Extrusion Processing of Rapeseed Press Cake-Starch Blends: Effect of Starch Type and Treatment Temperature on Protein, Fiber and Starch Solubility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Preparation of Blends
2.2. Extrusion Processing
2.3. Thermomechanical Treatment at Defined Temperatures and Shear Rates
2.4. Physicochemical Properties
2.4.1. Extractable Protein Content and Protein Solubility
2.4.2. Starch Gelatinization
2.4.3. Starch Hydrolysis
2.4.4. Soluble and Insoluble Dietary Fiber Analysis
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis
3.2. Extruder Response
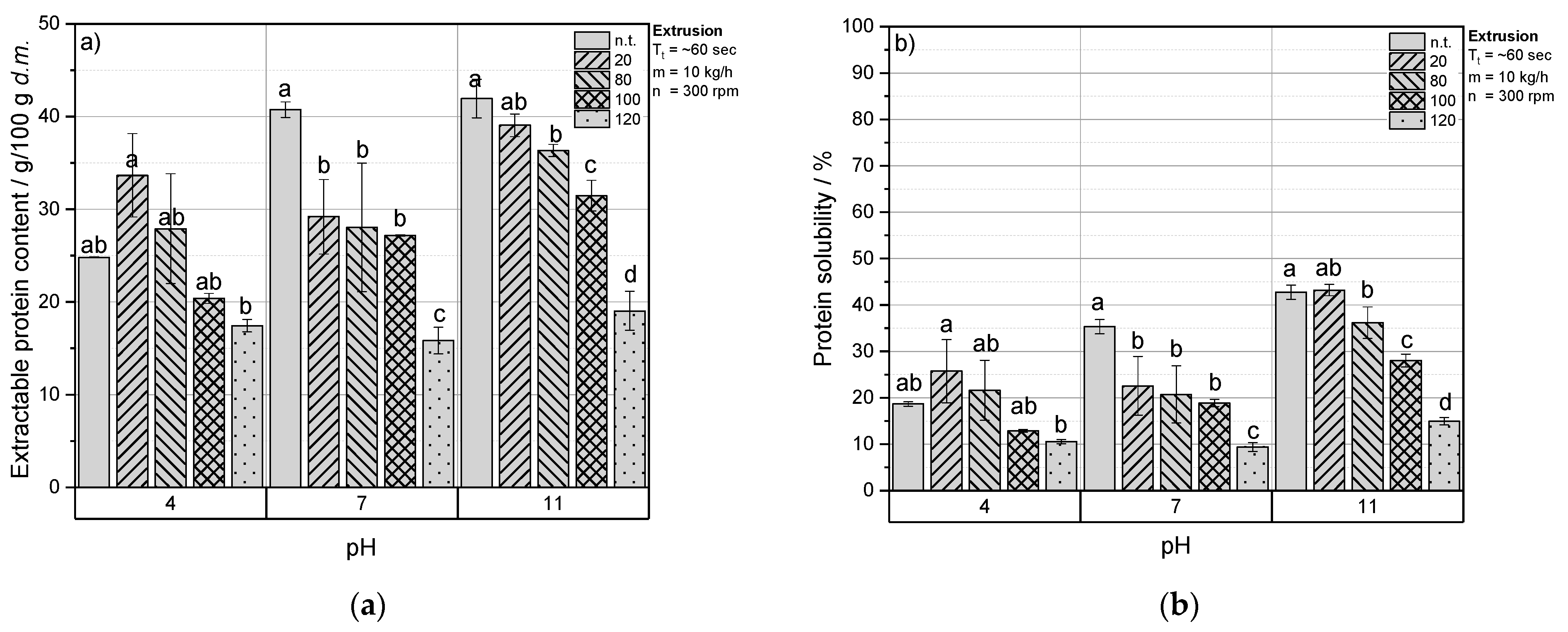
3.3. Impact of Thermomechanical Treatment on Protein Solubility of RPC
3.3.1. Extruded RPC
3.3.2. Thermomechanical Treatment of Rpc under Defined Conditions
3.3.3. Comparison of Extrusion-Processing and Defined Thermomechanical Conditions
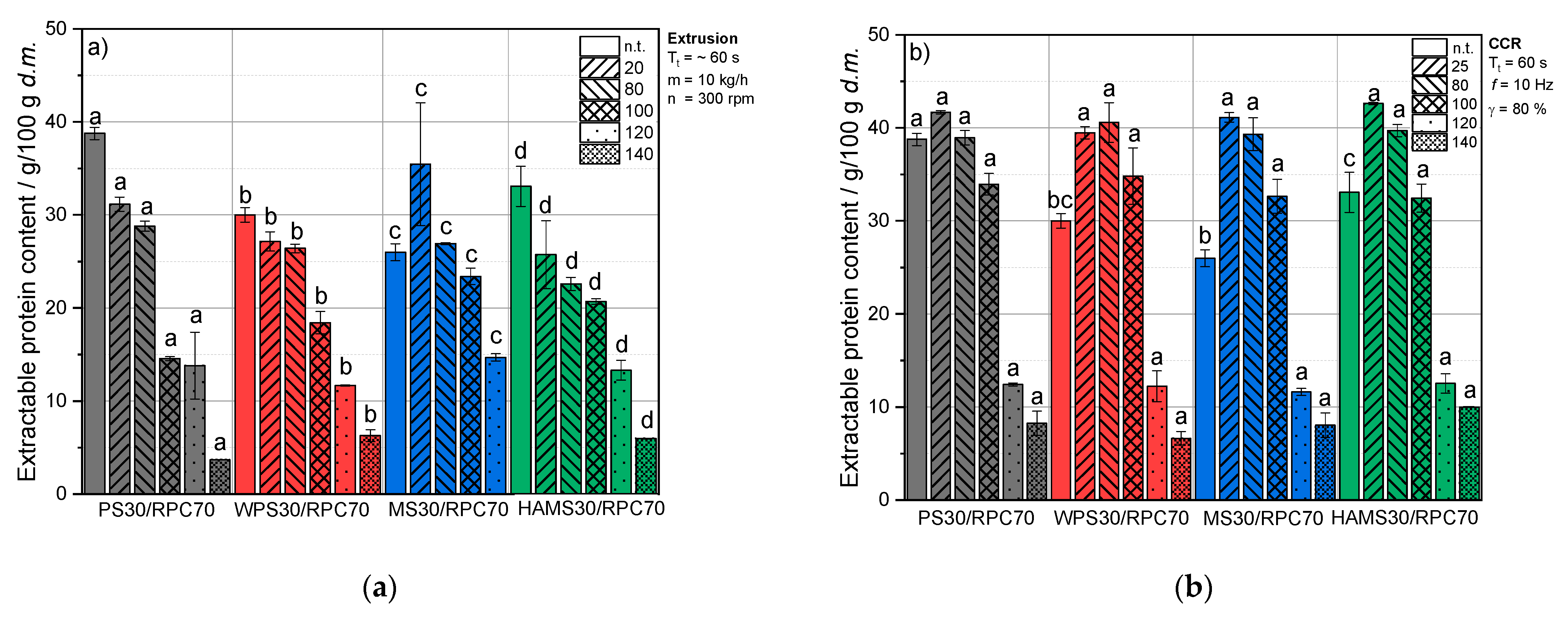
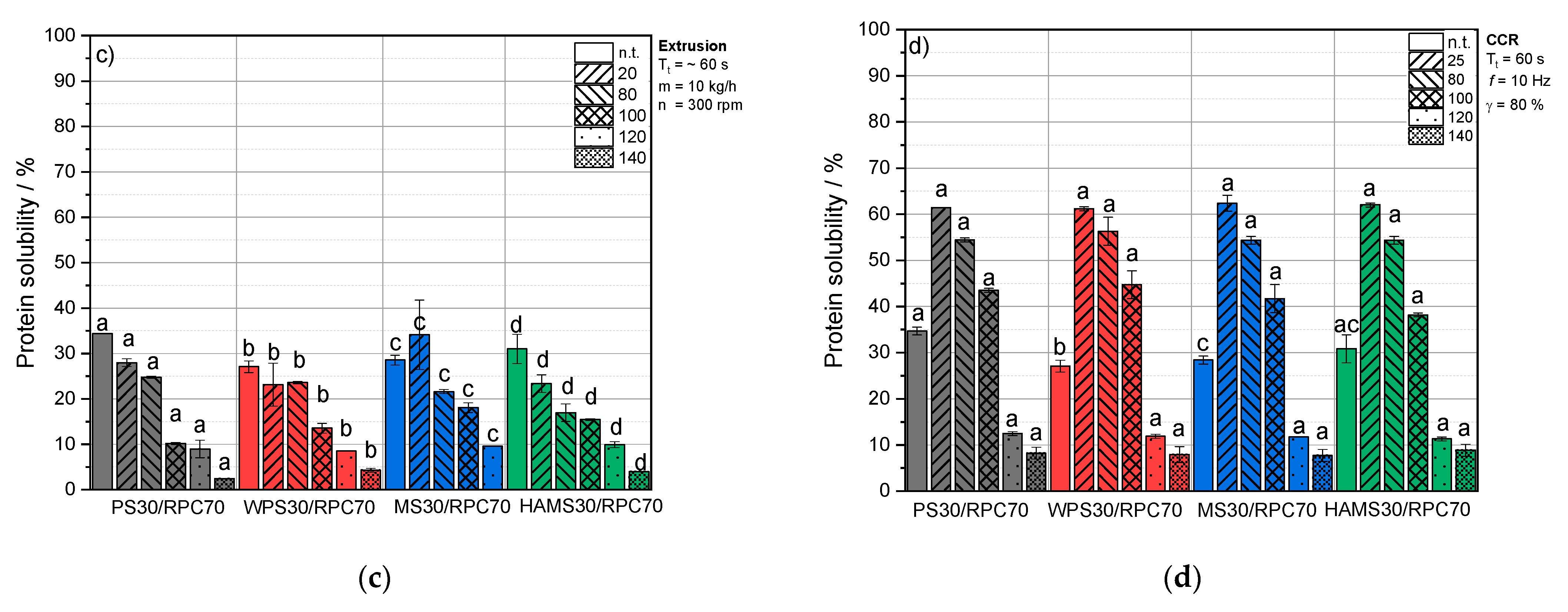
3.4. Impact of Thermomechanical Treatment and Starch Addition on Protein Solubility of RPC
3.4.1. Extractable Protein Content and Solubility of RPC/Starch Blends at Neutral pH
Untreated Blends
Extruded Blends
Thermomechanical Treatment under Defined Conditions
Comparison of Extrusion-Processing and Defined Thermomechanical Conditions
3.4.2. Extractable Protein Content of RPC/Starch Blends at Acidic and Alkaline pH
3.5. Impact of Extrusion on Starch Gelatinization and Hydrolysis
3.6. Impact of Extrusion on Total, Soluble, and Insoluble Dietary Fiber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Dgel | Degree of gelatinization (%) |
| d.m. | Dry matter (g/100g) |
| HAMS | High-amylose maize starch |
| ∆ Hgel | Gelatinization enthalpy (J/g) |
| IDF | Insoluble dietary fiber (g/100 g) |
| m | Mass flow rate (kg/h) |
| MS | Maize starch |
| n | Screw speed (rpm) |
| n.t. | Not treated |
| PS | Potato starch |
| RPC | Rapeseed press cake |
| SDF | Soluble dietary fiber (g/100 g) |
| SH | Total hydrolyzed starch (g/100 g) |
| SME | Specific mechanical energy (Wh/kg) |
| T | Temperature (°C) |
| TB | Barrel temperature (°C) |
| TDF | Total soluble dietary fiber (g/100 g) |
| Tgel | Gelatinization temperature (°C) |
| TT | Treatment temperature (°C) |
| Tt | Treatment time (s) |
| w.b. | Wet basis (g/100 g) |
| WPS | Waxy potato starch |
References
- Hoglund, E.; Eliasson, L.; Oliveira, G.; Almli, V.L.; Sozer, N.; Alminger, M. Effect of drying and extrusion processing on physical and nutritional characteristics of bilberry press cake extrudates. LWT Food Sci. Technol. 2018, 92, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Yagci, S.; Gogus, F. Response surface methodology for evaluation of physical and functional properties of extruded snack foods developed from food-by-products. J. Food Eng. 2008, 86, 122–132. [Google Scholar] [CrossRef]
- Da Silva, A.P.L.; Berrios, J.D.J.; Pan, J.; Ascheri, J.L.R. Passion fruit shell flour and rice blends processed into fiber-rich expanded extrudates. CyTA J. Food 2018, 16, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Karkle, E.L.; Alavi, S.; Dogan, H. Cellular architecture and its relationship with mechanical properties in expanded extrudates containing apple pomace. Food Res. Int. 2012, 46, 10–21. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kowalski, R.J.; Kang, Y.F.; Kiszonas, A.M.; Zhu, M.J.; Ganjyal, G.M. Impacts of the Particle Sizes and Levels of Inclusions of Cherry Pomace on the Physical and Structural Properties of Direct Expanded Corn Starch. Food Bioprocess. Tech. 2017, 10, 394–406. [Google Scholar] [CrossRef]
- Makila, L.; Laaksonen, O.; Diaz, J.M.R.; Vahvaselka, M.; Myllymaki, O.; Lehtomaki, I.; Laakso, S.; Jahreis, G.; Jouppila, K.; Larmo, P.; et al. Exploiting blackcurrant juice press residue in extruded snacks. LWT Food Sci. Technol. 2014, 57, 618–627. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Konstance, R.P. Extruded corn meal and whey protein concentrate: Effect of particle size. J. Food Process. Pres. 2006, 30, 475–487. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Smith, P.W.; Konstance, R.P.; Holsinger, V.H. Incorporation of whey products in extruded corn, potato or rice snacks. Food Res. Int. 2001, 34, 679–687. [Google Scholar] [CrossRef]
- Robin, F.; Dubois, C.; Pineau, N.; Schuchmann, H.P.; Palzer, S. Expansion mechanism of extruded foams supplemented with wheat bran. J. Food Eng. 2011, 107, 80–89. [Google Scholar] [CrossRef]
- Nikinmaa, M.; Nordlund, E.; Poutanen, K.; Sozer, N. From Underutilized Side-Streams to Hybrid Food Ingredients for Health. Cereal Food World 2018, 63, 137–142. [Google Scholar] [CrossRef]
- Martin, A.; Osen, R.; Greiling, A.; Karbstein, H.P.; Emin, A. Effect of of rapeseed press cake and peel on the extruder response and physical pellet quality in extruded fish feed. Aquaculture 2019, 512. [Google Scholar] [CrossRef]
- Tyapkova, O.; Osen, R.; Wagenstaller, M.; Baier, B.; Specht, F.; Zacherl, C. Replacing fishmeal with oilseed cakes in fish feed—A study on the influence of processing parameters on the extrusion behavior and quality properties of the feed pellets. J. Food Eng. 2016, 191, 28–36. [Google Scholar] [CrossRef]
- Smulikowska, S.; Czerwiński, J.; Mieczkowska, A. Nutritional value of rapeseed expeller cake for broilers: Effect of dry extrusion. J. Anim. Feed Sci. 2006, 15, 445–453. [Google Scholar] [CrossRef]
- Martin, A.; Osen, R.; Karbstein, H.P.; Emin, M.A. Impact of Rapeseed Press Cake on the Rheological Properties and Expansion Dynamics of Extruded Maize Starch. Foods 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Osen, R.; Karbstein, H.P.; Emin, M.A. Linking Expansion Behaviour of Extruded Potato Starch/Rapeseed Press Cake Blends to Rheological and Technofunctional Properties. Polymers 2021, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.Y.; Arntfield, S.D. Functional properties of raw and processed canola meal. LWT Food Sci. Technol. 2009, 42, 1119–1124. [Google Scholar] [CrossRef]
- Mahajan, A.; Dua, S. Nonchemical approach for reducing antinutritional factors in rapeseed (Brassica campestris var. Toria) and characterization of enzyme phytase. J. Agric. Food Chem. 1997, 45, 2504–2508. [Google Scholar] [CrossRef]
- Mahajan, A.; Dua, S.; Bhardwaj, S. Imbibition induced changes in antinutritional constituents and functional properties of rapeseed (Brassica campestris var. toria) meal. FASEB J. 1997, 11, A1105-A1105. [Google Scholar]
- Mawson, R.; Heaney, R.K.; Zdunczyk, Z.; Kozlowska, H. Rapeseed Meal-Glucosinolates and Their Antinutritional Effects.6. Taint in End-Products. Nahrung 1995, 39, 21–31. [Google Scholar] [CrossRef]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenges. Appl. Microbiol. Biot. 2012, 95, 1105–1114. [Google Scholar] [CrossRef]
- DellaValle, G.; Colonna, P.; Patria, A.; Vergnes, B. Influence of amylose content on the viscous behavior of low hydrated molten starches. J. Rheol. 1996, 40, 347–362. [Google Scholar] [CrossRef]
- Fetzer, A.; Herfellner, T.; Stabler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crop. Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Leming, R.; Lember, A. Chemical composition of expeller-extracted and cold-pressed rapeseed cake. Agraarteadus 2005, 16, 103–109. [Google Scholar]
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Drago, S.R.; González, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Effects of the addition of wild legumes (Lathyrus annuus and Lathyrus clymenum) on the physical and nutritional properties of extruded products based on whole corn and brown rice. Food Chem. 2011, 128, 961–967. [Google Scholar] [CrossRef]
- Day, L.; Swanson, B.G. Functionality of Protein-Fortified Extrudates. Compr. Rev. Food Sci. Food Saf. 2013, 12, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.; Jandt, U.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Investigation on the influence of high protein concentrations on the thermal reaction behaviour of beta-lactoglobulin by experimental and numerical analyses. Int. Dairy J. 2019, 97, 99–110. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Ying, D.; Sanguansri, L.; Augustin, M.A. Effect of extrusion conditions on the physico-chemical properties and in vitro protein digestibility of canola meal. Food Res. Int. 2017, 100, 658–664. [Google Scholar] [CrossRef]
- Matthey, F.P.; Hanna, M.A. Physical and Functional Properties of Twin-screw Extruded Whey Protein Concentrate–Corn Starch Blends. Lebensm Wiss Technol. 1997, 30, 359–366. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Zhang, B.; Drago, S.R.; Zhang, J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- Allen, K.E.; Carpenter, C.E.; Walsh, M.K. Influence of protein level and starch type on an extrusion-expanded whey product. Int. J. Food Sci. Technol. 2007, 42, 953–960. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Andersson, R.; Jonsäll, A.; Andersson, J.; Fredriksson, H. Effect of Different Extrusion Parameters on Dietary Fiber in Wheat Bran and Rye Bran. J. Food Sci. J. Food Sci. 2017, 82, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Naumann, S.; Schweiggert-Weisz, U.; Martin, A.; Schuster, M.; Eisner, P. Effects of extrusion processing on the physiochemical and functional properties of lupin kernel fibre. Food Hydrocoll. 2021, 111, 106222. [Google Scholar] [CrossRef]
- Vasanthan, T.; Gaosong, J.; Yeung, J.; Li, J. Dietary fiber profile of barley flour as affected by extrusion cooking. Food Chem. 2002, 77, 35–40. [Google Scholar] [CrossRef]
- German Food Act. Methods L. 16.01-2, L. 17.00-1, L. 17.00-3. In BVL Bundesamt für Verbraucherschutz und Lebensmittelsicherheit; Beuth Verlag GmbH: Berlin, Germany, 2005. [Google Scholar]
- AOAC International. Official Method 945.46—Ash Determination; The Scientific Association Dedicated to Analytical Excellence: Washington, DC, USA, 2016. [Google Scholar]
- AOAC International. Official Method 962.09—Fiber (Crude) in Animal Feed and Pet. Food; The Scientific Association Dedicated to Analytical Excellence: Washington, DC, USA, 1982. [Google Scholar]
- Beutler, H.O. Enzymatic Determination of Starch in Foods by Hexokinase Method. Starch Stärke 1978, 30, 309–312. [Google Scholar] [CrossRef]
- AACC. Hydration Capacity of Pregelatinized Cereal Products. Approved Methods of Analysis, 11th ed.; AACCI Method 56-20.01; American Association of Cereal Chemists International: St. Paul, MN, USA, 1999. [Google Scholar]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Koch, L.; Emin, M.A.; Schuchmann, H.P. Reaction behaviour of highly concentrated whey protein isolate under defined heat treatments. Int. Dairy J. 2017, 71, 114–121. [Google Scholar] [CrossRef]
- Emin, M.A.; Schuchmann, H.P. Analysis of the dispersive mixing efficiency in a twin-screw extrusion processing of starch based matrix. J. Food Eng. 2013, 115, 132–143. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein-protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Ebeling, M.E. The Dumas Method for Nitrogen in Feeds. J. AOAC Int. 1968, 51, 766–770. [Google Scholar] [CrossRef]
- Maki, K.C.; Pelkman, C.L.; Finocchiaro, E.T.; Kelley, K.M.; Lawless, A.L.; Schild, A.L.; Rains, T.M. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J. Nutr. 2012, 142, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hasjim, J.; Xie, F.; Halley, P.J.; Gilbert, R.G. Shear degradation of molecular, crystalline, and granular structures of starch during extrusion. Starch Stärke 2014, 66, 595–605. [Google Scholar] [CrossRef]
- Tzeng, Y.-M.; Diosady, L.L.; Rubin, L.J. Preparation of Rapeseed Protein Isolates Using Ultrafiltration, Precipitation and Diafiltration. Can. Inst. Food Sci. Technol. J. 1988, 21, 419–424. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Murray, E.D. The Influence of Processing Parameters on Food Protein Functionality I. Differential Scanning Calorimetry as an Indicator of Protein Denaturation. Can. Inst. Food Sci. Technol. J. 1981, 14, 289–294. [Google Scholar] [CrossRef]
- Perera, S.P.; McIntosh, T.C.; Wanasundara, J.P.D. Structural Properties of Cruciferin and Napin of Brassica napus (Canola) Show Distinct Responses to Changes in pH and Temperature. Plants 2016, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Canola Proteins for Human Consumption: Extraction, Profile, and Functional Properties. J. Food Sci. J. Food Sci. 2011, 76, R16–R28. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.Y.; Yu, C.; Liu, J.F.; Yang, Y.L.; Shen, X.C.; Liu, S.W.; Tang, X.Z. Physical properties and chemical forces of extruded corn starch fortified with soy protein isolate. Int. J. Food Sci. Tech. 2017, 52, 2604–2613. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Bruininx, E.M.A.M.; Gruppen, H.; Hendriks, W.H.; Carré, P.; Quinsac, A.; van der Poel, A.F.B. Physical and chemical changes of rapeseed meal proteins during toasting and their effects on in vitro digestibility. J. Anim. Sci. Biotechnol. 2016, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Mosenthin, R.; Messerschmidt, U.; Sauer, N.; Carré, P.; Quinsac, A.; Schöne, F. Effect of the desolventizing/toasting process on chemical composition and protein quality of rapeseed meal. J. Anim. Sci. Biotechnol. 2016, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Rommi, K.; Ercili-Cura, D.; Hakala, T.K.; Nordlund, E.; Poutanen, K.; Lantto, R. Impact of total solid content and extraction pH on enzyme-aided recovery of protein from defatted rapeseed (Brassica rapa L.) press cake and physicochemical properties of the protein fractions. J. Agric. Food Chem. 2015, 63, 2997–3003. [Google Scholar] [CrossRef]
- Miller, N.; Pretorius, H.E.; Du Toit, L.J. Phytic acid in sunflower seeds, pressed cake and protein concentrate. Food Chem. 1986, 21, 205–209. [Google Scholar] [CrossRef]
- Hídvégi, M.; Lásztity, R. Phytic acid content of cereals and legumes and interaction with proteins. Period. Polytech. Chem. Eng. 2002, 46, 59–64. [Google Scholar]
- Emin, M.A.; Quevedo, M.; Wilhelm, M.; Karbstein, H.P. Analysis of the reaction behavior of highly concentrated plant proteins in extrusion-like conditions. Innov. Food Sci. Emerg. 2017, 44, 15–20. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D.; Tan, S.; Alashi, A.M.; Pudel, F.; Blanchard, C. Proteins from Canola/Rapeseed: Current Status; Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–304. [Google Scholar]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Physical properties of pregelatinized and granular cold water swelling maize starches at different pH values. Int. J. Biol. Macromol. 2016, 91, 730–735. [Google Scholar] [CrossRef]
- Russell, P.L. Gelatinisation of starches of different amylose/amylopectin content. A study by differential scanning calorimetry. J. Cereal. Sci. 1987, 6, 133–145. [Google Scholar] [CrossRef]
- Eberstein, K.; Höpcke, R.; Kleve; Konieczny-Janda, G.; Stute, R. DSC-Untersuchungen an Stärke Teil I. Möglichkeiten thermoanalytischer Methoden zur Stärkecharakterisierung. Starch Stärke 1980, 32, 397–404. [Google Scholar] [CrossRef]
- Kibar, E.A.A.; Gönenç, İ.; Us, F. Gelatinization of Waxy, Normal and High Amylose Corn Starches. J. Food 2010, 35, 237–244. [Google Scholar]
- Ye, J.P.; Hu, X.T.; Luo, S.J.; Liu, W.; Chen, J.; Zeng, Z.R.; Liu, C.M. Properties of Starch after Extrusion: A Review. Starch Stärke 2018, 70. [Google Scholar] [CrossRef]
- Lai, L.S.; Kokini, J.L. The effect of extrusion operating conditions on the on-line apparent viscosity of 98% Amylopectin (Amioca) and 70% Amylose (Hylon 7) corn starches during extrusion. J. Rheol. 1990, 34, 1245–1266. [Google Scholar] [CrossRef]
- Lin, L.; Guo, D.; Zhao, L.; Zhang, X.; Wang, J.; Zhang, F.; Wei, C. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocolloid 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, B.; Chen, L.; Li, X.; Li, L.; Xie, F. Effect of planetary ball-milling on multi-scale structures and pasting properties of waxy and high-amylose cornstarches. Innov. Food Sci. Emerg. 2015, 30, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Waramboi, J.G.; Gidley, M.J.; Sopade, P.A. Influence of extrusion on expansion, functional and digestibility properties of whole sweetpotato flour. Lebensm Wiss Technol. 2014, 59, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, S.; Hanna, M.A. Extrusion Processing Conditions for Amylose Lipid Complexing. Cereal Chem. 1994, 71, 587–593. [Google Scholar]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of Extrusion Cooking on Functional Properties and in vitro Starch Digestibility of Barley-Based Extrudates from Fruit and Vegetable By-Products. J. Food Sci. J. Food Sci. 2009, 74, E77–E86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brennan, C.S. Rheological, pasting and microstructural studies of dairy protein-starch interactions and their application in extrusion-based products: A review. Starch Stärke 2017, 69, 1600273. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Tunick, M.H.; Thomas-Gahring, A.E. Pasting and Extrusion Properties of Mixed Carbohydrate and Whey Protein Isolate Matrices. J. Food Process. Pres. 2014, 38, 1577–1591. [Google Scholar] [CrossRef]
- Kim, C.H.; Maga, J.A. Properties of Extruded Whey Protein Concentrate and Cereal Flour Blends. Lebensm. Wiss. Technol. 1987, 20, 311–318UR. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=7641732 (accessed on 23 February 2021).
- Zhang, B.; Zhao, Y.; Li, X.; Zhang, P.; Li, L.; Xie, F.; Chen, L. Effects of amylose and phosphate monoester on aggregation structures of heat-moisture treated potato starches. Carbohydr. Polym. 2014, 103, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of cornstarch with different amylose/amylopectin content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Stevnebø, A.; Sahlström, S.; Svihus, B. Starch structure and degree of starch hydrolysis of small and large starch granules from barley varieties with varying amylose content. Anim. Feed Sci. Tech. 2006, 130, 23–38. [Google Scholar] [CrossRef]
- Leeman, M.A.; Karlsson, M.E.; Eliasson, A.-C.; Björck, I.M.E. Resistant starch formation in temperature treated potato starches varying in amylose/amylopectin ratio. Carbohydr. Polym. 2006, 65, 306–313. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M. Effects of Phytic Acid, Divalent Cations, and Their Interactions on Amylase Activity. J. Food Sci. J. Food Sci. 1984, 49, 516–519. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Thibault, J.-F.; Della Valle, G. Influence of extrusion-cooking on the physico-chemical properties of wheat bran. J. Cereal Sci. 1990, 11, 249–259. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Ahmed, A.; Asif Khan, M.; Niaz, B.; Tufail, T. Improving the physicochemical properties of partially enhanced soluble dietary fiber through innovative techniques: A coherent review. J. Food Process. Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]




| Extrusion Processing | Barrel Temperature TB of Barrel Segments 2–6 (°C) | Residence Time (s) | Shear Rate(s−1) | ||||
| 1 | 20 | 20 | 20 | 20 | 20 | Approx. 60 | 15–2430 * |
| 2 | 60 | 80 | 80 | 80 | 80 | Approx. 60 | 15–2430 * |
| 3 | 60 | 80 | 100 | 100 | 100 | Approx. 60 | 15–2430 * |
| 4 | 60 | 80 | 100 | 120 | 120 | Approx. 60 | 15–2430 * |
| 5 | 60 | 100 | 120 | 140 | 140 | Approx. 60 | 15–2430 * |
| Defined Thermomechanical Treatment | Treatment Temperature TT (°C) | Treatment Time Tt (s) | Shear Rate(s−1) | ||||
| 1 | 25 | 60 | 50 | ||||
| 2 | 80 | 60 | 50 | ||||
| 3 | 100 | 60 | 50 | ||||
| 4 | 120 | 60 | 50 | ||||
| 5 | 140 | 60 | 50 | ||||
| Chemical Composition | RPC100 | PS30/RPC70 | WPS30/RPC70 | MS30/RPC70 | HAMS30/RPC70 |
|---|---|---|---|---|---|
| Dry matter (d.m.) (%) | 95.10 ± 0.03 a | 92.18 ± 0.01 b | 91.96 ± 0.08 b | 93.50 ± 0.06 c | 93.41 ± 0.03 c |
| Protein (% d.m.) | 38.20 ± 0.30 a | 27.40 ± 0.11 b | 27.38 ± 0.29 b | 26.99 ± 0.038 b | 27.28 ± 0.029 b |
| Ash (% d.m.) | 7.30 ± 0.02 a | 4.72 ± 0.02 b | 4.79 ± 0.01 b | 4.67 ± 0.025 b | 4.41 ± 0.55 b |
| TDF (% d.m.) | 35.67 ± 5.19 a | 10.28 ± 2.82 b | 9.01 ± 1.11 b | 11.51 ± 0.14 b | 16.92 ± 0.37 c |
| SDF (% d.m.) | 4.17 ± 1.55 a | 1.82 ± 0.13 b | 1.95 ± 0.05 b | 3.38 ± 0.11 c | 2.97 ± 0.28 c |
| IDF (% d.m.) | 31.51 ± 4.96 a | 8.47 ± 2.81 b | 7.06 ± 1.11 b | 8.14 ± 0.07 b | 13.95 ± 0.24 c |
| Starch (% d.m.) | 3.00 ± 0.02 a | 29.66 ± 0.014 b | 28.93 ± 0.052 b | 29.39 ± 0.021 b | 27.26 ± 0.038 b |
| Lipid (% d.m.) | 23.40 ± 0.90 a | 16.65 ± 0.25 b | 16.16 ± 0.02 b | 15.71 ± 0.03 b | 16.17 ± 0.11 b |
| Particle size distribution (Dv0.5/µm) | 261.1 ± 4.5 a | 87.0 ± 1.4 b | 160.7 ± 13.1 c | 131.5 ± 6.7 d | 141.1 ± 6.8 c e |
| SME (Wh/kg) | |||||
| TB (°C) in segment 6 | RPC100 | PS30/RPC70 | WPS30/RPC70 | MS30/RPC70 | HAMS30/RPC70 |
| 20 | 61.23 ± 1.5 a | 98.13 ± 6.16 b | 104.32 ± 2.95 b | 60.68 ± 0.63 a | 69.16 ± 0.63 a |
| 80 | 38.08 ± 1.2 a | 76.73 ± 0.92 b | 83.70 ± 1.45 b | 47.76 ± 4.02 c | 53.22 ± 0.53 d |
| 100 | 37.37 ± 1.1 a | 74.89 ± 1.29 b | 78.52 ± 0.87 b | 45.85 ± 2.40 c | 46.25 ± 0.44 c |
| 120 | 36.57 ± 1.0 a | 72.486 ± 1.53 b | 74.82 ± 1.16 b | 45.42 ± 2.73 c | 40.74 ± 0.27 ac |
| 140 | - | 65.24 ± 2.25 a | 68.94 ± 1.42 a | 36.52 ± 1.10 b | 38.40 ± 0.50 b |
| Product Temperature (°C) | |||||
| TB (°C) in segment 6 | RPC100 | PS30/RPC70 | WPS30/RPC70 | MS30/RPC70 | HAMS30/RPC70 |
| 20 | 30.0 ± 0.5 a | 45.1 ± 0.5 b | 52.2 ± 0.5 ab | 43.7 ± 1.0 c | 42.0 ± 0.3 c |
| 80 | 72.1 ± 0.4 a | 85.5 ± 0.5 b | 90.2 ± 0.4 c | 80.8 ± 0.8 d | 72.0 ± 0.7 a |
| 100 | 88.1 ± 0.5 a | 104.0 ± 0.3 b | 100.0 ± 0.2 b | 98.0 ± 0.6 b | 90.3 ± 1.2 a |
| 120 | 108.2 ± 0.4 a | 116.1 ± 0.1 b | 108.8 ± 0.5 a | 111.3 ± 0.3 c | 100.0 ± 1.0 d |
| 140 | - | 135.3 ± 0.2 a | 123.4 ± 0.4 b | 124.3 ± 0.2 b | 112.3 ± 1.2 c |
| Barrel Temperature TB (°C) | PS30/RPC70 | WPS30/RPC70 | MS30/RPC70 | HAMS30/RPC70 |
|---|---|---|---|---|
| Degree of gelatinization Dgel (%) | ||||
| 20 | 58.14 a | 49.59 b | 81.71 c | No peak |
| 80 | 78.11 a | 93.31 b | 81.68 c | No peak |
| 100 | 94.97 a | No peak | 56.26 b | No peak |
| 120 | No peak | No peak | 91.39 | No peak |
| 140 | No peak | No peak | No peak | No peak |
| Gelatinization temperature Tgel (°C) | ||||
| Not extruded | 68.82 ± 0.49 a | 54.29 ± 0.51 b | 67.65 ± 0.48 c | No peak |
| 20 | 69.48 ± 1.08 a | 59.15 ± 0.49 b | 70.92 ± 1.24 c | No peak |
| 80 | 67.85 ± 0.14 a | 56.87 ± 0.12 b | 70.84 ± 1.01 c | No peak |
| 100 | 67.14 ± 0.11 a | No peak | 70.74 ± 0.88 b | No peak |
| 120 | No peak | No peak | 73.35 ± 0.64 | No peak |
| 140 | No peak | No peak | No peak | No peak |
| Gelatinization enthalpy ∆ Hgel (J/g) | ||||
| Not extruded | 1.74 ± 0.07 a | 8.12 ± 0.23 b | 1.16 ± 0.19 c | No peak |
| 20 | 0.73 ± 0.17 a | 4.09 ± 0.12 b | 0.21 ± 0.01 c | No peak |
| 80 | 0.38 ± 0.00 a | 0.54 ± 0.01 b | 0.21 ± 0.01 c | No peak |
| 100 | 0.08 ± 0.01 a | No peak | 0.50 ± 0.01 b | No peak |
| 120 | No peak | No peak | 0.09 ± 0.01 | No peak |
| 140 | No peak | No peak | No peak | No peak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, A.; Naumann, S.; Osen, R.; Karbstein, H.P.; Emin, M.A. Extrusion Processing of Rapeseed Press Cake-Starch Blends: Effect of Starch Type and Treatment Temperature on Protein, Fiber and Starch Solubility. Foods 2021, 10, 1160. https://doi.org/10.3390/foods10061160
Martin A, Naumann S, Osen R, Karbstein HP, Emin MA. Extrusion Processing of Rapeseed Press Cake-Starch Blends: Effect of Starch Type and Treatment Temperature on Protein, Fiber and Starch Solubility. Foods. 2021; 10(6):1160. https://doi.org/10.3390/foods10061160
Chicago/Turabian StyleMartin, Anna, Susanne Naumann, Raffael Osen, Heike Petra Karbstein, and M. Azad Emin. 2021. "Extrusion Processing of Rapeseed Press Cake-Starch Blends: Effect of Starch Type and Treatment Temperature on Protein, Fiber and Starch Solubility" Foods 10, no. 6: 1160. https://doi.org/10.3390/foods10061160








