Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium erinaceus Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Pretreatment Processes of H. erinaceus by the Design of Experiment
2.4. Biogenic Amines Extraction from H. erinaceus
2.5. Method Validation of Biogenic Amine Analysis in H. erinaceus Extract
2.6. Biogenic Amines Analysis Using Post-Column Derivatization High-Performance Liquid Chromatography (HPLC)
2.7. β-glucan Analysis
2.8. ABTS Radical Cation Scavenging Activity
2.9. Statistics Analysis
3. Results
3.1. Method Validation of Biogenic Amine in H. erinaceus Extract
3.2. Biogenic Amines Content in H. erinaceus Extract with Different Pretreatment Processes
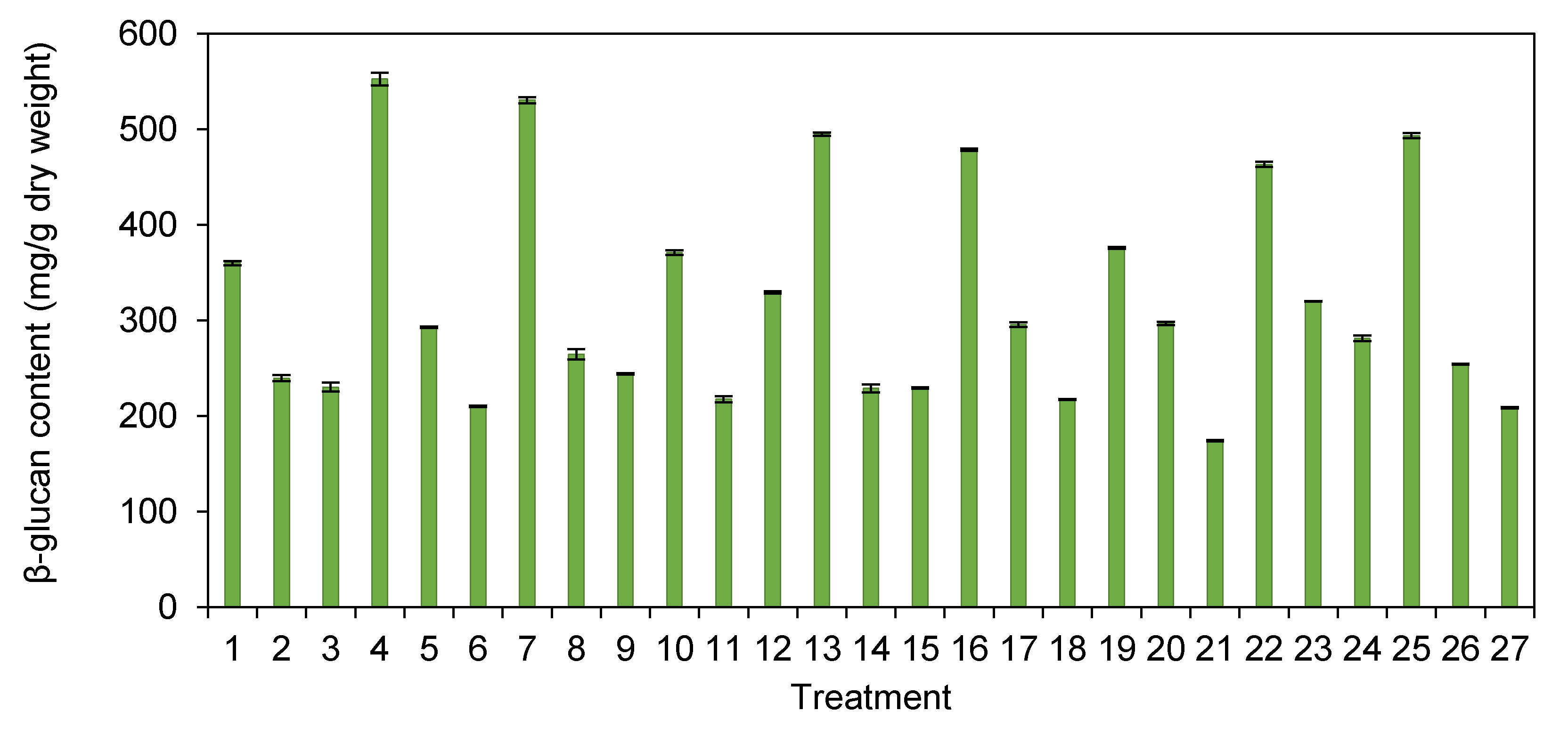
3.3. β-glucan Content in H. erinaceus Extract with Different Pretreatment Processes
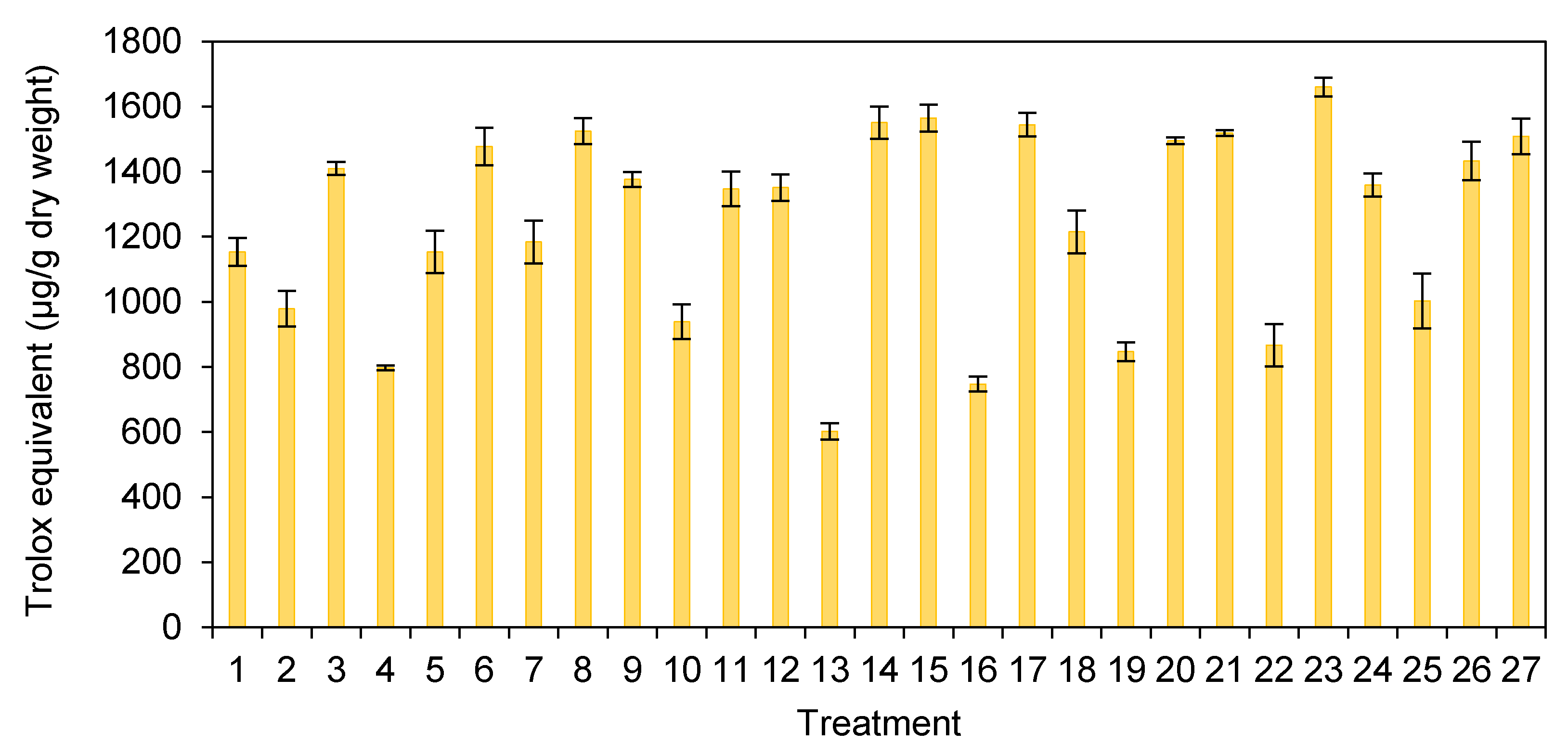
3.4. Antioxidant Activity in H. erinaceus Extract with Different Pretreatment Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, D.M.; Freitas, A.C.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J. Food Sci. Technol. 2015, 52, 6927–6939. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry nutrition and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Zhao, Z. Structures, biological activities and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complementary Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Sokol, S.; Golak-Siwulska, I.; Sobieralski, K.; Siwulski, M.; Górka, K. Biology cultivation and medicinal functions of the mushroom Hericium erinaceum. Acta Mycol. 2015, 50, 1–18. [Google Scholar]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Carballo, J.; Colmenero, F.J. Biogenic amines in pressurized vacuum-packaged cooked sliced ham under different chilled storage conditions. Meat Sci. 2007, 75, 397–405. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A survey on free biogenic amine content of fresh and preserved vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- Fish and Fishery Products Hazards and Controls Guidance. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Seafood/ucm2018426.htm (accessed on 17 March 2020).
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Visciano, P.; Schirone, M.; Paparella, A. An overview of histamine and other biogenic amines in fish and fish products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Lavizzari, T.; Veciana-Nogués, M.T.; Bover-Cid, S.; Mariné-Font, A.; Vidal-Carou, M.C. Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J. Chromatogr. A 2006, 1129, 67–72. [Google Scholar] [CrossRef]
- Mohedano, M.L.; Spano, G.; Spano, G.; López, P.; Russo, P. Controlling the formation of biogenic amines in fermented foods. In Advances in Fermented Foods and Beverages, 1st ed.; Holzapfel, W., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 273–310. [Google Scholar]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Content of biogenic amines and polyamines in some species of European wild-growing edible mushrooms. Eur. Food Res. Technol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic Amines in Plant-Origin Foods: Are they Frequently Underestimated in Low-Histamine Diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching–A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Paulsen, P.; Bauer, F. Spermine and spermidine concentrations in pork loin as affected by storage, curing and thermal processing. Eur. Food Res. Technol. 2007, 225, 921–924. [Google Scholar] [CrossRef]
- Hong, H.; Luo, Y.; Zhou, Z.; Bao, Y.; Lu, H.; Shen, H. Effects of different freezing treatments on the biogenic amine and quality changes of bighead carp (Aristichthys nobilis) heads during ice storage. Food Chem. 2013, 138, 1476–1482. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Effect of Processing on Antioxidants and Their Activity in Dietary Fiber Powder from Cabbage Outer Leaves. Dry. Technol. 2010, 28, 1063–1071. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Evolution of Anticarcinogenic Substance in Dietary Fibre Powder from Cabbage Outer Leaves During Drying. Food Chem. 2011, 127, 67–73. [Google Scholar] [CrossRef]
- International Council for Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology Q2(R1); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2005.
- Association of Official Analytical Chemists (AOAC). AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002; pp. 1–38. [Google Scholar]
- Pumtes, P.; Rojsuntornkitti, K.; Kongbangkerd, T.; Jittrepotch, N. Effects of different extracting conditions on antioxidant activities of Pleurotus flabellatus. Int. Food Res. J. 2016, 23, 173–179. [Google Scholar]
- Šimat, V.; Dalgaard, P. Use of small diameter column particles to enhance HPLC determination of histamine and other biogenic amines in seafood. Lwt-Food Sci. Technol. 2011, 44, 399–406. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Sławińska, A.; Stachniuk, A.; Stadnik, J. Determination of biogenic amines in processed and unprocessed mushrooms from the Polish market. J. Food Compos. Anal. 2020, 92, 103492. [Google Scholar] [CrossRef]
- Schindler, B.K.; Bruns, S.; Lach, G. Biogenic amines–A possible source for nicotine in mushrooms? A discussion of published literature data. Food Chem. 2015, 171, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Rejchrtová, E.; Sládková, P.; Zemánek, L.; Vymlátilová, L. Effect of some external factors on the content of biogenic amines and polyamines in a smear-ripened cheese. Dairy Sci. Technol. 2012, 92, 367–382. [Google Scholar] [CrossRef]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Vidal-Carou, M.C. Technological conditions influence aminogenesis during spontaneous sausage fermentation. Meat Sci. 2010, 85, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Schoppen, S.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Relationship between biogenic amine contents and the size of dry fermented sausages. Meat Sci. 1999, 51, 305–311. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Effect of controlled atmospheres enriched with O 2 in formation of biogenic amines in chilled hake (Merluccius merluccius L.). Eur. Food Res. Technol. 2001, 212, 546–550. [Google Scholar] [CrossRef]
- Preti, R.; Rapa, M.; Vinci, G. Effect of steaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaseolus vulgaris) varieties of different colours. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Scalzo, R.L.; Fibiani, M.; Francese, G.; D’Alessandro, A.; Rotino, G.L.; Conte, P.; Mennella, G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.). Food Chem. 2016, 194, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.D.; Noerrung, B.; Budka, H.; Andreoletti, O.; Buncic, S.; Griffin, J.; Hald, T.; Havelaar, A.; Hope, J.; Klein, G. Scientific opinion on risk based control of biogenic amine formation in fermented foods. Eur. Food Saf. Auth. J. 2011, 9, 2393. [Google Scholar]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Saldo, J.; Vidal-Carou, M.C. Effects of high hydrostatic pressure treatments on biogenic amine contents in goat cheeses during ripening. J. Agric. Food Chem. 2002, 50, 7288–7292. [Google Scholar] [CrossRef]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of drying technologies to ensure microbial safety of dried fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhang, M.; Adhikari, B. Drying of shiitake mushroom by combining freeze-drying and mid-infrared radiation. Food Bioprod. Process. 2015, 94, 507–517. [Google Scholar] [CrossRef]
- Pei, F.; Shi, Y.; Gao, X.; Wu, F.; Mariga, A.M.; Yang, W.; Zhao, L.; An, X.; Xin, Z.; Yang, F.; et al. Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem. 2014, 165, 547–554. [Google Scholar] [CrossRef]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef]
- Ren, F.; Perussello, C.A.; Zhang, Z.; Kerry, J.P.; Tiwari, B.K. Impact of ultrasound and blanching on functional properties of hot-air dried and freeze dried onions. Lwt Food Sci. Technol. 2018, 87, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Sheu, S.C.; Lyu, Y.; Lee, M.S.; Cheng, J.H. Immunomodulatory effects of polysaccharides isolated from Hericium erinaceus on dendritic cells. Process Biochem. 2013, 48, 1402–1408. [Google Scholar] [CrossRef]
- Gründemann, C.; Garcia-Käufer, M.; Sauer, B.; Scheer, R.; Merdivan, S.; Bettin, P.; Huber, R.; Lindequist, U. Comparative chemical and biological investigations of β-glucan-containing products from shiitake mushrooms. J. Funct. Foods 2015, 18, 692–702. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]


| Independent Variables | Code Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Sample size (X1) | whole | ripping | chopping |
| Heating process (X2) | non-heating | blanching | boiling |
| Drying method (X3) | nondrying | hot air drying | freeze drying |
| Operation | Time (Min) | Mobile Phase (%) | |
|---|---|---|---|
| A | B | ||
| elution | 0 | 70 | 30 |
| 10 | 70 | 30 | |
| 25 | 0 | 100 | |
| return | 28 | 70 | 30 |
| equilibration | 35 | 70 | 30 |
| Validation Parameters | PUT | SPD | |||
|---|---|---|---|---|---|
| Linearity | R2 | 0.9995 | 0.9981 | ||
| Accuracy | Recovery (%) | 80% | 105.94 | 101.62 | |
| 100% | 106.77 | 100.47 | |||
| 120% | 107.76 | 101.75 | |||
| Precision | RSD (%) | Intraday | 80% | 5.86 | 5.36 |
| 100% | 5.26 | 4.66 | |||
| 120% | 4.27 | 4.32 | |||
| Inter-day | 80% | 4.55 | 4.2 | ||
| 100% | 5.72 | 4 | |||
| 120% | 4.47 | 4.22 | |||
| LOD (mg/mL) | 0.01 | 0.55 | |||
| LOQ (mg/mL) | 0.04 | 1.67 | |||
| Treatment | Independent Variables | Biogenic Amines Content 1 | |||
|---|---|---|---|---|---|
| (µg/g Dry Weight) | |||||
| Sample Size | Heat Process | Drying Method | PUT | SPD | |
| 1 | −1 | −1 | −1 | 10.32 ± 0.56 | 102.52 ± 5.20 |
| 2 | −1 | −1 | 0 | 21.95 ± 1.69 | 31.10 ± 3.08 |
| 3 | −1 | −1 | 1 | 4.60 ± 0.18 * | 8.95 ± 0.71 * |
| 4 | −1 | 0 | −1 | 83.16 ± 7.82 | 137.97 ± 4.93 |
| 5 | −1 | 0 | 0 | 14.13 ± 1.22 | 6.73 ± 0.62 * |
| 6 | −1 | 0 | 1 | 2.84 ± 0.49 * | 13.23 ± 1.00 * |
| 7 | −1 | 1 | −1 | 70.43 ± 5.86 | 148.74 ± 5.06 |
| 8 | −1 | 1 | 0 | 7.96 ± 0.66 * | 9.37 ± 1.47 * |
| 9 | −1 | 1 | 1 | 2.52 ± 0.38 * | 10.87 ± 0.21 * |
| 10 | 0 | −1 | −1 | 11.24 ± 0.32 | 58.40 ± 5.20 |
| 11 | 0 | −1 | 0 | 9.00 ± 1.04 | 28.39 ± 2.14 |
| 12 | 0 | −1 | 1 | 3.44 ± 0.37 * | 5.29 ± 0.56 * |
| 13 | 0 | 0 | −1 | 45.22 ± 2.49 | 108.43 ± 10.05 |
| 14 | 0 | 0 | 0 | 2.36 ± 0.16 * | 15.48 ± 0.87 |
| 15 | 0 | 0 | 1 | 2.93 ± 0.28 * | 3.53 ± 0.35 * |
| 16 | 0 | 1 | −1 | 59.90 ± 5.19 | 91.90 ± 7.02 |
| 17 | 0 | 1 | 0 | 14.98 ± 2.00 | 4.90 ± 0.15 * |
| 18 | 0 | 1 | 1 | 5.12 ± 0.62 * | 8.92 ± 0.75 * |
| 19 | 1 | −1 | −1 | 7.27 ± 0.08 * | 39.89 ± 2.78 |
| 20 | 1 | −1 | 0 | 7.69 ± 0.44 * | 6.49 ± 0.18 * |
| 21 | 1 | −1 | 1 | 9.73 ± 0.23 | 8.56 ± 0.31 * |
| 22 | 1 | 0 | −1 | 11.02 ± 0.44 | 46.14 ± 4.63 |
| 23 | 1 | 0 | 0 | 0.81 ± 0.11 * | 12.95 ± 0.44 * |
| 24 | 1 | 0 | 1 | 1.04 ± 0.12 * | 9.45 ± 0.58 * |
| 25 | 1 | 1 | −1 | 28.45 ± 1.13 | 69.80 ± 1.30 |
| 26 | 1 | 1 | 0 | 2.92 ± 0.56 * | 5.34 ± 0.44 * |
| 27 | 1 | 1 | 1 | 2.41 ± 0.24 * | 7.14 ± 0.38 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhamrueang, N.; Sirilun, S.; Sirithunyalug, J.; Chaiyana, W.; Wangcharoen, W.; Peerajan, S.; Chaiyasut, C. Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium erinaceus Extract. Foods 2021, 10, 996. https://doi.org/10.3390/foods10050996
Makhamrueang N, Sirilun S, Sirithunyalug J, Chaiyana W, Wangcharoen W, Peerajan S, Chaiyasut C. Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium erinaceus Extract. Foods. 2021; 10(5):996. https://doi.org/10.3390/foods10050996
Chicago/Turabian StyleMakhamrueang, Netnapa, Sasithorn Sirilun, Jakkapan Sirithunyalug, Wantida Chaiyana, Wiwat Wangcharoen, Sartjin Peerajan, and Chaiyavat Chaiyasut. 2021. "Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium erinaceus Extract" Foods 10, no. 5: 996. https://doi.org/10.3390/foods10050996







