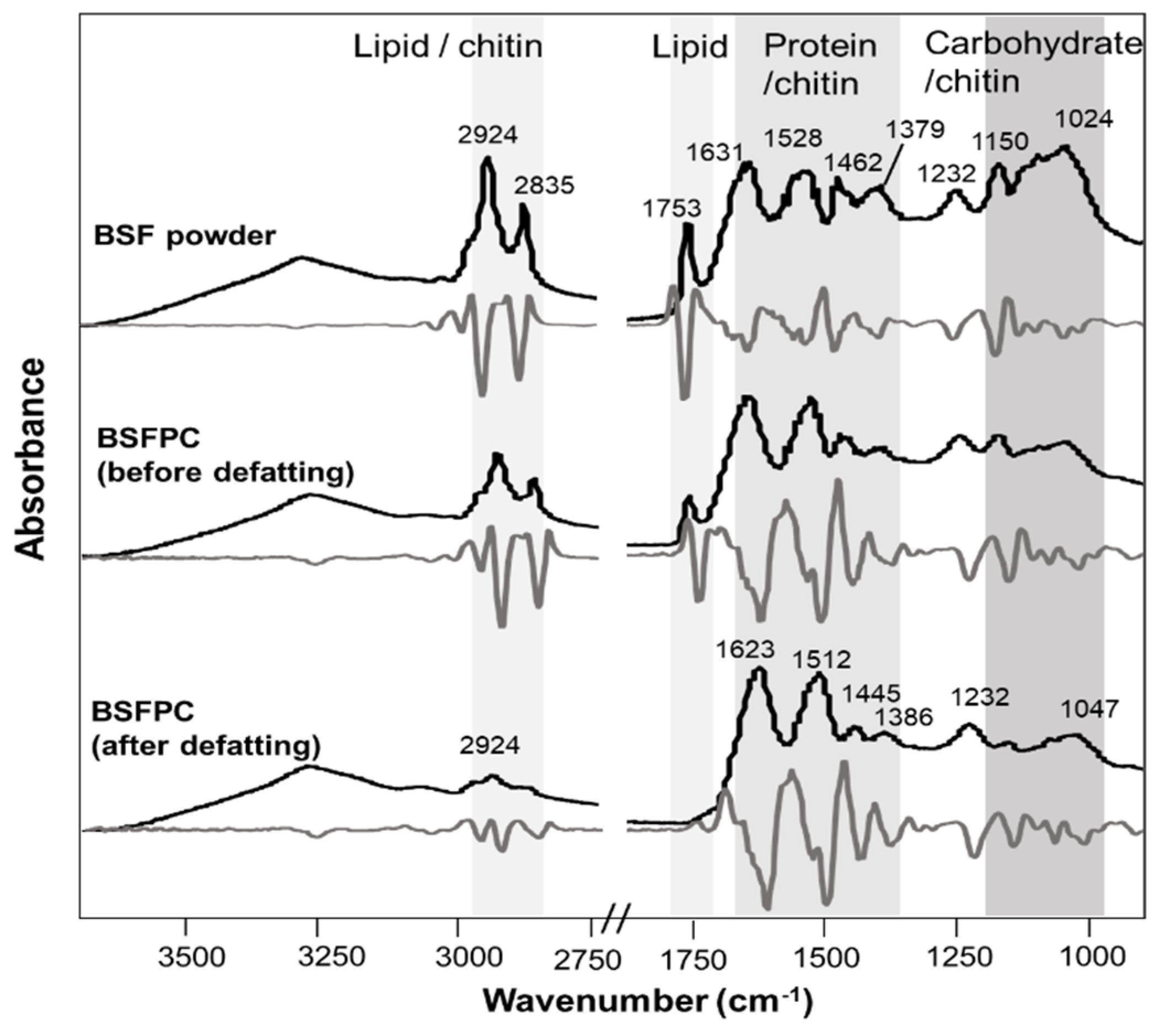
3.1.2. Attenuated total Reflectance Fourier Transform Mid-Infrared Spectroscopy (ATR-FT-MIR)
3.2. Techno-Functional Properties
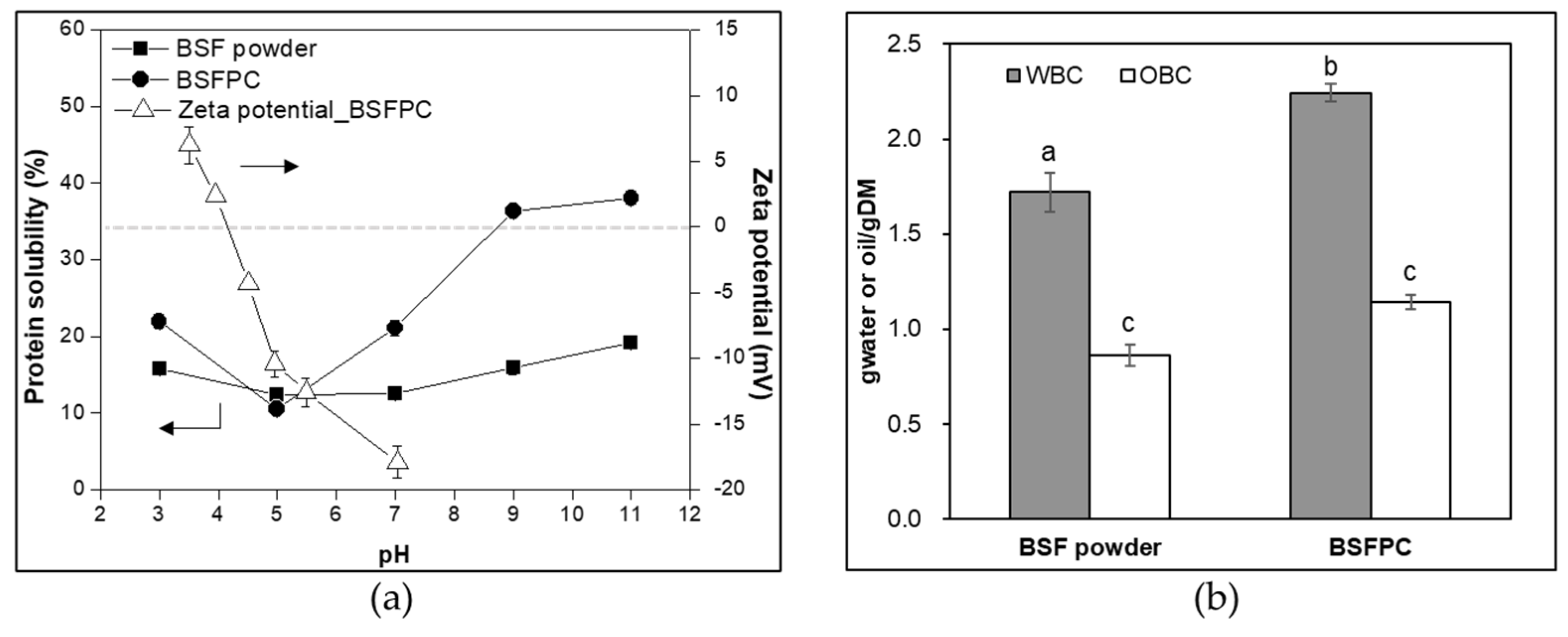
3.2.1. Protein Solubility and Isoelectric Point (pI)
3.2.2. WBC and OBC
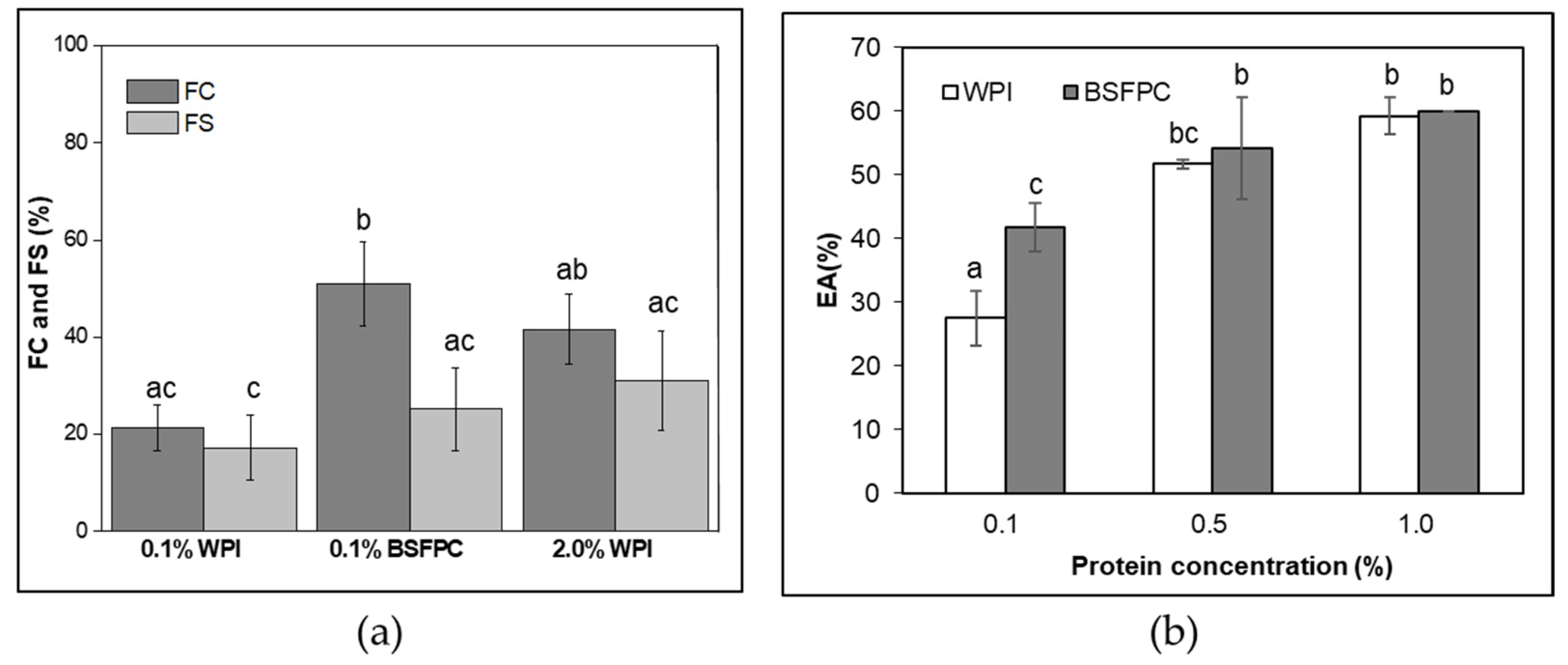
3.2.3. FC and FS
3.2.4. EA
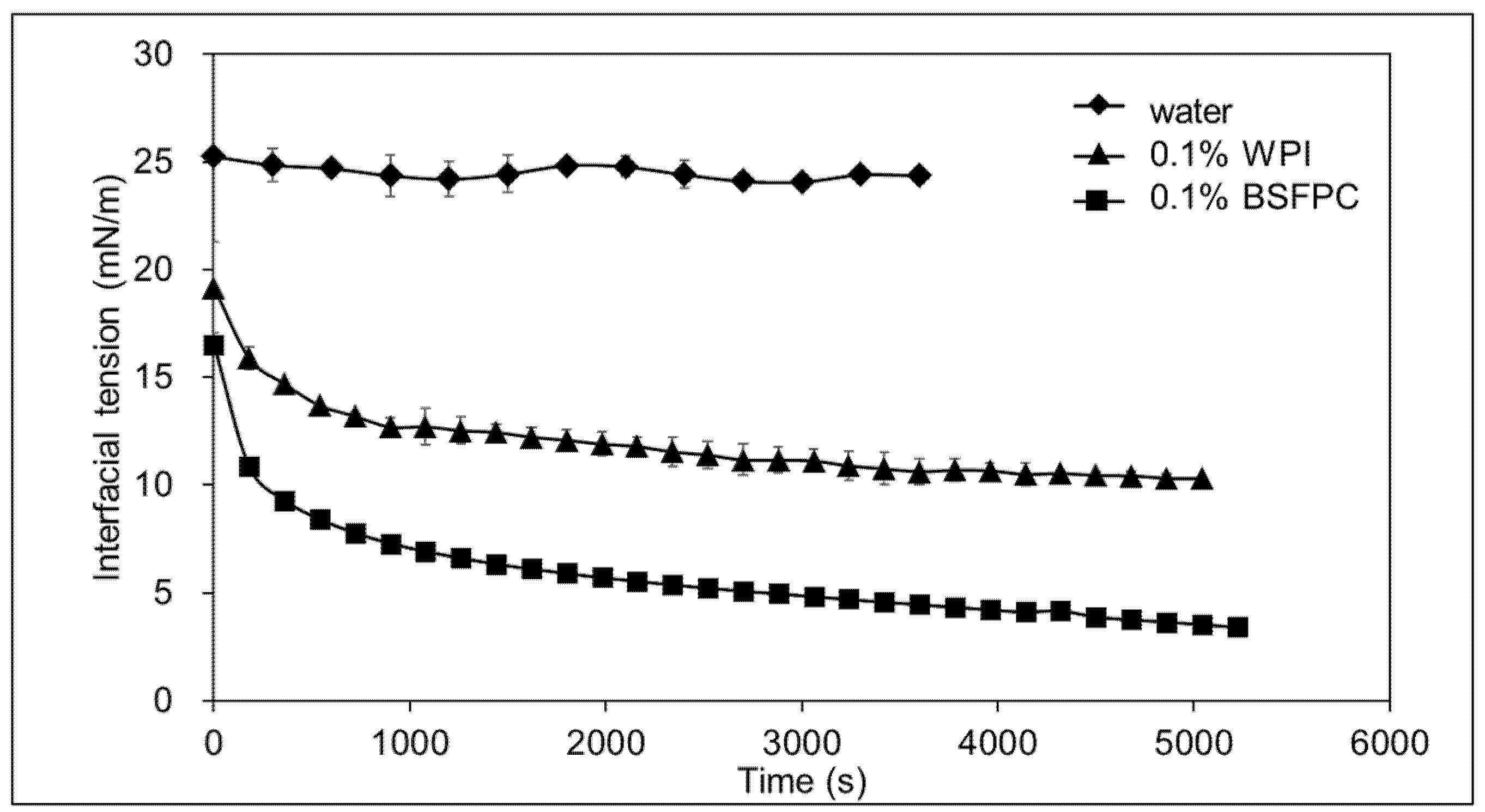
3.2.5. Interfacial Tension
3.3. Dynamic Membrane of Tunable Pore Size (DMTS) Emulsification
3.3.1. Droplet Size Distribution
3.3.2. Productivity
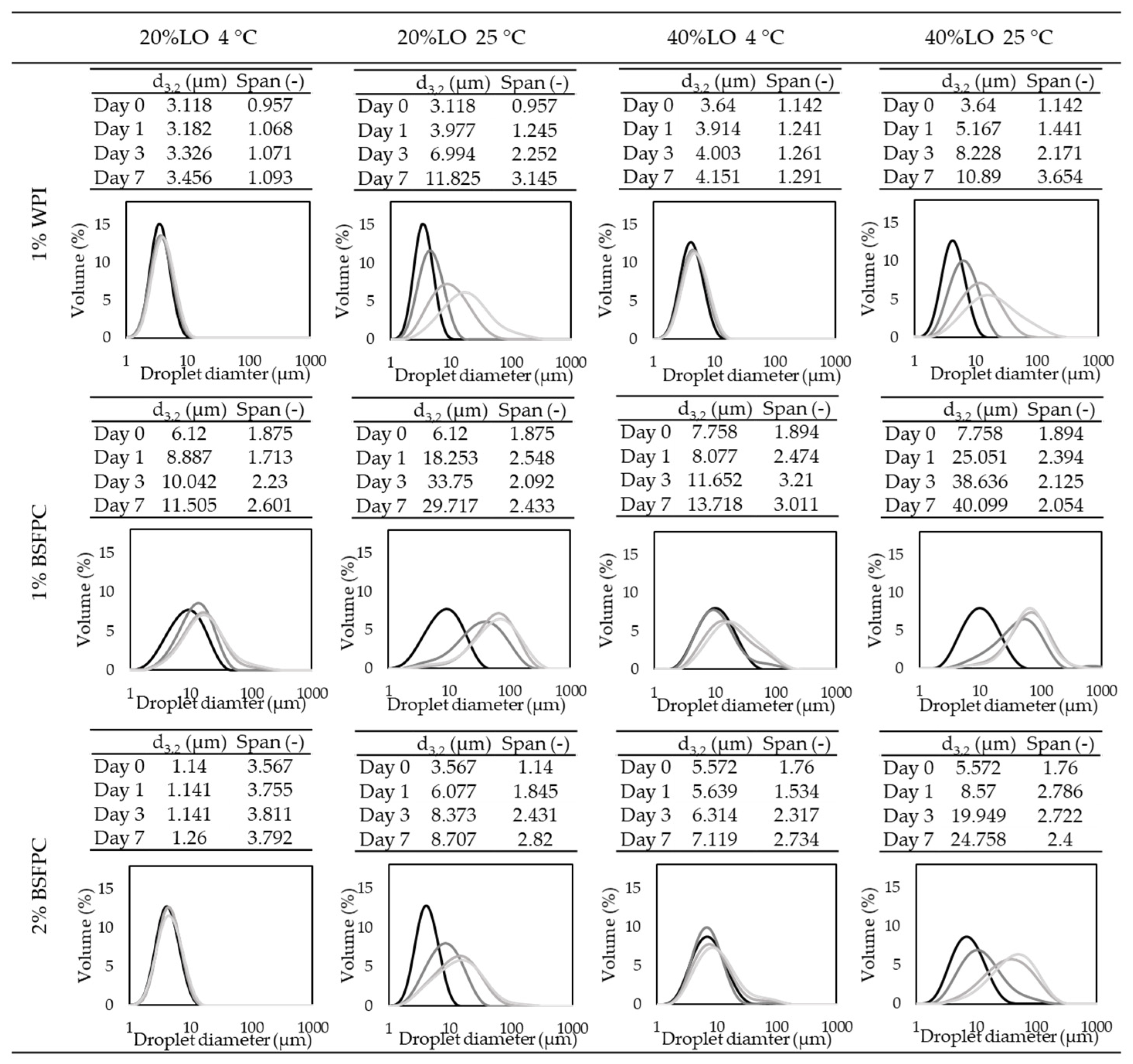
3.3.3. Stability of the Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050 the 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Charlton, A.J.; Dickinson, M.; Wakefield, M.E.; Fitches, E.; Kenis, M.; Han, R.; Zhu, F.; Kone, N.; Grant, M.; Devic, E.; et al. Exploring the chemical safety of fly larvae as a source of protein for animal feed. J. Insects Food Feed 2015, 1, 7–16. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italt, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Van Krimpen, M.; Bikker, P.; Van der Meer, I.; Van der Peet-Schwering, C.; Vereijken, J. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Lelystat, The Netherlands, 2013. [Google Scholar]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Amato, M. Insects as Food: A Cross-Cultural Comparison of Consumers’ Intention and Behaviour. Ph.D. Thesis, Università degli Studi di Napoli Federico II, Napoli, Italy, 2017; p. 121. [Google Scholar]
- DeFoliart, G.R. Insects as food: Why the Western Attitude Is Important. Annu. Rev. Entomol. 1999, 44, 21–50. [Google Scholar] [CrossRef]
- Hartmann, C.; Shi, J.; Giusto, A.; Siegrist, M. The psychology of eating insects: A cross-cultural comparison between Germany and China. Food Qual. Prefer. 2015, 44, 148–156. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.H.; Tinchan, P.; Stieger, M.; Steenbekkers, L.P.A.; van Trijp, H.C.M. Insects as food: Exploring cultural exposure and individual experience as determinants of acceptance. Food Qual. Prefer. 2015, 42, 78–89. [Google Scholar] [CrossRef]
- Yen, A.L. Edible insects: Traditional knowledge or western phobia? Entomol. Res. 2009, 39, 289–298. [Google Scholar] [CrossRef]
- Balzan, S.; Fasolato, L.; Maniero, S.; Novelli, E. Edible insects and young adults in a north-east Italian city an exploratory study. Br. Food J. 2016, 118, 318–326. [Google Scholar] [CrossRef]
- Schösler, H.; de Boer, J.; Boersema, J.J. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Gould, J.; Wolf, B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocoll. 2018, 77, 57–65. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Nugroho, D.S.; Cheng, Y.S.; Chang, J.Y. Development and Characterization of Nano-emulsions Based on Oil Extracted from Black Soldier Fly Larvae. Appl. Biochem. Biotechnol. 2020, 191, 331–345. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Martinez, J.-J.J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Tanzmeister, H.; Meinlschmidt, P.; Baumgartner, S.; Lauter, K.; Jäger, H. Recovery of soluble proteins from migratory locust (Locusta migratoria) and characterisation of their compositional and techno-functional properties. Food Res. Int. 2018, 106, 271–279. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef]
- Kim, H.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Glorieux, S.; Van de Walle, D.; Dewettinck, K.; Fraeye, I. The effect of temperature on structure formation in three insect batters. Food Res. Int. 2019, 122, 411–418. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Manuel, J.; Moreno, P.; Prado, E.E.; Perez, M.A.; Otero, J.L.; Guevara, O.L. De Nutritional Value of Edible Insects from the State of Oaxaca, Mexico. J. Food Compos. Anal. 1997, 157, 142–157. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Memb. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Kaade, W.; Güell, C.; Ballon, A.; Mellado-Carretero, J.; De Lamo-Castellví, S.; Ferrando, M. Dynamic membranes of tunable pore size for lemon oil encapsulation. LWT 2020, 123, 109090. [Google Scholar] [CrossRef]
- Wang, J.; Martínez-Hernández, A.; de Lamo-Castellví, S.; Romero, M.-P.; Kaade, W.; Ferrando, M.; Güell, C. Low-energy membrane-based processes to concentrate and encapsulate polyphenols from carob pulp. J. Food Eng. 2020, 281, 109996. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Berton-Carabin, C.; Chibane, M.; Schroën, K.; Ladjal Ettoumi, Y.; Berton-Carabin, C.; Chibane, M.; Schroën, K. Legume Protein Isolates for Stable Acidic Emulsions Prepared by Premix Membrane Emulsification. Food Biophys. 2017, 12, 119–128. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes—Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.D.S.; Lanfer Marquez, U.M.; Lourenco, S.O.; Barbarino, E.; De-Paula, J.C.; da S. Pereira, L.O.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Mellado-Carretero, J.; García-Gutiérrez, N.; Ferrando, M.; Güell, C.; García-Gonzalo, D.; De Lamo-Castellví, S. Rapid discrimination and classification of edible insect powders using ATR-FTIR spectroscopy combined with multivariate analysis. J. Insects Food Feed 2020, 6, 141–148. [Google Scholar] [CrossRef]
- Kaade, W.; Ferrando, M.; Khanmohammed, A.; Torras, C.; De Lamo-Castellví, S.; Güell, C. Low-energy high-throughput emulsification with nickel micro-sieves for essential oils encapsulation. J. Food Eng. 2019, 263, 326–336. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.P.; Van Den Broek, L.A.M.M.; Fogliano, V.; Lakemond, C.M.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Oshimura, E.; Sakamoto, K. Amino acids, peptides, and proteins. Cosmet. Sci. Technol. Theor. Princ. Appl. 2017, 285–303. [Google Scholar] [CrossRef]
- Burns, A.; Olszowy, P.; Ciborowski, P. Biomolecules. In Proteomic Profiling and Analytical Chemistry: The Crossroads, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 7–24. ISBN 9780444636881. [Google Scholar]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Boekel, M.A.J.S.V. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of conventional and sustainable lipid extraction methods for the production of oil and protein isolate from edible insect meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Protein evaluation, amino acid scoring and the Food and Drug Administration’s proposed food labeling regulations. J. Nutr. 1991, 121, 145–150. [Google Scholar] [CrossRef]
- Chatsuwan, N.; Nalinanon, S.; Puechkamut, Y.; Lamsal, B.P.; Pinsirodom, P. Characteristics, Functional Properties, and Antioxidant Activities of Water-Soluble Proteins Extracted from Grasshoppers, Patanga succincta and Chondracris roseapbrunner. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies Contents, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Haque, M.A.; Timilsena, Y.P.; Adhikari, B. Food Proteins, Structure, and Function. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–8. ISBN 9780081005965. [Google Scholar]
- Son, Y.J.; Lee, J.C.; Hwang, I.K.; Nho, C.W.; Kim, S.H. Physicochemical properties of mealworm (Tenebrio molitor) powders manufactured by different industrial processes. LWT 2019, 116, 108514. [Google Scholar] [CrossRef]
- Chandra, S. Assessment of functional properties of different flours. Afr. J. Agric. Res. 2013, 8, 4849–4852. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Onwulata, C.I. Microencapsulation and functional bioactive foods. J. Food Process. Preserv. 2013, 37, 510–532. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Boom, R.M.; Schroën, K. Droplet break-up mechanism in premix emulsification using packed beds. Chem. Eng. Sci. 2013, 92, 190–197. [Google Scholar] [CrossRef]
- Sahin, S.; Sawalha, H.; Schroën, K. High throughput production of double emulsions using packed bed premix emulsification. Food Res. Int. 2014, 66, 78–85. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Impact of lemon oil composition on formation and stability of model food and beverage emulsions. Food Chem. 2012, 134, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Treesuwan, W.; Neves, M.A.; Uemura, K.; Nakajima, M.; Kobayashi, I. Preparation characteristics of monodisperse oil-in-water emulsions by microchannel emulsification using different essential oils. LWT Food Sci. Technol. 2017, 84, 617–625. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant. Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Cheong, M.W.; Chong, Z.S.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Characterisation of calamansi (Citrus microcarpa). Part I: Volatiles, aromatic profiles and phenolic acids in the peel. Food Chem. 2012, 134, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s-1. J. Food Process. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Trentin, A.; Ferrando, M.; López, F.; Güell, C. Premix membrane O/W emulsification: Effect of fouling when using BSA as emulsifier. Desalination 2009, 245, 388–395. [Google Scholar] [CrossRef]
- Surh, J.; Jeong, Y.G.; Vladisavljević, G.T. On the preparation of lecithin-stabilized oil-in-water emulsions by multi-stage premix membrane emulsification. J. Food Eng. 2008, 89, 164–170. [Google Scholar] [CrossRef]
- Sawalha, H.; Sahin, S.; Schroën, K. Preparation of polylactide microcapsules at a high throughput with a packed-bed premix emulsification system. J. Appl. Polym. Sci. 2016, 133, 3–9. [Google Scholar] [CrossRef]
- Zecca, E. Investigating the Role of Surface Hydrophobicity in Protein Aggregation. Ph.D. Thesis, University of Connecticut Graduate School, Storrs, CT, USA, 2017. [Google Scholar]
 d3,2-1% WPI (whey protein isolate);
d3,2-1% WPI (whey protein isolate);  d3,2-1% BSFPC (black soldier fly protein concentrate);
d3,2-1% BSFPC (black soldier fly protein concentrate);  d3,2-2% BSFPC;
d3,2-2% BSFPC;  span-1%WPI;
span-1%WPI;  span-1% BSFPC;
span-1% BSFPC;  span-2% BSFPC).
span-2% BSFPC).
 d3,2-1% WPI (whey protein isolate);
d3,2-1% WPI (whey protein isolate);  d3,2-1% BSFPC (black soldier fly protein concentrate);
d3,2-1% BSFPC (black soldier fly protein concentrate);  d3,2-2% BSFPC;
d3,2-2% BSFPC;  span-1%WPI;
span-1%WPI;  span-1% BSFPC;
span-1% BSFPC;  span-2% BSFPC).
span-2% BSFPC).
 d3,2-1 % WPI (whey protein isolate);
d3,2-1 % WPI (whey protein isolate);  d3,2-1 % BSFPC (black soldier fly protein concentrate);
d3,2-1 % BSFPC (black soldier fly protein concentrate);  d3,2-2 % BSFPC;
d3,2-2 % BSFPC;  span-1%WPI;
span-1%WPI;  span-1% BSFPC;
span-1% BSFPC;  span-2% BSFPC).
span-2% BSFPC).
 d3,2-1 % WPI (whey protein isolate);
d3,2-1 % WPI (whey protein isolate);  d3,2-1 % BSFPC (black soldier fly protein concentrate);
d3,2-1 % BSFPC (black soldier fly protein concentrate);  d3,2-2 % BSFPC;
d3,2-2 % BSFPC;  span-1%WPI;
span-1%WPI;  span-1% BSFPC;
span-1% BSFPC;  span-2% BSFPC).
span-2% BSFPC).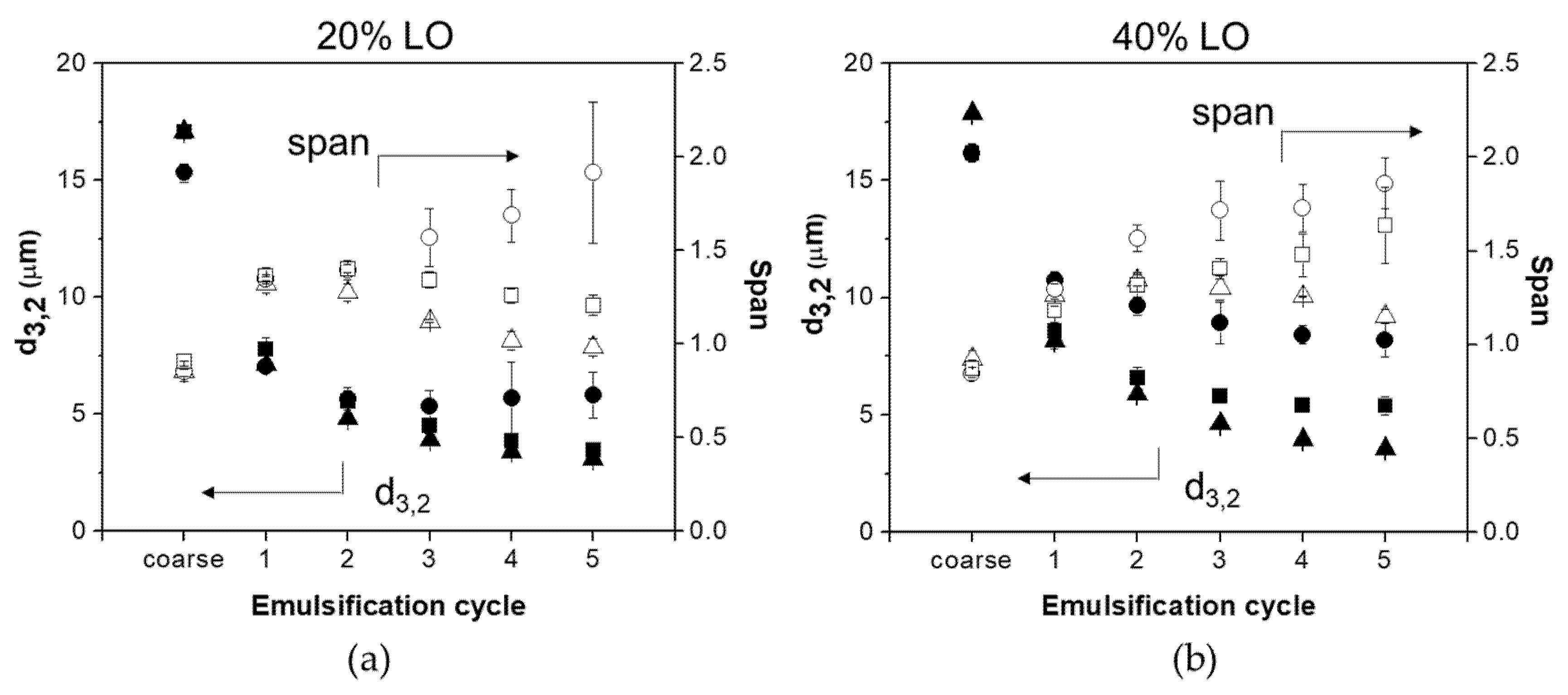

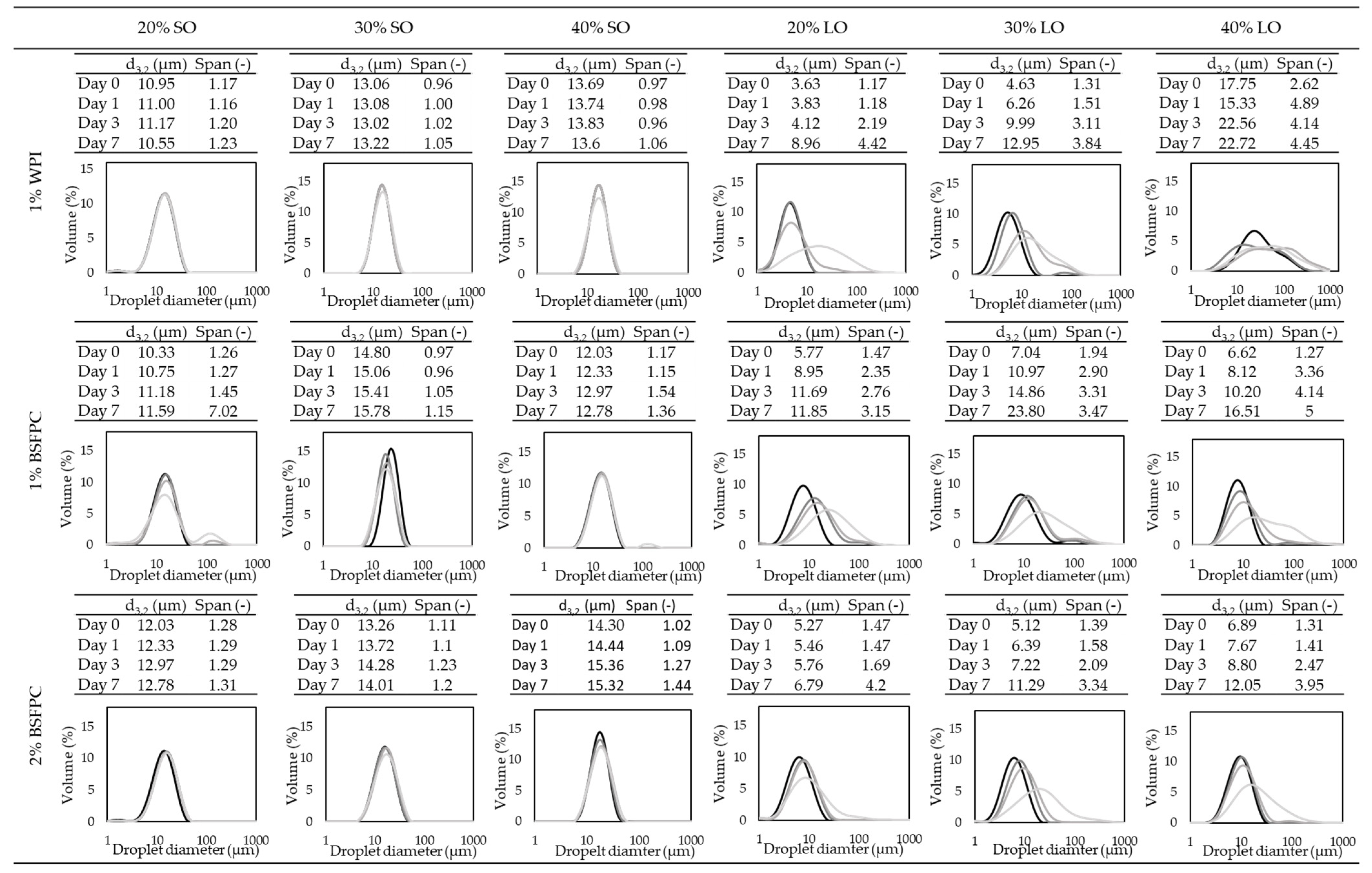
 1% WPI (whey protein isolate);
1% WPI (whey protein isolate);  1% BSFPC (black soldier fly protein concentrate); and 2% BSFPC).
1% BSFPC (black soldier fly protein concentrate); and 2% BSFPC).
 1% WPI (whey protein isolate);
1% WPI (whey protein isolate);  1% BSFPC (black soldier fly protein concentrate); and 2% BSFPC).
1% BSFPC (black soldier fly protein concentrate); and 2% BSFPC).
| Oil Phase | Oil Fraction | Emulsifier in Water Phase | Water Phase Medium |
|---|---|---|---|
| Sunflower oil or lemon oil | 20 wt.%, 30 wt.%, or 40 wt.% | 1% WPI | Deionized water or 0.2 M phosphate buffer pH7 1 |
| 1% BSFPC | |||
| 2% BSFPC |
| BSF Powder (% DM) | BSFPC (% DM) | BSF Powder (mg/g Protein) | BSFPC (mg/g Protein) | 2013 FAO (mg/g Protein) | Soybean (mg/g Protein) 5 | Casein (mg/g Protein) 5 | |
|---|---|---|---|---|---|---|---|
| Essential amino acid | |||||||
| His | 1.71 | 2.60 | 43.8 | 41.6 | 16 | 25 | 32 |
| Ile | 2.12 | 3.68 | 54.4 | 58.9 | 30 | 47 | 54 |
| Leu | 3.36 | 5.80 | 86.9 | 92.9 | 61 | 85 | 95 |
| Lys | 3.06 | 5.12 | 78.5 | 82.0 | 48 | 63 | 85 |
| Met+Cys 3 | 1.38 | 1.76 | 35.4 | 28.2 | 23 | 24 | 35 |
| Phe+Tyr 4 | 5.23 | 9.43 | 134.1 | 151.1 | 41 | 97 | 111 |
| Thr | 1.96 | 3.21 | 50.3 | 51.4 | 25 | 38 | 42 |
| Val | 2.71 | 4.79 | 69.5 | 76.7 | 40 | 49 | 63 |
| Trp | 1.87 1 | nd 2 | nd 2 | Nd 2 | 6.6 | 11 | 14 |
| Non-essential amino acid | |||||||
| Asp | 4.79 | 8.07 | 122.8 | 129.2 | |||
| Glu | 6.17 | 8.53 | 158.2 | 136.6 | |||
| Ala | 3.08 | 4.84 | 80.0 | 77.5 | |||
| Arg | 2.52 | 3.92 | 64.6 | 62.8 | |||
| Gly | 2.66 | 3.72 | 68.2 | 59.6 | |||
| Pro | 2.74 | 4.10 | 70.3 | 65.7 | |||
| Ser | 1.99 | 3.16 | 51.0 | 50.6 | |||
| Kp | 4.71 | 5.36 | |||||
| Total Nitrogen (%) 3 | 8.28 | 11.65 | |||||
| Protein content (%) | 39.00 | 62.44 | |||||
| Extraction yield (%) | 35.8 | ||||||
| Protein yield (%) | 59.3 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. https://doi.org/10.3390/foods10051048
Wang J, Jousse M, Jayakumar J, Fernández-Arteaga A, de Lamo-Castellví S, Ferrando M, Güell C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods. 2021; 10(5):1048. https://doi.org/10.3390/foods10051048
Chicago/Turabian StyleWang, Junjing, Morane Jousse, Jitesh Jayakumar, Alejandro Fernández-Arteaga, Silvia de Lamo-Castellví, Montserrat Ferrando, and Carme Güell. 2021. "Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology" Foods 10, no. 5: 1048. https://doi.org/10.3390/foods10051048
APA StyleWang, J., Jousse, M., Jayakumar, J., Fernández-Arteaga, A., de Lamo-Castellví, S., Ferrando, M., & Güell, C. (2021). Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods, 10(5), 1048. https://doi.org/10.3390/foods10051048








