A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Honey Sample Preparation
2.3. Honey NMR Spectroscopy
2.4. Extraction of Propolis and Cerumen. Sample Preparation
2.5. GC-MS Analysis
2.6. Biological Tests
2.6.1. Microbial Strains
2.6.2. Determination of Minimal Inhibitory Concentration (MIC)
2.6.3. Effect of Extract on Bacterial Biofilm Formation
2.6.4. Quorum Sensing Inhibition (QSI) Activity on Chromobacterium violaceum CV026
2.6.5. Violacein Inhibition Assay on C. violaceum CV12472
2.6.6. Swarming and Swimming Motility Inhibition on P. aeruginosa PA01
3. Results
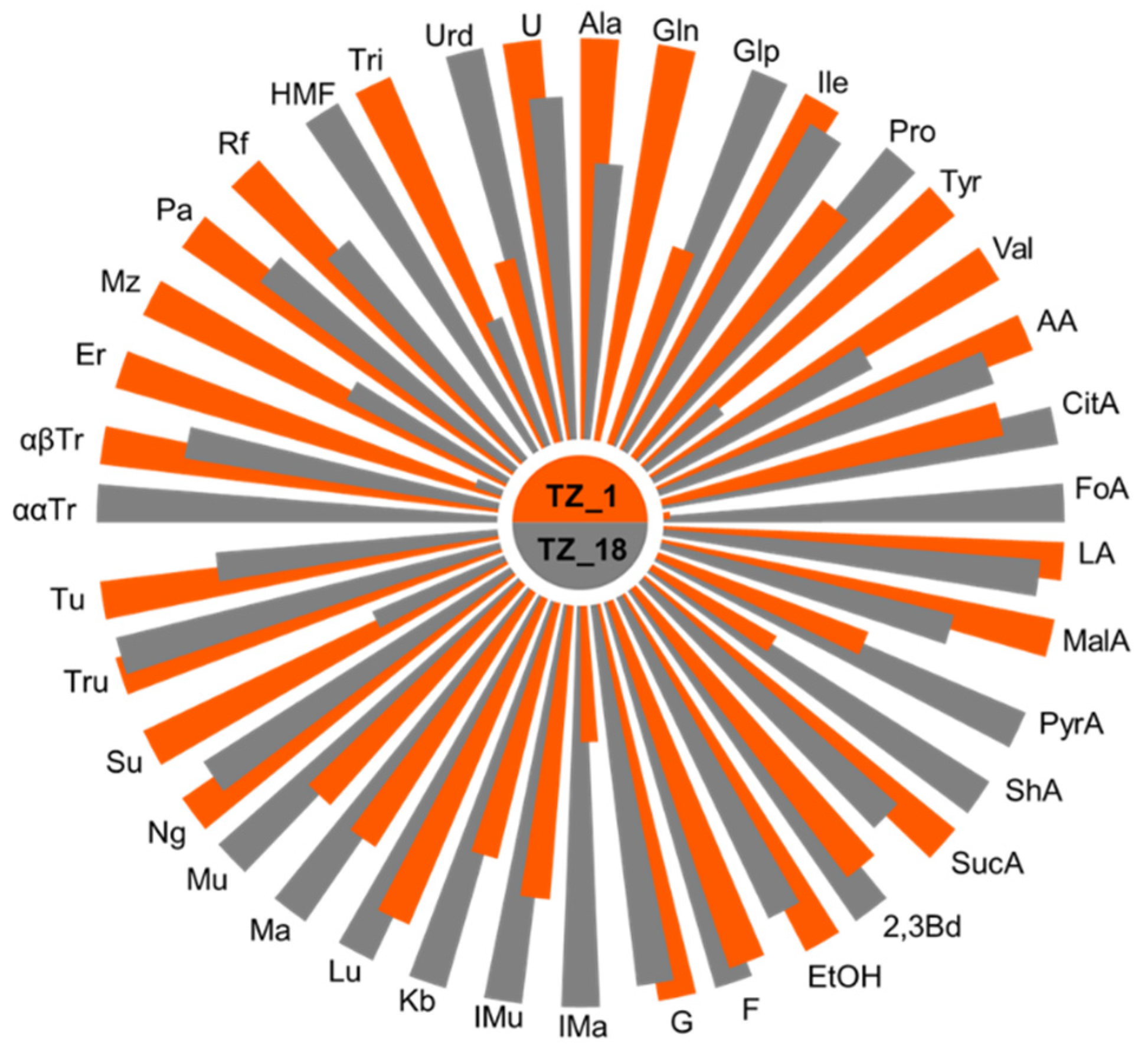
3.1. Honey Chemical Composition
3.2. Propolis/Cerumen Chemical Composition
3.3. Propolis/Cerumen Antimicrobial Activity
3.3.1. Determination of MIC
3.3.2. Inhibitory Potential against Violacein Synthesis
3.3.3. Antibiofilm Activity
3.3.4. Quorum Sensing Inhibition (QSI) Activity on C. Violaceum CV026
3.3.5. Inhibition Assay of Swarming and Swimming Motility
4. Discussion
4.1. Honey Chemical Profile
4.2. Propolis/Cerumen Chemical Profiles
4.3. Propolis/Cerumen Antimicrobial and Anti-Quorum Sensing Activity
4.3.1. Determination of MIC
4.3.2. Inhibitory Potential against Violacein Synthesis
4.3.3. Antibiofilm Activity
4.3.4. Quorum Sensing Inhibition (QSI) Activity on C. violaceum CV026
4.3.5. Inhibition Assay of Swarming and Swimming Motility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hrncir, M.; Jarau, S.; Barth, F.G.J. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef]
- Sanches, M.A.; Pereira, A.M.S.; Serrão, J.E. Pharmacological actions of extracts of propolis of stingless bees (Meliponini). J. Apicult. Res. 2017, 56, 50–57. [Google Scholar] [CrossRef]
- Chanchao, C. Antimicrobial activity by Trigona laeviceps (stingless bee) honey from Thailand. Pak. J. Med. Sci. 2009, 25, 364–369. [Google Scholar]
- Lavinas, F.C.; Macedo, E.H.B.; Sá, G.B.; Amaral, A.C.F.; Silva, J.R.; Azevedo, M.M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Hartfelder, K.; Imperatriz-Fonseca, V.L. Stingless bees: Biology and management. Apidologie 2006, 37, 121–123. [Google Scholar] [CrossRef]
- Leonhardt, S.D. Chemical ecology of stingless bees. J. Chem. Ecol. 2017, 43, 385–402. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Roubik, D.W. Stingless bee nesting biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Kajobe, R. Nesting biology of equatorial afrotropical stingless bees (Apidae; Meliponini) in Bwindi Impenetrable National Park, Uganda. J. Apicult. Res. 2007, 46, 245–255. [Google Scholar] [CrossRef]
- Cockburn, C.L.; Kwapong, P.K.; Wubah, D.A.; Wubah, J.A. Shelf-life and variances in antimicrobial properties of honey from Meliponula bocandei and Meliponula ferruginea in central Ghana. J. Young Investig. 2013, 25, 10–14. [Google Scholar]
- Zuccato, V.; Finotello, C.; Menegazzo, I.; Peccolo, G.; Schievano, E. Entomological authentication of stingless bee honey by 1H NMR-based metabolomics approach. Food Control 2017, 82, 145–153. [Google Scholar] [CrossRef]
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR profiling of North Macedonian and Bulgarian honeys for detection of botanical and geographical origin. Molecules 2020, 25, 4687. [Google Scholar] [CrossRef]
- Excel How to. Available online: https://www.excelhowto.com/nightingale-rose-diagram-excel-template/ (accessed on 27 April 2021).
- Sartorius. Available online: https://umetrics.com/kb/simca-15 (accessed on 27 April 2020).
- Clinical Laboratory Standards Institute. Quality Control Minimal Inhibitory Concentration (MIC) Limits for Broth Dilution and MIC Interpretative Breakpoints (Supplement M27-s2); Clinical Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Current Protoc. Microbiol. 2005. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Duru, M.E. HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O Kuntze and Curcuma longa L. LWT 2020, 133, 110150. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Intern. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Fallico, B.; Arena, E.; Zappala, M. Prediction of honey shelf life. J. Food. Qual. 2009, 32, 352–368. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B. Standard methods for Apis mellifera propolis research. J. Apicult. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef]
- Rosli, F.N.; Hazemi, M.H.F.; Akbar, M.A.; Basir, S.; Kassim, H.; Bunawan, H. Stingless bee honey: Evaluating its antibacterial activity and bacterial diversity. Insects 2020, 11, 500. [Google Scholar] [CrossRef]
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Compost. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Vit, P.; Bogdanov, S.; Kilchenmann, V. Composition of Venezuelan honeys from stingless bees (Apidae: Meliponinae) and Apis mellifera L. Apidologie 1994, 25, 278–288. [Google Scholar] [CrossRef][Green Version]
- Ngalimat, M.S.; Raja Abd Rahman, R.N.Z.; Yusof, M.T.; Syahir, A.; Sabri, S. Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis. PeerJ 2019, 7, e7478. [Google Scholar] [CrossRef]
- Souza, E.C.A.; Menezes, C.; Flach, A. Stingless bee honey (Hymenoptera, Apidae, Meliponini): A review of quality control, chemical profile, and biological potential. Apidologie 2021, 52, 113–132. [Google Scholar] [CrossRef]
- Razali, M.T.A.; Zainal, Z.A.; Maulidiani, M.; Shaari, K.; Zamri, Z.; Mohd Idrus, M.Z.; Ismail, I.S. Classification of raw stingless bee honeys by bee species origins using the NMR-and LC-MS-based metabolomics approach. Molecules 2018, 23, 2160. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Effects of long-term storage on stingless bee (Hymenoptera: Apidae: Meliponini) honey. J. Apicult. Res. 2015, 54, 441–451. [Google Scholar] [CrossRef]
- Hamada, S. Role of sweeteners in the etiology and prevention of dental caries. Pure Appl. Chem. 2002, 74, 1293–1300. [Google Scholar] [CrossRef]
- Wach, W.; Rose, T.; Klingeberg, M.; Peters, S.; Dörr, T.; Theis, S.; Kowalczyk, J.; Hausmanns, S. Trehalulose-Containing Composition, Its Preparation and Use. International Patent Number WO/2010/118866, 21 October 2010. [Google Scholar]
- Fletcher, M.; Hungerford, N.L.; Webber, D.; de Jesus, M.C.; Zhang, J.; Stone, I.S.; Zawawi, N. Stingless bee honey, a novel source of trehalulose: A biologically active disaccharide with health benefits. Sci. Rep. 2020, 10, 12128. [Google Scholar] [CrossRef]
- Byrne, D.N.; Hendrix, D.L.; Williams III, L.H. Presence of trehalulose and other oligosaccharides in hemipteran honeydew, particularly Aleyrodidae. Physiol. Entomol. 2003, 28, 144–149. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Riethof, M.L.; Kushnir, I. Composition of honey VI. The effect of storage on carbohydrates, acidity and diastase content. J. Food Sci. 1961, 26, 63–71. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef]
- Muli, E.; Maingi, J.M.; Macharia, J. Antimicrobial properties of propolis and honey from the Kenyan stingless bee, Dactylurina schimidti. Apiacta 2008, 43, 49–61. [Google Scholar]
- Anibijuwon, I.; Gbala, I.; Adeyemi, J.; Abioye, J. Antibacterial activity of stingless bee (Dactylurina studingeri) propolis on bacteria isolated from wound. SMU Med. J. 2017, 4, 43–50. [Google Scholar]
- Tanzania Forest Service Agency. Forests, West Kilimanjaro. Available online: https://www.tfs.go.tz/index.php/en/forests/west-kilimanjaro (accessed on 23 April 2021).
- Otto, A.; Wilde, V. Sesqui-, di-, and triterpenoids as chemosystematic markers in extant conifers—A review. Bot. Rev. 2001, 67, 141–238. [Google Scholar] [CrossRef]
- Peter, C.M.; Waller, S.B.; Picoli, T.; Osório, L.G.; Zani, J.L.; Meireles, M.C.A.; Fischer, G. Chemical and cytotoxic analyses of three varieties of Brazilian propolis (green propolis, jataí propolis and brown propolis) and its anti-Sporothrix brasiliensis in vitro activity. Arq. Bras. Med. Vet. Zootec 2019, 71, 819–827. [Google Scholar] [CrossRef]
- Hroboňová, K.; Lehotay, J.; Čižmárik, J. Determination of organic acids in propolis by HPLC using two columns with an on-line SPE system. J. Liq. Chromatogr. Relat. Technol. 2008, 32, 125–135. [Google Scholar] [CrossRef]
- Dutra, R.P.; Bezerra, J.L.; da Silva, M.C.P.; Batista, M.C.A.; Patrício, F.J.B. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farmacogn. 2019, 29, 287–293. [Google Scholar] [CrossRef]
- Bhatia, A.; Bharti, S.K.; Tripathi, T.; Mishra, A.; Sidhu, O.P.; Roy, R.; Nautiyal, C.S. Metabolic profiling of Commiphora wightii (guggul) reveals a potential source for pharmaceuticals and nutraceuticals. Phytochemistry 2015, 110, 29–36. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, L.R.; Ferreres, F.; Guedes de Pinho, P.; Valentao, P.; Silva, B.M.; Andrade, P.B. Chemical assessment and in vitro antioxidant capacity of Ficus carica latex. J. Agric. Food Chem. 2010, 58, 3393–3398. [Google Scholar] [CrossRef]
- Gallardo, D.G.; Fajardo, M.; Alonso, Y.N.; Echemendía, L.S.M.; Copello, J.M. Physical-chemical characterization and thin layer chromatographic comparison of a local propolis with the Mangifera indica resin. Pharm. Biosci. J. 2020, 8, 15–21. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2019, 153098. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Koendhori, E.B.; Tsvetkova, I.; Naydenski, C.; Bankova, V. Indonesian propolis: Chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2011, 25, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Dimitrova, R.; Al-Lawati, H.T.; Tsvetkova, I.; Najdenski, H.; Bankova, V. Omani propolis: Chemical profiling, antibacterial activity and new propolis plant sources. Chem. Cent. J. 2013, 7, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrera-López, M.G.; Rubio-Hernández, E.I.; Richomme, P.; Schinkovitz, A.; Calvo-Irabién, L.M.; Rodríguez, L.M.P. Resorcinolic lipids from Yucatecan propolis. J. Braz. Chem. Soc. 2020, 31, 186–192. [Google Scholar] [CrossRef]
- Bauer, H.; Claeys, M.; Vermeylen, R.; Schueller, E.; Weinke, G.; Berger, A.; Puxbaum, H. Arabitol and mannitol as tracers for the quantification of airborne fungal spores. Atmos. Environ. 2008, 42, 588–593. [Google Scholar] [CrossRef]
- Morais, P.B.; Calaça, P.S.S.T.; Rosa, C.A. Microorganisms associated with stingless bees. In Pot-Honey; Vit, P., Pedro, S.R.M., Roubik, D.W., Eds.; Springer: New York, NY, USA, 2013; pp. 173–186. [Google Scholar]
- Tomadoni, B.; Moreira, M.R.; Ponce, A. Anti-quorum sensing activity of natural compounds against Chromobacterium violaceum. Ann. Food. Sci. Nut. 2016, 1, 43–48. [Google Scholar]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef]
- Ceylan, O.; Tamfu, A.N.; Doğaç, Y.I.; Teke, M. Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech. 2020, 10, 315. [Google Scholar] [CrossRef]
- Hentzer, M.; Eberl, L.; Nielsen, J.; Givskov, M. Quorum sensing. BioDrugs 2003, 17, 241–250. [Google Scholar] [CrossRef]
- Silva, A.C.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.; Pisciottano, M.N.; Amorim, E.L.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complement. Alternat. Med. 2013, 308980. [Google Scholar] [CrossRef]
- Bankova, V.; Marcucci, M.C.; Simova, S.; Nikolova, N.; Kujumgiev, A.; Popov, S. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. C J. Biosci. 1996, 51, 277–280. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Marcucci, M.C.; Tsvetkova, I.; Kujumgiev, A. Chemical composition and biological activity of propolis from Brazilian meliponinae. Z. Naturforsch. C J. Biosci. 2000, 55, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Singh, P.; Chenia, H.Y. Quorum sensing inhibitory potential and molecular docking studies of sesquiterpene lactones from Vernonia blumeoides. Phytochemistry 2016, 126, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bakkiyaraj, D.; Pandian, S.T.K. In vitro and in vivo antibiofilm activity of a coral associated actinomycete against drug resistant Staphylococcus aureus biofilms. Biofouling 2010, 26, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Guerrini, A.; Marino, A.; Tacchini, M.; Di Giulio, M.; Grandini, A.; Akin, M.; Cellini, L.; Bisignano, G.; Saraçoğlu, H.T. In vitro activity of plant extracts against biofilm-producing food-related bacteria. Int. J. Food Microbiol. 2016, 238, 33–39. [Google Scholar] [CrossRef]
- Savka, M.A.; Dailey, L.; Popova, M.; Mihaylova, R.; Merritt, B.; Masek, M.; Le, P.; Nor, S.R.M.; Ahmad, M.; Hudson, A.O.; et al. Chemical composition and disruption of quorum sensing signaling in geographically diverse United States propolis. Evid. Based Complementary Altern. Med. 2015, 472593. [Google Scholar] [CrossRef]
- Jose, R.; Singh, V. Swarming in Bacteria: A tale of plasticity in motility behavior. J. Indian I. Sci. 2020, 100, 515–524. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Attenuation of quorum sensing controlled virulence factors and biofilm formation in Pseudomonas aeruginosa by pentacyclic triterpenes, betulin and betulinic acid. Microb. Pathog. 2018, 118, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Godelmann, R.; Kost, C.; Patz, C.D.; Ristow, R.; Wachter, H. Quantitation of compounds in wine using 1H NMR spectroscopy: Description of the method and collaborative study. J. AOAC Int. 2016, 99, 1295–1304. [Google Scholar] [CrossRef]






| Compound Class | KC1-1 | KC1-2 | KC2-1 | KC2-2 |
|---|---|---|---|---|
| Sugars and sugar derivatives | 15.6 | 9.1 | 35.1 | 54.1 |
| Aromatic acids | - | 1.8 | 0.2 | - |
| Fatty acids | 2.0 | 4.6 | 3.6 | 0.9 |
| Diterpenes (acids) | 60.6 | - | - | 11.9 |
| Cardanol C17:1 | - | 0.1 | - | - |
| Resorcinols | - | 4.1 | - | 0.2 |
| Anacardic acids | - | 2.8 | - | 0.3 |
| Quinic acid | 0.4 | 15.5 | 1.2 | 2.1 |
| Caffeoylquinic acids | - | 7.6 | - | - |
| Triterpenes | 0.7 | 37.3 | 24.4 | 26.7 |
| Unknown M+ = 570 | 3.9 | - | - | - |
| Unknown M+ = 568 | 8.0 | - | - | - |
| Micro-Organism | KC 1-1 | KC 2-1 |
|---|---|---|
| S. aureus ATCC 25923 | 0.625 | 1.25 |
| E. faecalis ATCC 29212 | 0.3125 | 0.625 |
| L. monocytogenes ATCC 7644 | 0.1563 | 0.625 |
| C. albicans ATCC 10239 | 0.625 | 1.25 |
| E. coli ATCC 25922 | 0.625 | 1.25 |
| P. aeruginosa ATCC 27853 | 0.3125 | 0.1563 |
| S. typhi ATCC 14028 | 2.5 | 1.25 |
| Concentration | KC1-1 | KC2-1 | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| 0.625 | 2.5 | 2.5 | >5 | |
| Violacein Inhibition (%) | ||||
| MIC | 100 ± 0.0 | 100 ± 0.0 | ||
| MIC/2 | 100 ± 0.0 | 100 ± 0.0 | ||
| MIC/4 | 100 ± 0.0 | 100 ± 0.0 | ||
| MIC/8 | 100 ± 0.0 | 89.6 ± 0.3 | ||
| MIC/16 | 61.4 ± 5.2 | 50.1 ± 2.0 | ||
| MIC/32 | 44.2 ± 1.5 | 28.9 ± 0.4 | ||
| MIC/64 | 21.6 ± 1.1 | 13.0 ± 0.5 | ||
| MIC/128 | 11.8 ± 2.4 | - | ||
| MIC/256 | - | - | ||
| Concentration | KC1-1 (MIC = 2.5 mg/mL) | KC2-1 (MIC = 5 mg/mL) |
|---|---|---|
| MIC | 16.5 ± 2.3 | 10.5 ± 0.5 |
| MIC/2 | 13.8 ± 1.5 | - |
| MIC/4 | 10.0 ± 4.5 | - |
| MIC/8 | - | - |
| Concentration (µg/mL) | KC1-1 | KC2-1 | ||
|---|---|---|---|---|
| Swarming | Swimming | Swarming | Swimming | |
| 100 | 42.13 ± 1.75 | 27.32 ± 2.11 | 57.14 ± 0.50 | 29.28 ± 4.18 |
| 75 | 20.70 ± 4.21 | 6.77 ± 1.00 | 42.86 ± 5.10 | 14.27 ± 2.50 |
| 50 | 6.43 ± 0.50 | - | 20.70 ± 1.00 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea. Foods 2021, 10, 997. https://doi.org/10.3390/foods10050997
Popova M, Gerginova D, Trusheva B, Simova S, Tamfu AN, Ceylan O, Clark K, Bankova V. A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea. Foods. 2021; 10(5):997. https://doi.org/10.3390/foods10050997
Chicago/Turabian StylePopova, Milena, Dessislava Gerginova, Boryana Trusheva, Svetlana Simova, Alfred Ngenge Tamfu, Ozgur Ceylan, Kerry Clark, and Vassya Bankova. 2021. "A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea" Foods 10, no. 5: 997. https://doi.org/10.3390/foods10050997
APA StylePopova, M., Gerginova, D., Trusheva, B., Simova, S., Tamfu, A. N., Ceylan, O., Clark, K., & Bankova, V. (2021). A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula ferruginea. Foods, 10(5), 997. https://doi.org/10.3390/foods10050997