Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GCP
2.2. Cell Viability Assay
2.3. Experimental Design and Samples Collection
2.4. Serum Biochemical Index Detection
2.5. Measurement of SCFAs
2.6. DNA Extraction of Cecum Contents and High-Throughput Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Cell Viability Assay
3.2. Effect of GCP on the Body Weight of Normal Mice
3.3. Effect of GCP on the Serum Biochemistry of Normal Mice
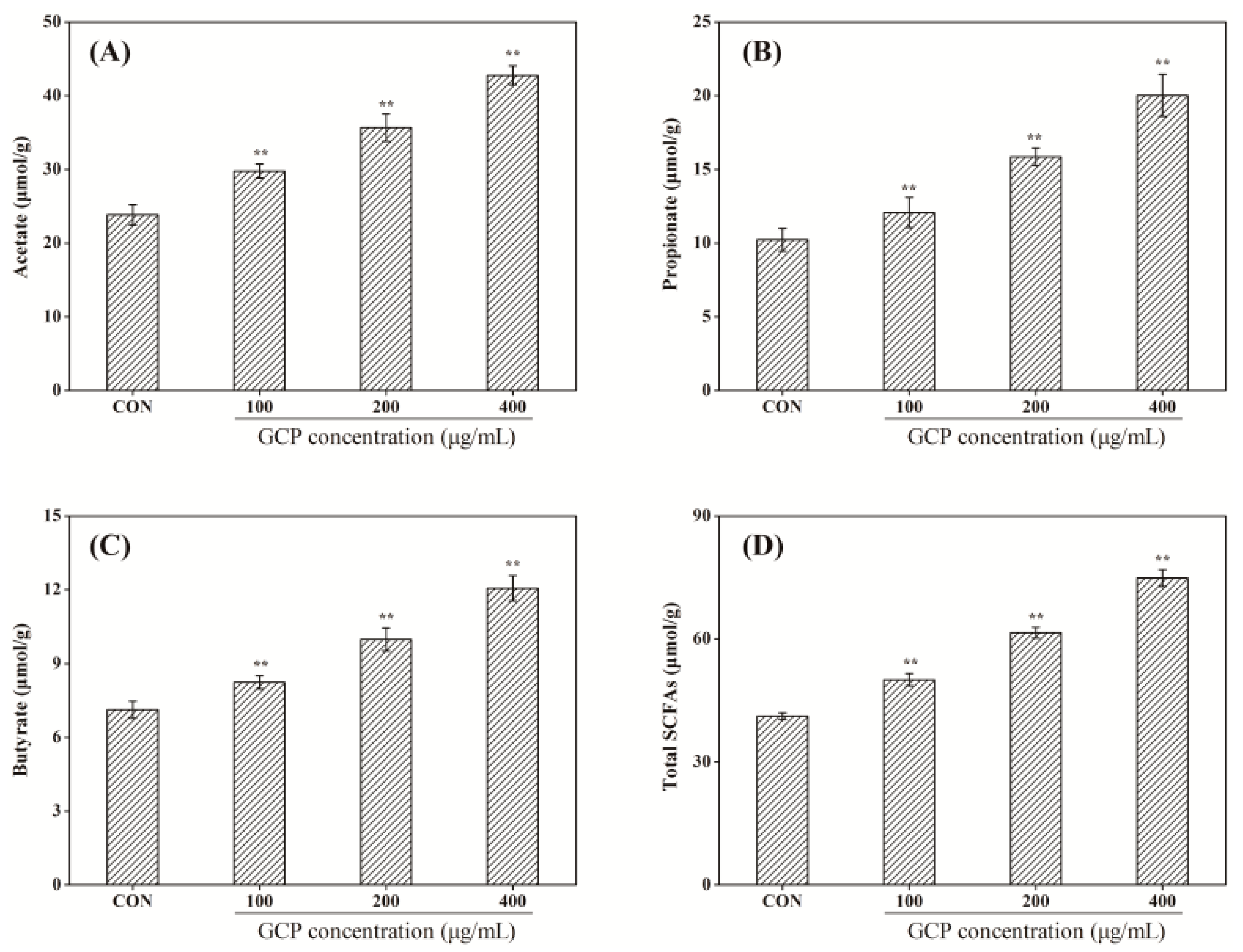
3.4. Effect of GCP on the SCFAs of Normal Mice
3.5. Effect of GCP on the Composition and Diversity of Gut Microbiota
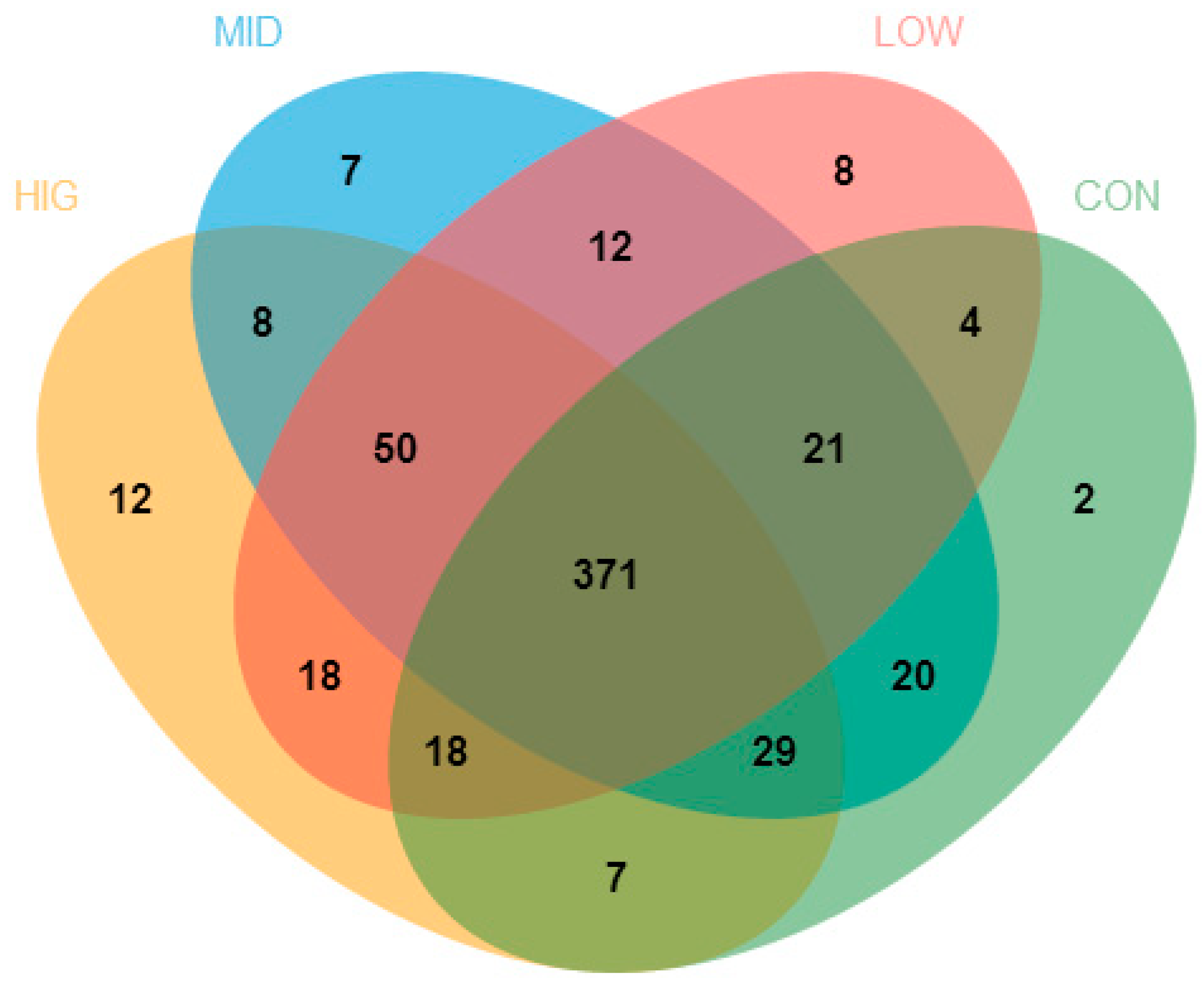
3.5.1. Diversity Analysis of the Structure of Gut Microbiota
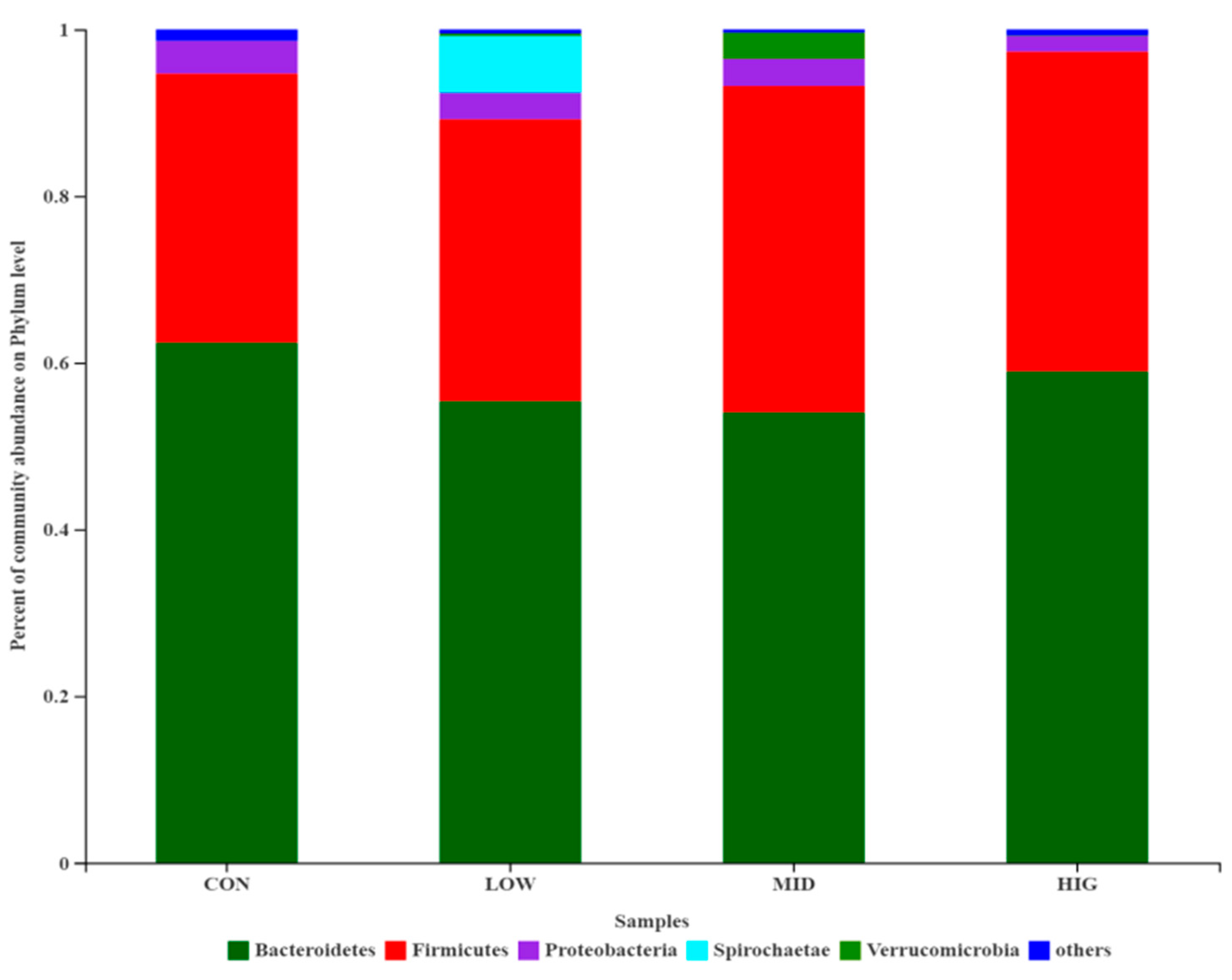
3.5.2. Composition Analysis of the Gut Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rimal, B.; Nichols, R.G.; Tian, Y.; Smith, P.B.; Hatzakis, E.; Chang, S.-C.; Butenhoff, J.L.; Peters, J.M.; Patterson, A.D. Perfluorooctane sulfonate alters gut microbiota-host metabolic homeostasis in mice. Toxicology 2020, 431, 152365. [Google Scholar] [CrossRef]
- Li, Z.-T.; Zhu, L.; Zhang, W.-L.; Zhan, X.-B.; Gao, M.-J. New dynamic digestion model reactor that mimics gastrointestinal function. Biochem. Eng. J. 2020, 154, 107431. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Hu, B.; Ye, C.; Leung, E.L.-H.; Zhu, L.; Hu, H.; Zhang, Z.; Zheng, J.; Liu, H. Bletilla striata oligosaccharides improve metabolic syndrome through modulation of gut microbiota and intestinal metabolites in high fat diet-fed mice. Pharmacol. Res. 2020, 159, 104942. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liao, W.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Interactions between resveratrol and gut microbiota affect the development of hepatic steatosis: A fecal microbiota transplantation study in high-fat diet mice. J. Funct. Foods 2020, 67, 103883. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the Gut Microbiota Is Associated with HIV Disease Progression and Tryptophan Catabolism. Sci. Transl. Med. 2013, 5, 193ra91. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c− Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Zheng, H.; Ma, G.; Zhao, L.; Hu, Q. Tuber indicum polysaccharide relieves fatigue by regulating gut microbiota in mice. J. Funct. Foods 2019, 63, 103580. [Google Scholar] [CrossRef]
- Al-Khafaji, A.H.; Jepsen, S.D.; Christensen, K.R.; Vigsnæs, L.K. The potential of human milk oligosaccharides to impact the microbiota-gut-brain axis through modulation of the gut microbiota. J. Funct. Foods 2020, 74, 104176. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and per-spectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef]
- van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.B.; Levin, S.A.S.; Bonhoeffer, S.; Laxminarayan, R.R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Wu, Z.; Yin, W.; Schwarz, S.; Tyrrell, J.M.; Zheng, Y.; Wang, S.; Shen, Z.; et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017, 2, 16260. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ruan, B.; Jiang, Y.; Xue, T.; Wang, Z.; Lu, H.; Wei, M.; Wang, S.; Ye, Z.; Zhai, D.; et al. Antibiotics-induced gut microbiota dysbiosis promotes tumor initiation via affecting APC-Th1 development in mice. Biochem. Biophys. Res. Commun. 2017, 488, 418–424. [Google Scholar] [CrossRef]
- Fang, Q.; Hu, J.; Nie, Q.; Nie, S. Effects of polysaccharides on glycometabolism based on gut microbiota alteration. Trends Food Sci. Technol. 2019, 92, 65–70. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Li, W.; Li, R.; Wang, X.; Qiao, H.; Sun, Q.; Zhang, H. Antibacterial mechanism of the polysaccharide produced by Chaetomium globosum CGMCC 6882 against Staphylococcus aureus. Int. J. Biol. Macromol. 2020, 159, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, R.; Cui, J.; Wang, J.; Fan, W.; Zhang, H.; Zhan, X. Antibacterial activity of a polysaccharide produced from Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2019, 125, 376–382. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.; Guo, X.; Huang, L.; Yang, J.; Yu, Q.; Chen, Y.; Xie, J. Cyclocaryapaliurus polysaccharide alleviates liver in-flammation in mice via beneficial regulation of gut microbiota and TLR4/MAPK signaling pathways. Int. J. Biol. Macromol. 2020, 160, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, B.; Wang, X.; Chen, K.; Zhang, K.; Zhang, L.; Fei, C.; Wang, C.; Liu, Y.; Xue, F.; et al. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. Int. J. Biol. Macromol. 2020, 158, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Kan, J.; Sun, R.; Tang, S.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int. J. Biol. Macromol. 2020, 154, 773–787. [Google Scholar] [CrossRef]
- Caillot, A.R.C.; Bezerra, I.D.L.; Palhares, L.C.G.F.; Santana-Filho, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of blackberry wine polysaccharides and immunomodulatory effects on LPS-activated RAW 264.7 macrophages. Food Chem. 2018, 257, 143–149. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control. 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; An, W.; Zhang, J.; Cao, X.; Mi, J.; Zhao, J.; Zhang, Y.; Li, J. Lyciumruthenicum diet alters the gut microbiota and partially enhances gut barrier function in male C57BL/6 mice. J. Funct. Foods 2019, 52, 516–528. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Z.; Deng, Q.; Huang, Q.; Wang, X.; Huang, F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res. Int. 2020, 131, 108994. [Google Scholar] [CrossRef]
- Wei, T.; Bao, J.-Y.; Yang, H.-H.; Lin, J.-F.; Zheng, Q.-W.; Ye, Z.-W.; Zou, Y.; Li, X.; Jiang, Z.-L.; Guo, L.-Q. Musa basjoo regulates the gut microbiota in mice by rebalancing the abundance of probiotic and pathogen. Microb. Pathog. 2019, 131, 205–211. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, B.; Wang, H.; Kong, L.; Han, H.; Li, K.; Sun, S.; Lei, Z.; Shimizu, K.; Zhang, Z. Supplementation with nanobubble water alleviates obesity-associated markers through modulation of gut microbiota in high-fat diet fed mice. J. Funct. Foods 2020, 67, 103820. [Google Scholar] [CrossRef]
- Pan, L.; Han, Y.; Zhou, Z. In Vitro prebiotic activities of exopolysaccharide from Leuconostocpseudomesenteroides XG5 and its effect on the gut microbiota of mice. J. Funct. Foods 2020, 67, 103853. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut mi-crobiome: The role of sex. J. Autoimm. 2018, 92, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Fu, Z.; Han, L.; Zhang, P.; Mao, H.; Zhang, H.; Wang, Y.; Gao, X.; Liu, E. Cistanche polysaccharides enhance echinacoside absorption In Vivo and affect the gut microbiota. Int. J. Biol. Macromol. 2020, 149, 732–740. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Singh, A.; Zapata, R.C.; Pezeshki, A.; Reidelberger, R.D.; Chelikani, P.K. Inulin fiber dose-dependently modulates energy balance, glucose tolerance, gut microbiota, hormones and diet preference in high-fat-fed male rats. J. Nutr. Biochem. 2018, 59, 142–152. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2018, 26, 351–361. [Google Scholar] [CrossRef]

| Mice Weight | Control Group | GCP Concentration (μg/mL) | ||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| 0 day (g) | 20.2 ± 1.57 a | 20.4 ± 1.38 a | 20.0 ± 2.23 a | 20.8 ± 2.42 a |
| 7 day (g) | 23.7 ± 2.27 b | 23.5 ± 2.09 b | 22.9 ± 3.37 b | 22.5 ± 2.45 b |
| 14 day (g) | 26.5 ± 2.34 c | 26.1 ± 1.99 c | 25.7 ± 2.54 c | 25.4 ± 2.08 c |
| 21 day (g) | 28.1 ± 2.14 d | 27.9 ± 2.23 d | 27.4 ± 2.15 d | 26.9 ± 2.64 d |
| 28 day (g) | 30.7 ± 2.61 e | 30.1 ± 2.45 e | 29.1 ± 2.38 e | 29.2 ± 3.17 e |
| Weight gain (g) | 10.5 ± 1.21 a | 9.7 ± 1.09 b | 9.1 ± 0.88 c | 8.4 ± 1.17 d |
| Serum Biochemistry | Control Group | GCP Concentration (μg/mL) | ||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Aspartate transaminase (U/L) | 38.5 ± 3.37 a | 37.3 ± 2.98 a | 36.2 ± 3.29 b | 35.4 ± 1.87 b |
| Alanine aminotransferase (U/L) | 121.5 ± 6.43 a | 115.3 ± 9.31 b | 111.9 ± 11.34 c | 105.4 ± 10.91 d |
| Total protein (g/L) | 53.5 ± 3.04 a | 54.5 ± 5.09 a | 54.8 ± 4.10 a | 53.9 ± 2.08 a |
| Albumin (g/L) | 31.7 ± 1.63 a | 32.1 ± 3.14 a | 31.9 ± 2.68 a | 31.2 ± 2.10 a |
| Globulin (g/L) | 20.8 ± 1.41 a | 21.1 ± 2.15 a | 20.9 ± 3.32 a | 20.7 ± 2.47 a |
| Urea (mmol/L) | 11.62 ± 0.83 a | 11.75 ± 1.22 a | 11.69 ± 0.91 a | 11.66 ± 1.13 a |
| High density lipoprotein (mmol/L) | 1.66 ± 0.03 a | 1.68 ± 0.02 a | 1.63 ± 0.03 a | 1.65 ± 0.06 a |
| Low density lipoprotein (mmol/L) | 0.11 ± 0.008 a | 0.09 ± 0.004 a | 0.12 ± 0.012 a | 0.10 ± 0.007 a |
| Glucose (mmol/L) | 5.74 ± 0.41 a | 5.84 ± 0.12 a | 5.76 ± 0.51 a | 5.79 ± 0.32 a |
| Diversity Index | Control Group | GCP Concentration (μg/mL) | ||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Sobs | 360.66 ± 18.64 a | 368.66 ± 15.21 b | 382.00 ± 21.35 c | 379.50 ± 26.33 c |
| ACE | 401.41 ± 29.32 a | 408.41 ± 35.03 b | 416.06 ± 27.06 c | 414.54 ± 38.09 c |
| Chao1 | 404.96 ± 25.54 a | 418.25 ± 34.17 b | 426.88 ± 29.13 c | 424.96 ± 34.10 c |
| Simpson | 0.074 ± 0.09 a | 0.057 ± 0.01 b | 0.049 ± 0.05 c | 0.043 ± 0.09 d |
| Shannon | 3.84 ± 0.19 a | 3.94 ± 0.27 b | 4.19 ± 0.31 c | 4.30 ± 0.39 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, Z.; Hu, X.; Zhao, C.; Zhang, X.; Zhang, H. Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice. Foods 2021, 10, 1084. https://doi.org/10.3390/foods10051084
Sun X, Wang Z, Hu X, Zhao C, Zhang X, Zhang H. Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice. Foods. 2021; 10(5):1084. https://doi.org/10.3390/foods10051084
Chicago/Turabian StyleSun, Xincheng, Zichao Wang, Xuyang Hu, Chengxin Zhao, Xiaogen Zhang, and Huiru Zhang. 2021. "Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice" Foods 10, no. 5: 1084. https://doi.org/10.3390/foods10051084
APA StyleSun, X., Wang, Z., Hu, X., Zhao, C., Zhang, X., & Zhang, H. (2021). Effect of an Antibacterial Polysaccharide Produced by Chaetomium globosum CGMCC 6882 on the Gut Microbiota of Mice. Foods, 10(5), 1084. https://doi.org/10.3390/foods10051084






