Construction and Application of EGCG-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Conditions of Lysozyme/Pectin Nanoparticles (LY/Ps NPs)
2.3. Preparation of EGCG-Loaded Lysozyme/Pectin Nanoparticles (EGCG-LY/Ps NPs)
2.4. Determination of Interaction of LY/Ps NPs with EGCG by Fluorescence Spectroscopy
2.5. Calculation of Binding Constants and Thermodynamic Parameters
2.6. Determination of Interaction between LY/Ps NPs and EGCG by Circular Dichroism Chromatography
2.7. Determination of EGCG-LY/Ps NPs Particle Size Distribution
2.8. Determination of EGCG-LY/Ps NPs Loading Rate
2.9. Heat Stress Assay
2.10. Oxidative Stress Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Interaction of EGCG with Lysozyme and Lysozyme/Pectin Nanoparticles (LY/Ps NPs)
3.2. Binding Sites, Binding Constants, and Action Forces of EGCG Interaction with LY and NPs
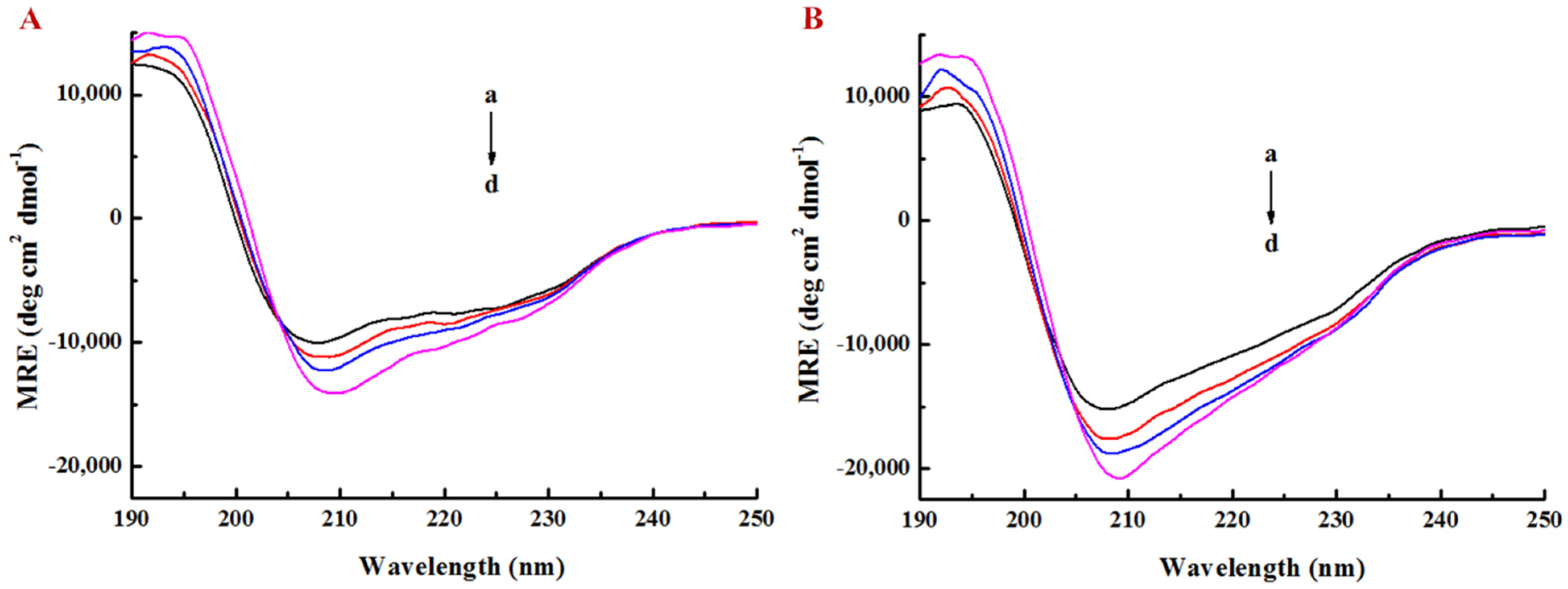
3.3. Effect of EGCG on Secondary Structure of Lysozyme
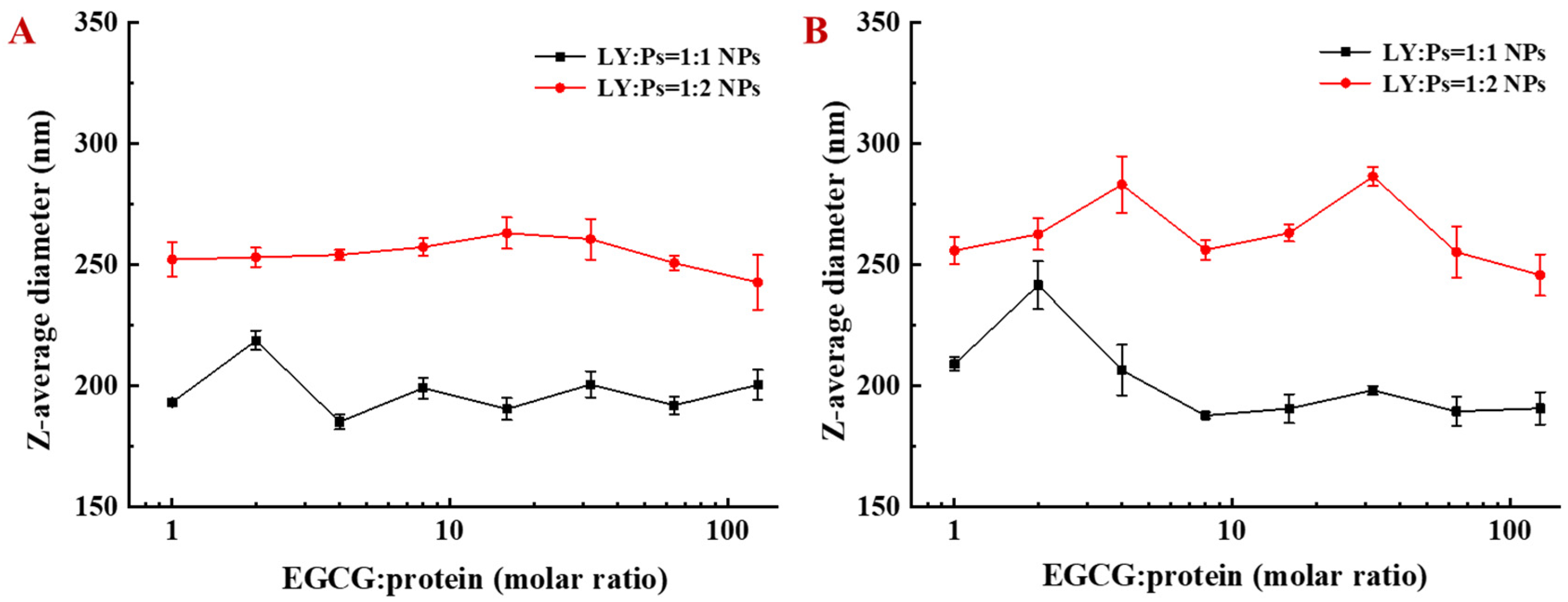
3.4. Particle Size Variation and Encapsulation Rate of EGCG by LY/Ps NPs
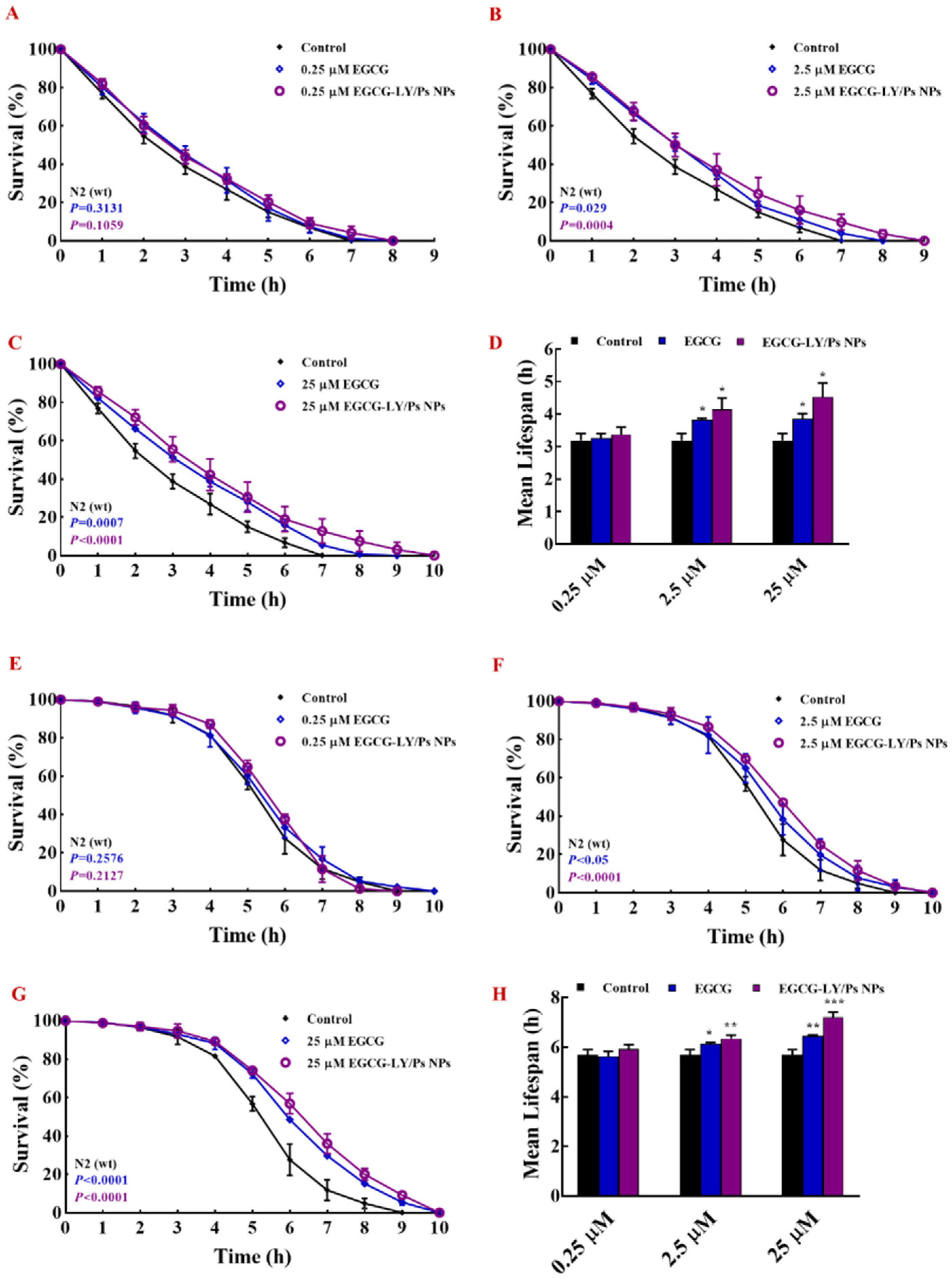
3.5. EGCG-LY/Ps NPs Enhanced the Resistance of C. Elegans to Heat Stress and Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sosa, P.M.; de Souza, M.A.; Mello-Carpes, P.B. Green Tea and Red Tea from Camellia sinensis Partially Prevented the Motor Deficits and Striatal Oxidative Damage Induced by Hemorrhagic Stroke in Rats. Neural Plast. 2018, 2018, 5158724. [Google Scholar] [CrossRef]
- Yang, J.E.; Rhoo, K.Y.; Lee, S.; Lee, J.T.; Park, J.H.; Bhak, G.; Paik, S.R. EGCG-mediated Protection of the Membrane Disruption and Cytotoxicity Caused by the ‘Active Oligomer’ of alpha-Synuclein. Sci. Rep. 2017, 7, 17945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, L.; Yu, J.; Wang, Y.; Peng, B. Anti-osteoclastogenic effect of epigallocatechin gallate-functionalized gold nanoparticles in vitro and in vivo. Int. J. Nanomed. 2019, 14, 5017. [Google Scholar] [CrossRef] [Green Version]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2017, 12, 908–931. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Kumar, S.; Dhingra, D.; Dilbaghi, N. Chitosan-Gellan Gum Bipolymeric Nanohydrogels—A Potential Nanocarrier for the Delivery of Epigallocatechin Gallate. BioNanoScience 2017, 7, 508–520. [Google Scholar] [CrossRef]
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Dev. 2016, 10, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, R.; Liu, J.; Meng, D.; Zhou, Z.; Zhang, Y.; Blanchard, C. Fabrication, structure, and function evaluation of the ferritin based nano-carrier for food bioactive compounds. Food Chem. 2019, 299, 125097. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Gao, C.; Chen, L.; He, Y.; Ma, W.; Lin, Z. Recent advances in biomolecule immobilization based on self-assembly: Organic–inorganic hybrid nanoflowers and metal–organic frameworks as novel substrates. J. Mater. Chem. B 2018, 6, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and Polysaccharide-Based Magnetic Composite Materials for Medical Applications. Int. J. Mol. Sci. 2020, 21, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaber, M.; Mabrouk, M.T.; Freag, M.S.; Khiste, S.K.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Protein-polysaccharide nanohybrids: Hybridization techniques and drug delivery applications. Eur. J. Pharm. Biopharm. 2018, 133, 42–62. [Google Scholar] [CrossRef]
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Chapter 22—Use of Hen Egg White Lysozyme in the Food Industry. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 233–242. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Wada, T.; Kasai, T.; Shiratori, T.; Minami, Y.; Shimada, Y.; Otsuka, Y.; Komatsu, K.; Goto, S. Effects of ionic and reductive atmosphere on the conformational rearrangement in hen egg white lysozyme prior to amyloid formation. Colloids Surf. B Biointerfaces 2020, 190, 110845. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef]
- Khotimchenko, M. Pectin polymers for colon-targeted antitumor drug delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocoll. 2018, 77, 152–162. [Google Scholar] [CrossRef]
- Chevalier, L.M.; Rioux, L.-E.; Angers, P.; Turgeon, S.L. Study of the interactions between pectin in a blueberry puree and whey proteins: Functionality and application. Food Hydrocoll. 2019, 87, 61–70. [Google Scholar] [CrossRef]
- Lin, L.; Xu, W.; Liang, H.; He, L.; Liu, S.; Li, Y.; Li, B.; Chen, Y. Construction of pH-sensitive lysozyme/pectin nanogel for tumor methotrexate delivery. Colloids Surf. B Biointerfaces 2015, 126, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Li, Y.; Xia, Q.; Zhang, X.; Huang, Q. Glycosylation of bovine serum albumin via Maillard reaction prevents epigallocatechin-3-gallate-induced protein aggregation. Food Hydrocoll. 2015, 43, 228–235. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Karaś, M.; Zięba, E.; Mężyńska, M.; Wiącek, A.E. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll. 2020, 101, 105539. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Chen, J.; Zhou, Q.-H.; Shi, Y.-Q.; Wang, Y.-Q. Study on the interaction between cinnamic acid and lysozyme. J. Mol. Struct. 2011, 987, 7–12. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Chen, T.-T.; Zhang, H.-M. Investigation of the interactions of lysozyme and trypsin with biphenol A using spectroscopic methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1130–1137. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Liu, Y.; Sun, T.-Q.; Bai, A.-M.; Lü, J.-Q.; Pi, Z.-B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Yang, J. Protein determination using methylene blue in a synchronous fluorescence technique. Talanta 2010, 81, 760–765. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Y.; Li, J.; Zhou, H.; Wang, X. Binding of curcumin with bovine serum albumin in the presence of ι-carrageenan and implications on the stability and antioxidant activity of curcumin. J. Agric. Food Chem. 2013, 61, 7150–7155. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Barratt, E.; Bingham, R.J.; Warner, D.J.; Laughton, C.A.; Phillips, S.E.; Homans, S.W. Van der Waals interactions dominate ligand− protein association in a protein binding site occluded from solvent water. J. Am. Chem. Soc. 2005, 127, 11827–11834. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, S.; Shareghi, B.; Momeni, L.; Abou-Zied, O.K.; Sirotkin, V.A.; Tachiya, M.; Saboury, A.A. Insights into the molecular interaction between sucrose and α-chymotrypsin. Int. J. Biol. Macromol. 2018, 114, 950–960. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.-J.; Feng, S.; Zeng, C.-M. Quinopeptide formation associated with the disruptive effect of epigallocatechin-gallate on lysozyme fibrils. Int. J. Biol. Macromol. 2015, 78, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.I.; Hassan, F.; Anwer, R.; Juan, F.; Lai, D. Comparative analysis of bacteriophytochrome agp2 and its engineered photoactivatable NIR fluorescent proteins pairfp1 and pairfp2. Biomolecules 2020, 10, 1286. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hosseini, A.; Taghavi, F.; Jafari, S.; Lotfabadi, A.; Ejtehadi, M.R.; Shahbazi, S.; Fattahi, A.; Ghasemi, A.; Barzegari, E. Molecular interaction of fibrinogen with zeolite nanoparticles. Sci. Rep. 2019, 9, 1558. [Google Scholar] [CrossRef]
- Fan, Q.; Ji, Y.; Wang, J.; Wu, L.; Li, W.; Chen, R.; Chen, Z. Self-assembly behaviours of peptide–drug conjugates: Influence of multiple factors on aggregate morphology and potential self-assembly mechanism. R. Soc. Open Sci. 2018, 5, 172040. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Yao, X.; Zhang, Y.; Zhao, M.; Zhang, K.; Jiang, F. Complexation of Bovine Serum Albumin and Sugar Beet Pectin: Structural Transitions and Phase Diagram. Langmuir 2012, 28, 10164–10176. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.J. Longevity and heat stress regulation in Caenorhabditis elegans. Mech. Ageing Dev. 2003, 124, 43–48. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Candido, E.P.M. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: Relationship to the cellular stress response. J. Exp. Zool. 1999, 284, 147–157. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species-sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.; Wink, M. Green tea extract induces the resistance of Caenorhabditis elegans against oxidative stress. Antioxidants 2014, 3, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile synthesis of nano-nanocarriers from chitosan and pectin with improved stability and biocompatibility for anthocyanins delivery: An in vitro and in vivo study. Food Hydrocoll. 2020, 109, 106114. [Google Scholar] [CrossRef]
- Butt, H.; Mehmood, A.; Ejaz, A.; Humayun, S.; Riazuddin, S. Epigallocatechin-3-gallate protects Wharton’s jelly derived mesenchymal stem cells against in vitro heat stress. Eur. J. Pharm. 2020, 872, 172958. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef] [PubMed]
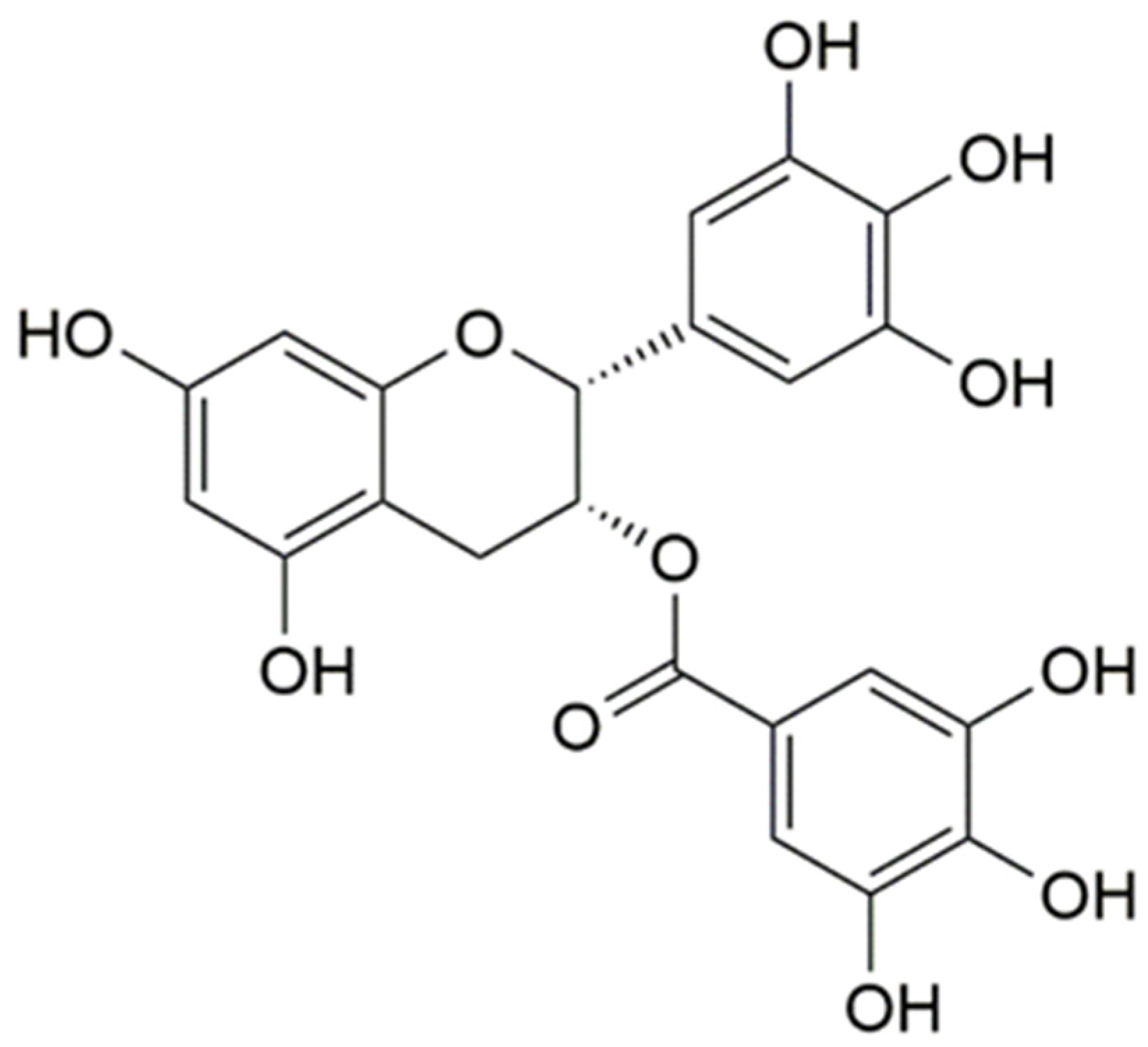
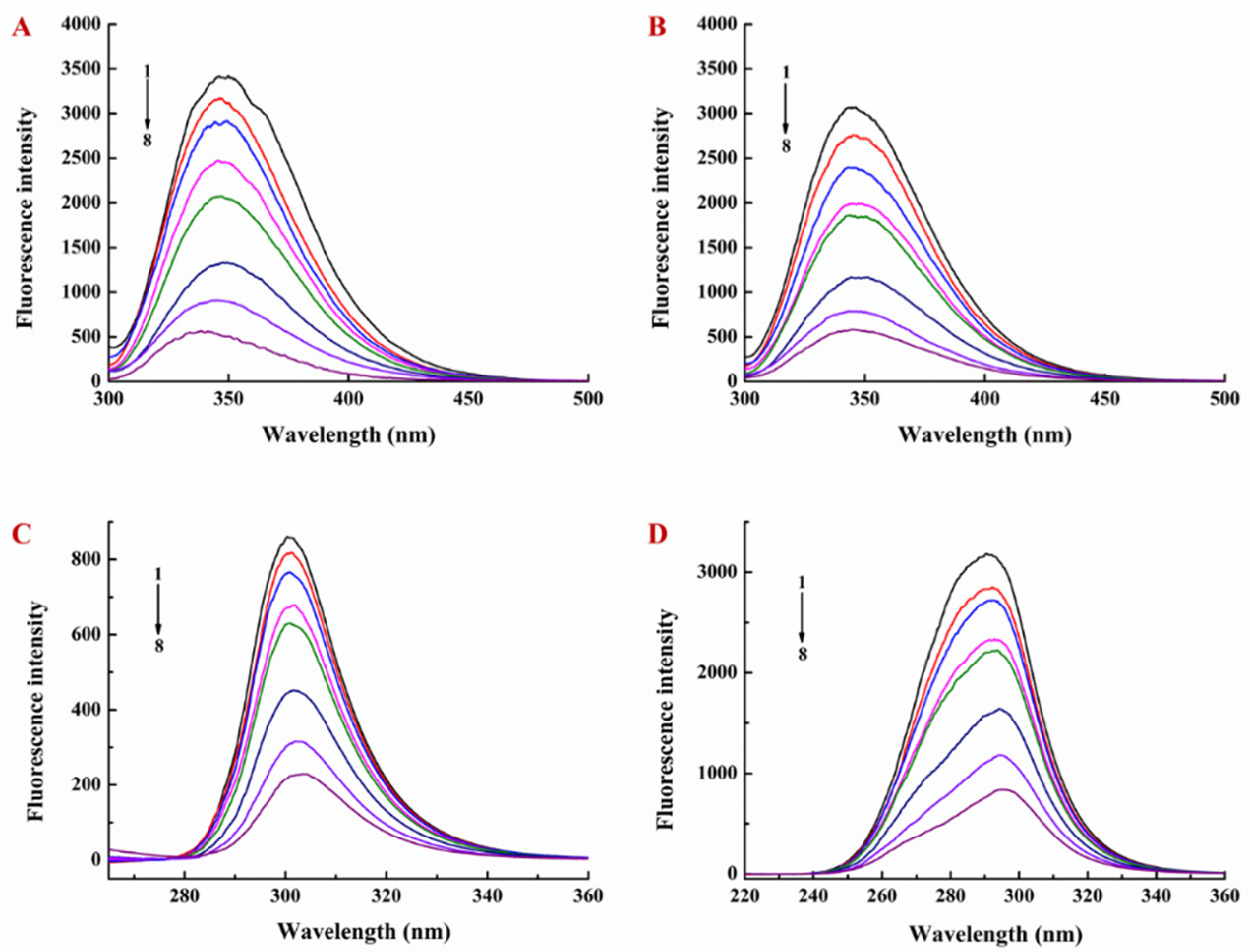
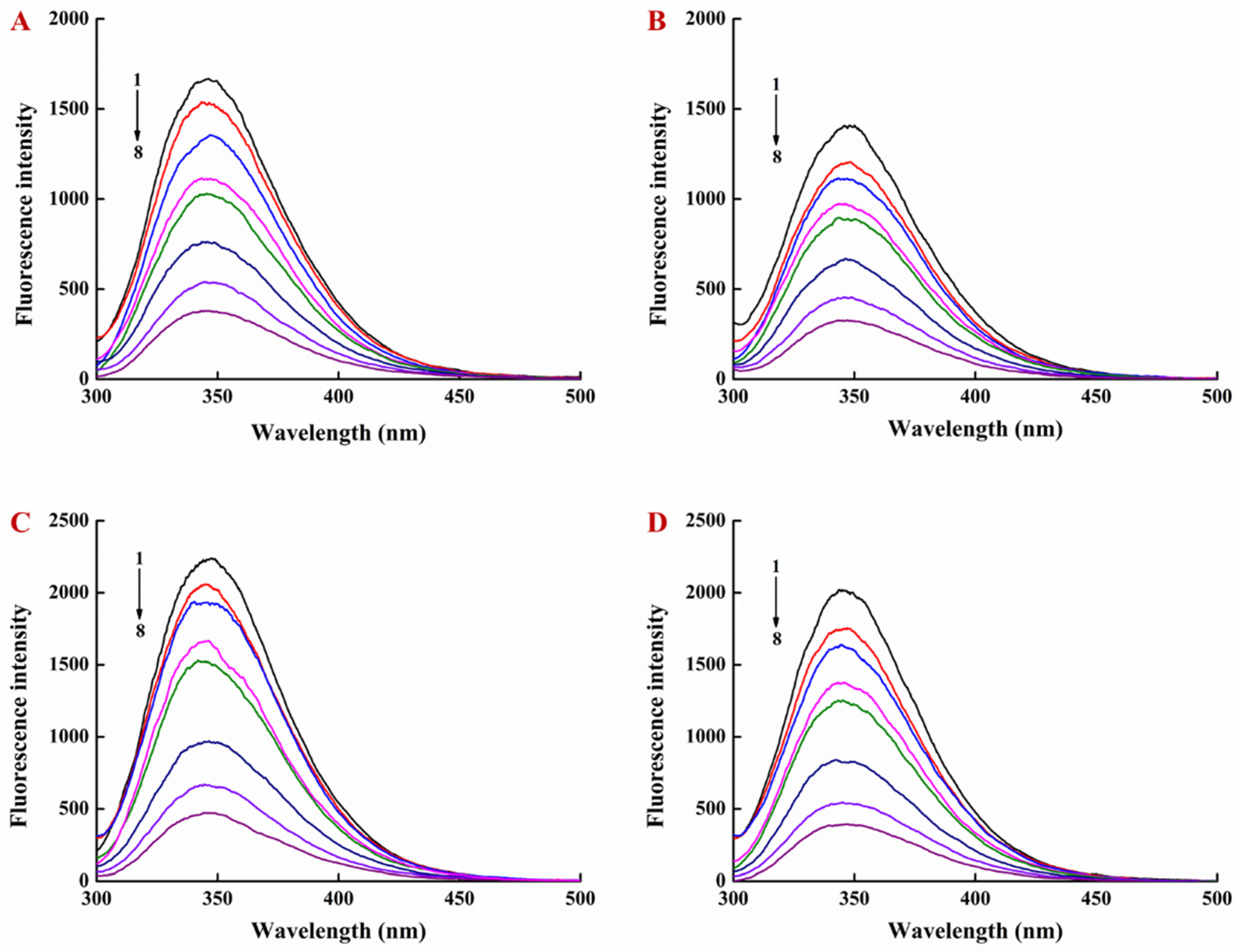
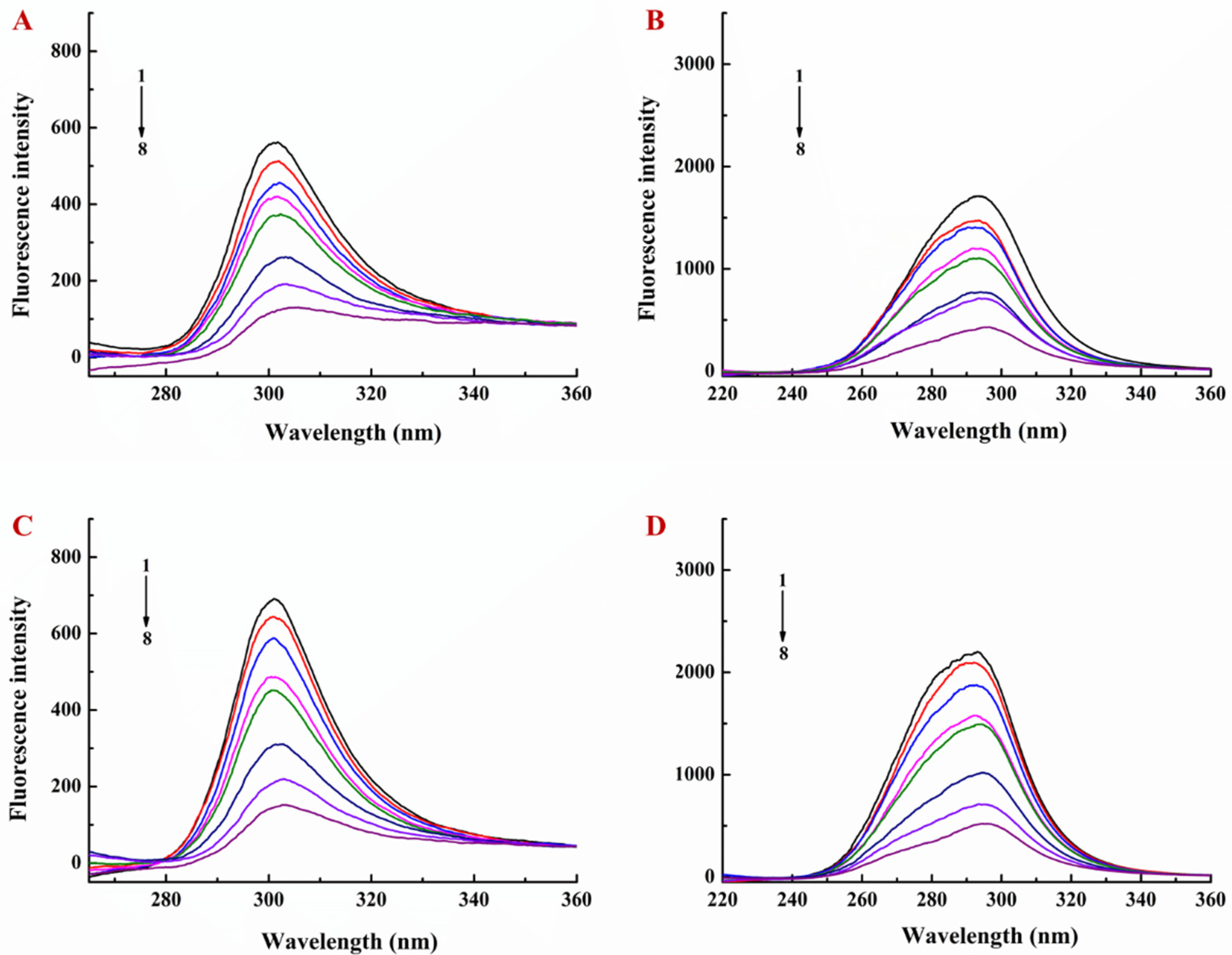
| Sample | T (K) | Ka (L·mol−1) | n | R2 | ΔG (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔH (kJ·mol−1) |
|---|---|---|---|---|---|---|---|
| Native Protein | 298 | 1.12 × 106 | 1.4121 | 0.9995 | −34.52 | −324.57 | −131.24 |
| 308 | 2.01 × 105 | 1.2217 | 0.9964 | −31.27 | −324.57 | ||
| LY:Ps = 1:1 | 298 | 9.01 × 104 | 1.1677 | 0.9955 | −28.26 | −62.55 | −46.90 |
| 308 | 4.87 × 104 | 1.1001 | 0.9976 | −27.64 | −62.53 | ||
| LY:Ps = 1:2 | 298 | 2.64 × 105 | 1.2740 | 0.9937 | −30.93 | −4.73 | −32.35 |
| 308 | 1.73 × 105 | 1.2170 | 0.9936 | −30.88 | −4.74 |
| Molar Ratio (EGCG: Protein) | EGCG-Nanoparticles | |
|---|---|---|
| Entrapment Efficiency (%) | EGCG Loading (μg/mg) | |
| 0:1 | ND | ND |
| 1:1 | 71.77 ± 8.01 | 11.48 ± 1.28 |
| 2:1 | 65.18 ± 5.01 | 20.85 ± 1.60 |
| 4:1 | 57.00 ± 4.08 | 36.48 ± 2.61 |
| 8:1 | 51.59 ± 3.31 | 66.03 ± 4.23 |
| 16:1 | 47.90 ± 3.05 | 122.62 ± 7.80 |
| 32:1 | 37.48 ± 2.70 | 191.90 ± 13.82 |
| 64:1 | 31.95 ± 1.59 | 327.17 ± 16.28 |
| 128:1 | 28.98 ± 1.28 | 593.51 ± 26.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, L.; Cui, H.; Li, B.; Tian, J. Construction and Application of EGCG-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses. Foods 2021, 10, 1127. https://doi.org/10.3390/foods10051127
Zhang Y, Lin L, Cui H, Li B, Tian J. Construction and Application of EGCG-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses. Foods. 2021; 10(5):1127. https://doi.org/10.3390/foods10051127
Chicago/Turabian StyleZhang, Yu, Liufeng Lin, Hao Cui, Bin Li, and Jing Tian. 2021. "Construction and Application of EGCG-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses" Foods 10, no. 5: 1127. https://doi.org/10.3390/foods10051127
APA StyleZhang, Y., Lin, L., Cui, H., Li, B., & Tian, J. (2021). Construction and Application of EGCG-Loaded Lysozyme/Pectin Nanoparticles for Enhancing the Resistance of Nematodes to Heat and Oxidation Stresses. Foods, 10(5), 1127. https://doi.org/10.3390/foods10051127







