An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Poly(Styrene) Samples
2.3. Food Simulants and Testing Conditions
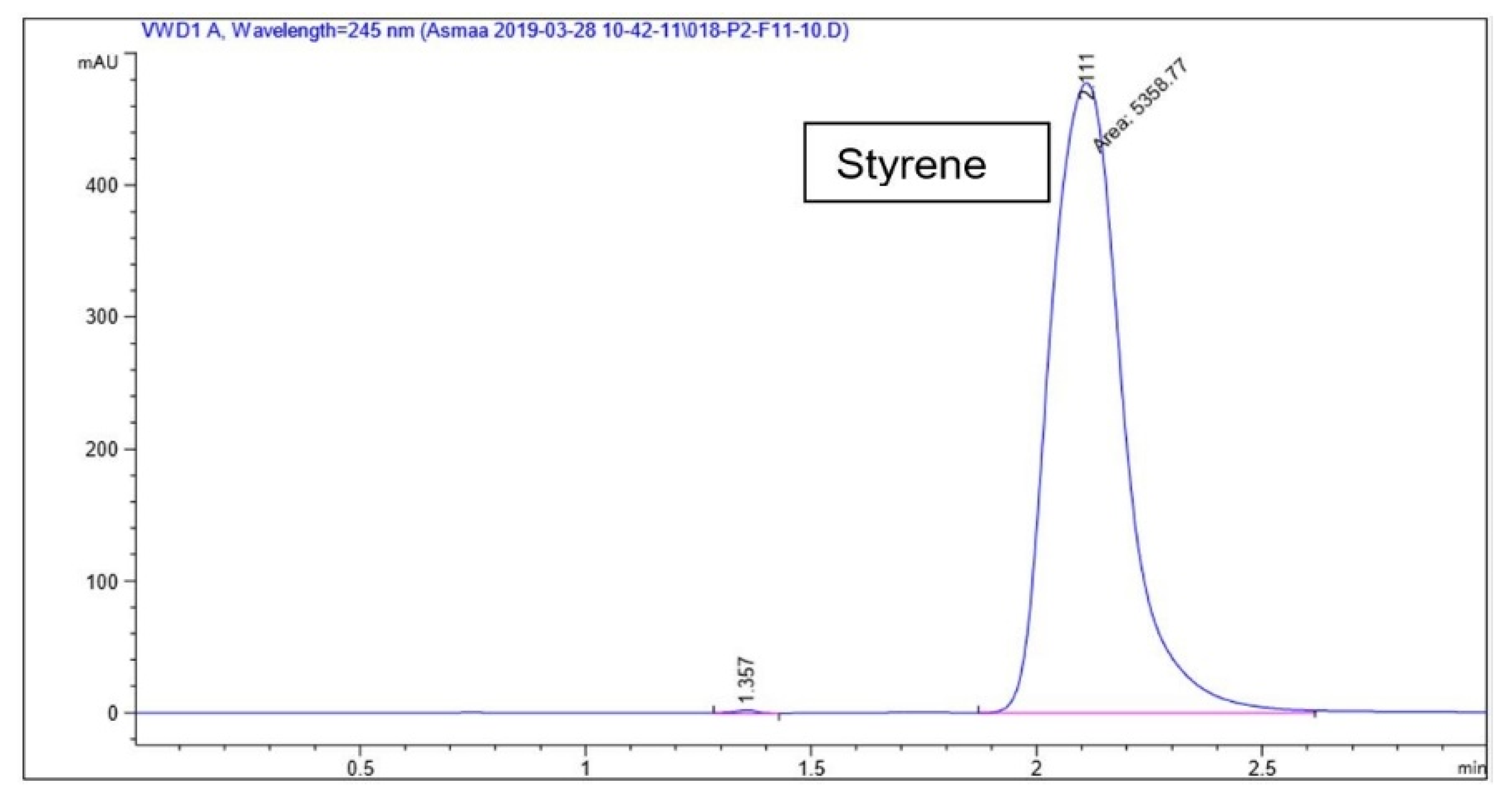
2.4. HPLC Conditions
2.5. Experimental Procedure
2.5.1. Preparing the Calibration Curves
2.5.2. Sample Preparation
2.5.3. Nile Red
3. Results
3.1. Drinking Cup Samples 1–4
3.2. Food Container Samples 5–7
3.3. Calibration Curves
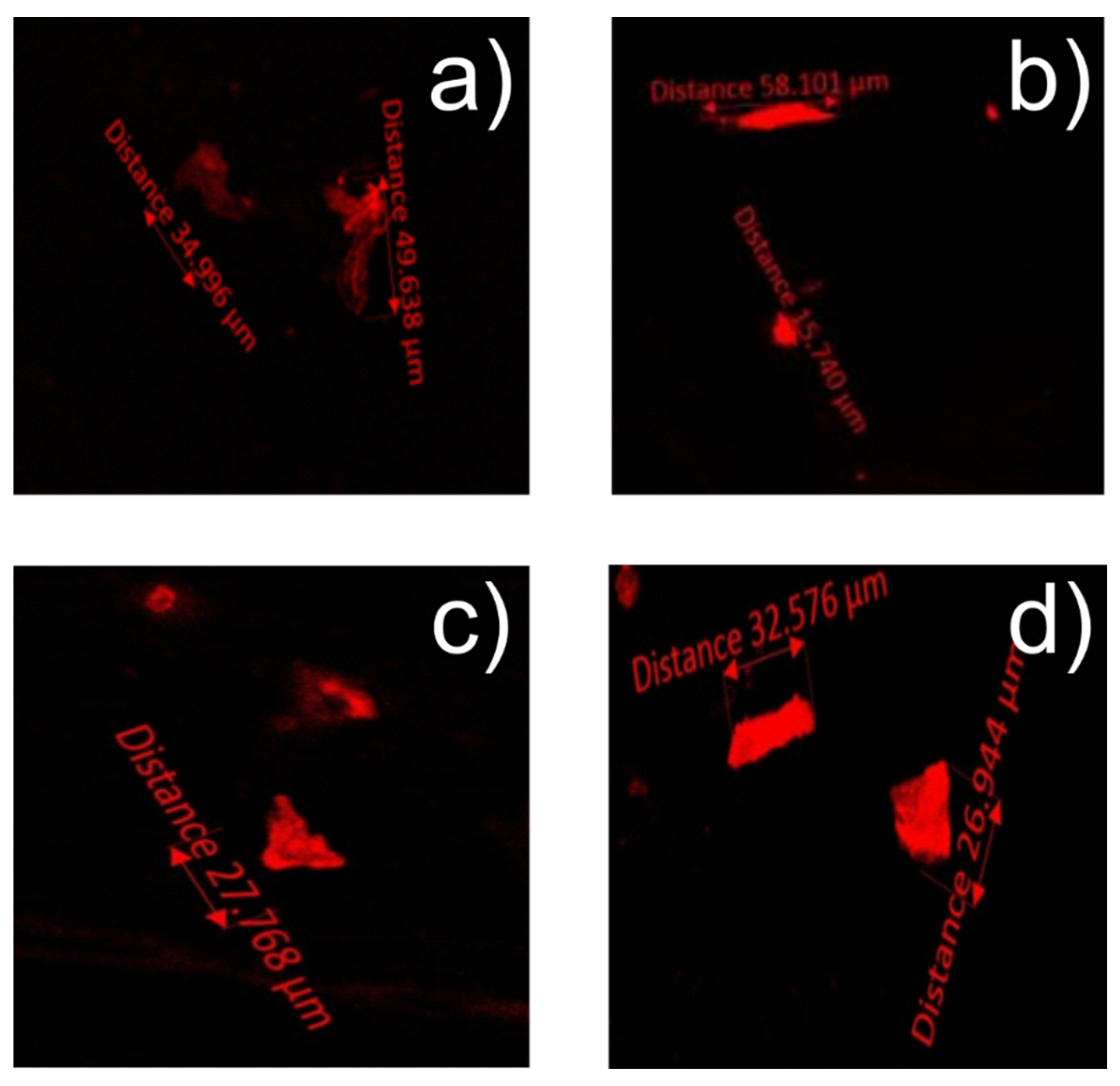
3.4. Nile Red
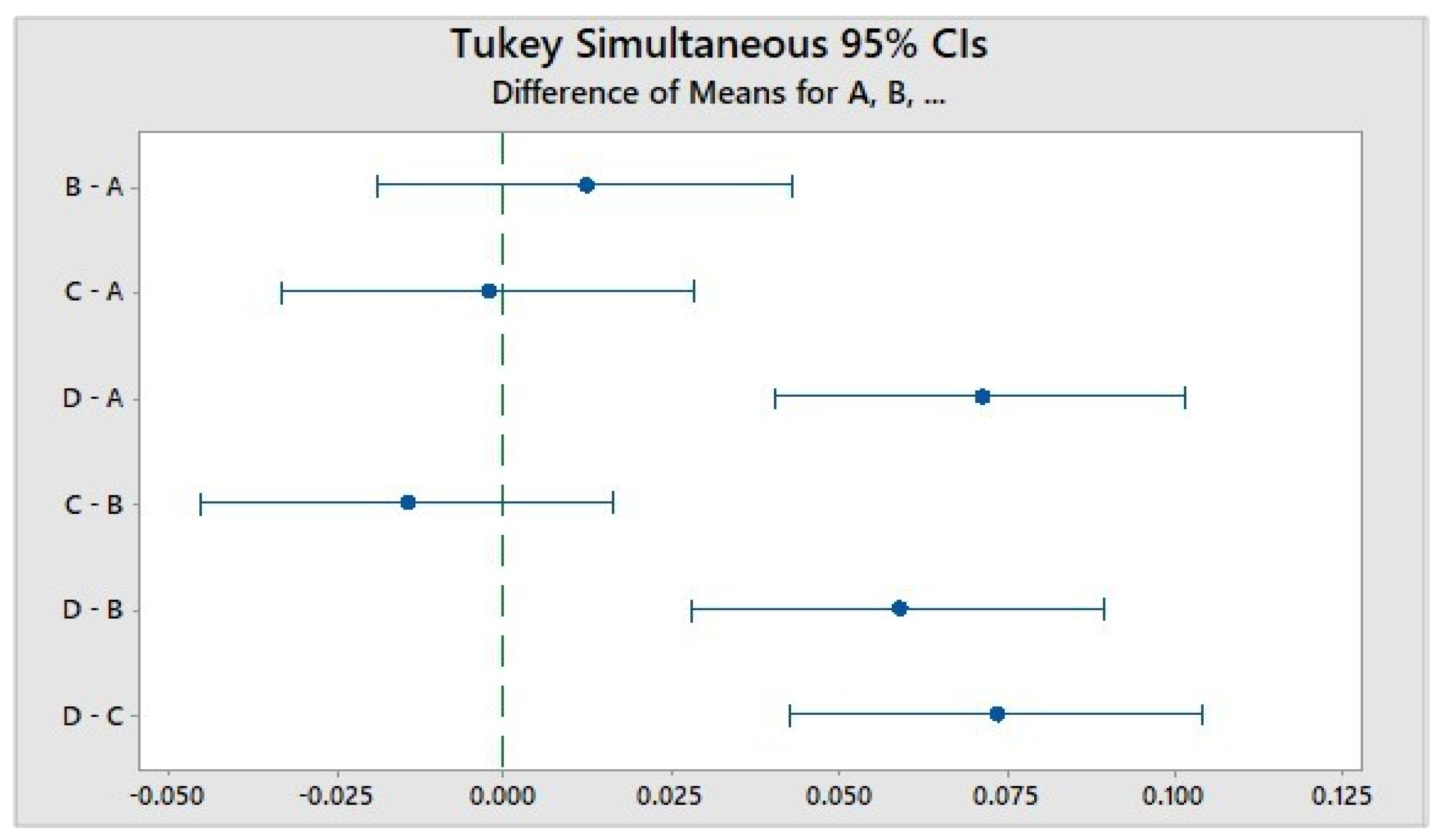
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouellette, R.J.; Rawn, J.D. 15-Synthetic Polymers. In Principles of Organic Chemistry; Ouellette, R.J., Rawn, J.D., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 397–419. ISBN 978-0-12-802444-7. [Google Scholar]
- Podzimek, S. Polymers Synthetic. In Encyclopaedia of Analytical Science, 4th ed.; Academic Press: Oxford, UK, 2019; pp. 360–370. ISBN 978-0-08-101984-9. [Google Scholar]
- Market Data: Plastics Europe. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 22 March 2021).
- About Plastics Packaging. British Plastic Federation. Available online: https://www.bpf.co.uk/plastipedia/applications/about_plastics packaging.aspx (accessed on 22 March 2021).
- Marsh, K.; Bugusu, B. Food Packaging Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- First Plastics Pact in the Pacific Region Galvanises Organisations across Australia, New Zealand, and the Pacific Island Nations for a Circular Economy for Plastic. Circular Economy-UK, USA, Europe, Asia & South America-The Ellen MacArthur Foundation. Available online: https://www.ellenmacarthurfoundation.org/ (accessed on 19 May 2021).
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans R. Soc. Lond Ser. B Bio. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Nidhin, S.; Ananthakrishnan, U.; Amith, S.; Hasif Ali Kalody, B. Rajesh Menon. Review on recent development and future scope in using biodegradable polymers materials for food packaging. Int. J. Eng. Res. Tech. (IJERT) 2019, 8, 474–478. [Google Scholar]
- Hahladakis, J.N.; Velis, C.A.; Weber, R. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Union POotE. Food Contact Materials. 2016. Available online: https://publications.europa.eu/en/publication-detail/-/publication/1c258f12-c3f7-11e5-8d08-01aa75ed71a1 (accessed on 22 March 2021).
- Paraskevopoulou, D.; Achilias, D.S.; Paraskevopoulou, A. Migration of styrene from plastic packaging based on poly(styrene) into food simulants. Polym. Inter. 2012, 61, 141–148. [Google Scholar] [CrossRef]
- Trusted Science for Safe Food. Available online: https://www.efsa.europa.eu/en/corporate/pub/strategy2020 (accessed on 22 March 2021).
- Food Contact Materials. 2016. Available online: https://ec.europa.eu/food/safety/chemical_safety/food_contact_materials_en (accessed on 22 March 2021).
- Ekaterina, K. Food Contact Materials-Regulation (EC) 1935/2004; European Implementation Assessment Study. Available online: http://www.europarl.europa.eu/RegData/etudes/STUD/2016/581411/EPRS_STU(2016)581411_EN.pdf (accessed on 22 March 2021).
- EUR-Lex, Access to European Union Law. Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0010 (accessed on 22 March 2021).
- Satterthwaite, K. Chapter 12, Plastics Based on Styrene. In Brydson’s Plastic Material; Gilbert, M., Ed.; Butterworth Heinemann: Oxford, UK, 2017; pp. 311–328. [Google Scholar]
- Ping, L. Ku Poly(styrene) and Styrene Copolymers. I. Their Manufacture and Application. 1988. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adv.1988.060080204 (accessed on 22 March 2021).
- Khaksar, M.R.; Ghazi-Khansari, M. Determination of migration monomer styrene from GPPS (general purpose polystyrene) and HIPS (high impact polystyrene) cups to hot drinks. Toxicol. Mech. Methods 2009, 19, 257–261. [Google Scholar] [CrossRef]
- Lickly, T.D.; Lehr, K.M.; Welsh, G.C. Migration of styrene from poly(styrene) foam food-contact articles. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1995, 33, 475–481. [Google Scholar] [CrossRef]
- Cohen, J.T.; Carlson, G.; Charnley, G. A comprehensive evaluation of the potential health risks associated with occupational and environmental exposure to styrene. J. Toxicol. Environ. Health Part B Crit. Rev. 2002, 5, 335. [Google Scholar] [CrossRef]
- Groh KJ and Muncke, J. In Vitro Toxicity Testing of Food Contact Materials: State-of-the-Art and Future Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1123–1150. [Google Scholar] [CrossRef] [Green Version]
- Styrene: Health Effects, Incident Management and Toxicology. Available online: https://www.gov.uk/government/publications/styrene-properties-incident-management-and-toxicology (accessed on 22 March 2021).
- El-Alfy ATGupta, R.G. Reproductive and Developmental Toxicology. Academic Press, Elsevier; 2011, 1202pp, $250 (hardcover). Am. J. Pharm. Educ. 2013, 77, 20. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.T.; Tuohy, J.J.; Franz, R. Comparison of milk and ethanol/water mixtures with respect to monostyrene migration from a poly(styrene) packaging material. Inter. Dairy J. 1994, 4, 271–283. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiesa, L.M.; Panseri, S.; Soncin, S.; Vallone, L.; Dragoni, I. Determination of styrene in Gorgonzola PDO cheese by headspace solid phase micro-extraction (HS-SPME) and gas chromatography mass-spectrometry (GC-MS). Vet. Res. Commun. 2010, 34, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerin, C.; Rubio, C.; Cacho, J.; Salafranca, J. Determination of styrene in olive oil by an automatic purge-and-trap system coupled to gas chromatography-mass-spectrometry. Chromatographia 1995, 41, 216–220. [Google Scholar] [CrossRef]
- Cristina, N.; Carlos, R.; Juan, C.; Jesus, S. Parts-per-trillion determination of styrene in yogurt by purge-and-trap gas chromatography mass-spectrometry detection. Food Addit. Contam. 1998, 15, 346–354. [Google Scholar] [CrossRef]
- David, H.; Steele, M.J.; Thornburg, J.S.; Stanley, R.R.; Miller, R.B.; Janette, R.C.; George, C. Determination of styrene in selected foods. J. Agric. Food Chem. 1994, 42, 1661–1665. [Google Scholar]
- Patricia, S.; Bernd, R.; Rainer, K.; Dirk, B. A sensitive, viable-colony staining method using Nile Red for direct screening of bacteria that accumulate of polyhdroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 µm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- ICH Guidelines. 2018. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/ich-guidelines (accessed on 22 April 2021).
- Tawfik MS and Huyghebaert, A. Poly(styrene) cups and containers: Styrene migration. Food Addit. Contam. 1998, 15, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Environmental Chemistry. Colin Baird, Michael Cann. Hardcover–9781464113499. 23 March 2012. Available online: https://www.macmillanihe.com/page/detail/environmental-chemistry/?k=9781464113499&loc=uk (accessed on 22 March 2021).
- Yee, A.F.; Sue, H.J. Impact Resistance. In Encyclopaedia of Polymer Science and Technology; Interscience: New York, NY, USA, 2002; ISBN 978-0-471-44026-0. [Google Scholar]
- Lau, O.W.; Wong, S.K. Contamination in food from packaging material. J. Chromatogr. A 2000, 16, 255–270. [Google Scholar] [CrossRef]
- Sandra Rainieri and Alejandro Barranco. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA) US. The Safety of Styrene-Based Polymers for Food-Contact Use. 2013. Available online: https://www.plasticfoodservicefacts.com/wp-content/uploads/2017/10/Poly(styrene)-Report.pdf (accessed on 19 May 2021).
- Galgani, F.; Pham, C.K.; Reisser, J. Editorial: Plastic Pollution. Front. Mar. Sci. 2017, 4, 307. [Google Scholar] [CrossRef] [Green Version]





| Simulants | Contact Foods |
|---|---|
| A-10% aq. Ethanol/distilled Water | Aqueous foods (pH > 4.5) |
| B-3% aq. Acetic acid | Acidic food (pH < 4.5) |
| C-50% aq. Ethanol | Diary food products |
| D-95% aq. Ethanol | High fat content foods |
| Parameters | Value |
|---|---|
| Accuracy | 98.3 ± 3.29 |
| Slope | 633.113 |
| Intercept | 12.529 |
| Linearity range | 0.02–0.08 µg/mL |
| Correlation coefficient (r) | 0.9996 |
| Standard Error | 1.6650 |
| LOD | 0.0086 µg/mL |
| LOQ | 0.0263 µg/mL |
| Parameters | Value |
| Accuracy | 100.34 ± 1.95 |
| Slope | 524.81 |
| Intercept | 80.51 |
| Linearity range | 2–10 µg/mL |
| Correlation coefficient (r) | 0.9996 |
| Standard Error | 10.608 |
| LOD | 0.066 µg/mL |
| LOQ | 0.202 µg/mL |
| Article | Food Simulant | Time (h) | Temperature | Concentration µg/mL |
|---|---|---|---|---|
| Sample 1: HIPS Poly(styrene) cup | A-Water | 1 h | 60 °C | 0.0074 |
| B-Acetic acid 3% | 1 h | 60 °C | 0.0121 | |
| C-Ethanol 10% | 1 h | 60 °C | 0.0004 | |
| D-Ethanol 50% | 1 h | 60 °C | 0.0773 | |
| Sample 2: EPS Styrofoam cup | A-Water | 1 h | 60 °C | 0.0074 |
| B-Acetic acid 3% | 1 h | 60 °C | 0.0025 | |
| C-Ethanol 10% | 1 h | 60 °C | 0.0080 | |
| D-Ethanol 50% | 1 h | 60 °C | 0.0779 | |
| Sample 3: HIPS Coffee lid | A-Water | 1 h | 60 °C | 0.0039 |
| B-Acetic acid 3% | 1 h | 60 °C | 0.0172 | |
| C-Ethanol 10% | 1 h | 60 °C | 0.0010 | |
| D-Ethanol 50% | 1 h | 60 °C | 0.1105 | |
| Sample 4: HIPS Tumbler cup | A-Water | 1 h | 60 °C | 0.0039 |
| B-Acetic acid 3% | 1 h | 60 °C | 0.0167 | |
| C-Ethanol 10% | 1 h | 60 °C | 0.0033 | |
| D-Ethanol 50% | 1 h | 60 °C | 0.0406 |
| Article | Food Simulant | Time (h) | Temperature | Concentration µg/mL |
|---|---|---|---|---|
| Sample 5: EPS | Ethanol 10% | 2 | 60 °C | 0.00880 = <LOQ |
| Takeaway Container | 2 | 70 °C | 0.00950 = <LOQ | |
| Acetic Acid 3% | 2 | 60 °C | 0.00480 = <LOD | |
| 2 | 70 °C | 0.0260 | ||
| Ethanol 50% | 2 | 60 °C | 0.126 | |
| 2 | 70 °C | 0.190 | ||
| Ethanol 95% | 2 | 60 °C | 1.31 | |
| 2 | 70 °C | 5.57 | ||
| Sample: 6 EPS | Ethanol 10% | 240 | 5 °C | 0.000300 = <LOD |
| Meat Tray | Ethanol 95% | 240 | 5 °C | 0.411 |
| Sample 7 XPS | Ethanol 10% | 240 | 5 °C | 0.00810 |
| Disposable Foam | 2 | 70 °C | 0.029 | |
| Plate | Acetic Acid 3% | 2 | 60 °C | 0.0079 |
| 2 | 70 °C | 0.0091 | ||
| Ethanol 50% | 240 | 5 °C | 0.0390 | |
| 2 | 60 °C | 2.88 | ||
| 2 | 70 °C | 6.04 | ||
| Ethanol 95% | 240 | 5 °C | 1.51 | |
| 2 | 60 °C | 3.01 | ||
| 2 | 70 °C | 6.42 |
| Microplastic Size in μm | Poly(Styrene) Type | Simulant and Temperature | Microplastic Pieces Per cm2 |
|---|---|---|---|
| 52.9 | EPS takeaway container | 3% Acetic acid at 60 °C | 1–6 |
| 17.6 | EPS meat tray | 50% Ethanol at 60 °C | 2–6 |
| 6.40 | EPS meat tray | 50% Ethanol at 70 °C | 4–10 |
| 104 | EPS meat tray | 95% Ethanol at 70 °C | 2–10 |
| 58.9 | XPS disposable plate | 50% Ethanol at 5 °C | 1–4 |
| 11.8 | XPS disposable plate | 95% Ethanol at 5 °C | 1–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajaj, A.; J’Bari, S.; Ononogbo, A.; Buonocore, F.; Bear, J.C.; Mayes, A.G.; Morgan, H. An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging. Foods 2021, 10, 1136. https://doi.org/10.3390/foods10051136
Ajaj A, J’Bari S, Ononogbo A, Buonocore F, Bear JC, Mayes AG, Morgan H. An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging. Foods. 2021; 10(5):1136. https://doi.org/10.3390/foods10051136
Chicago/Turabian StyleAjaj, Asmaa, Shayma J’Bari, Anthonia Ononogbo, Federico Buonocore, Joseph C. Bear, Andrew G. Mayes, and Huda Morgan. 2021. "An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging" Foods 10, no. 5: 1136. https://doi.org/10.3390/foods10051136
APA StyleAjaj, A., J’Bari, S., Ononogbo, A., Buonocore, F., Bear, J. C., Mayes, A. G., & Morgan, H. (2021). An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging. Foods, 10(5), 1136. https://doi.org/10.3390/foods10051136







