Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Developing FAD2DR Backcross Population and Genotyping by PCR
2.2. Phenotyping by Total Fatty Acid Profiling Using Gas Chromatography
2.3. Rice Grain Polishing and Starch Extraction
2.4. Quantitative Analysis of Lysophospholipids Using Liquid Chromatography−Mass Spectrometry
2.5. Total Starch, Amylose and Protein Content of Rice Flour
2.6. Starch Gelatinization Characterisation Using Differential Scanning Calorimetry and Swelling Power
2.7. Determination of Starch Molecular Structure Using FACE
2.8. Statistical Analysis
3. Results
3.1. Genotyping and Single Grain Total Fatty Acid Profiling
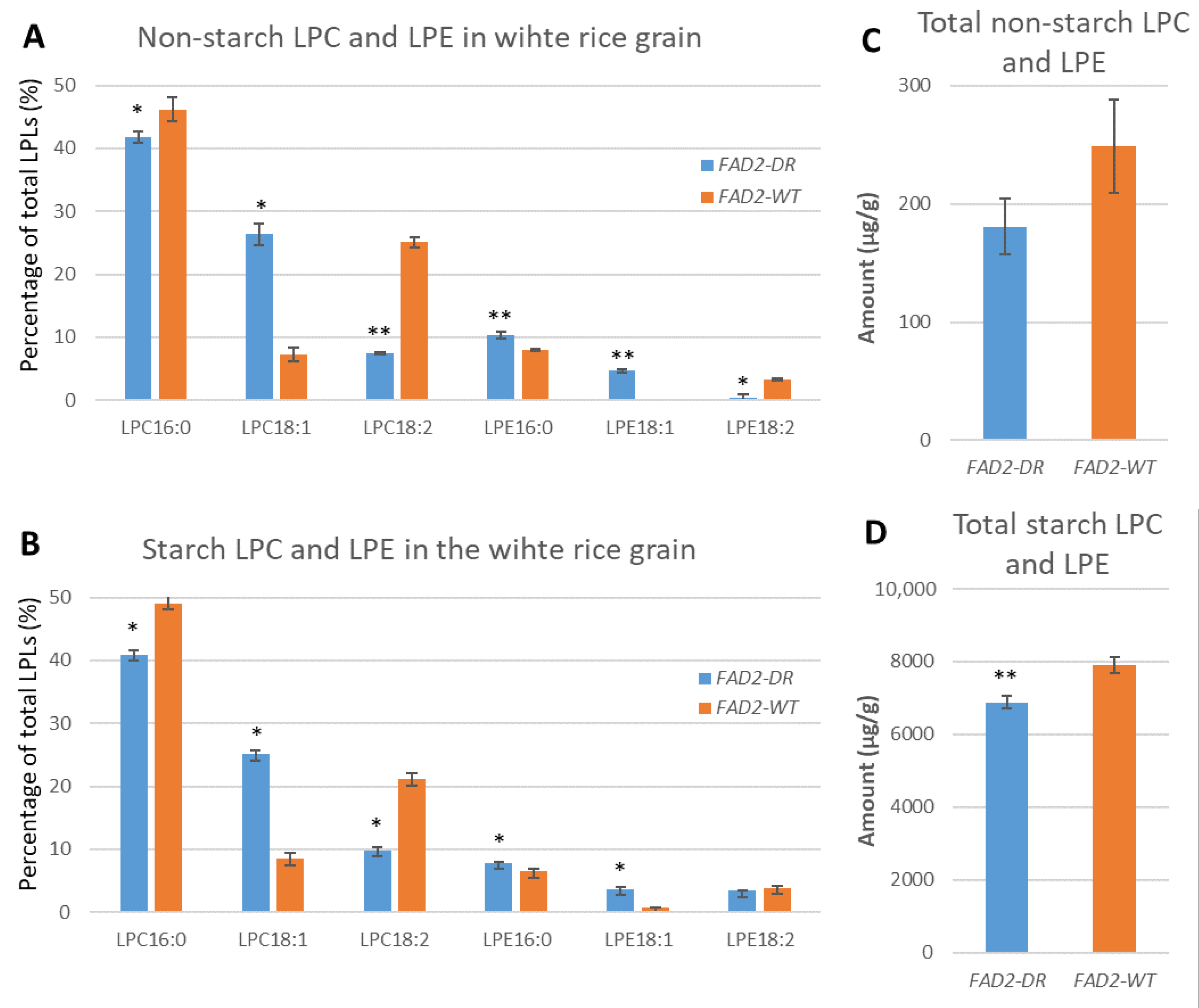
3.2. Characterisation of Non-Starch Lipids and Starch Lipids
3.3. White Rice Flour Composition and Starch Properties
3.4. Starch Molecular Structure Analyses
4. Discussion
4.1. Indication of Lipid Accumulation in White Rice Grain
4.2. Formation of Amylose–Lipid Complex and Functional Properties of High-Oleic Rice Flour
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoshida, H.; Tanigawa, T.; Kuriyama, I.; Yoshida, N.; Tomiyama, Y.; Mizushina, Y. Variation in fatty acid distribution of different acyl lipids in rice (Oryza sativa L.) brans. Nutrients 2011, 3, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Blanchard, C.; Helliwell, S.; Robards, K. Fatty acid composition of three rice varieties following storage. J. Cereal Sci. 2003, 37, 327–335. [Google Scholar] [CrossRef]
- Morrison, W.R. Lipids in cereal starches: A review. J. Cereal Sci. 1988, 8, 1–15. [Google Scholar] [CrossRef]
- Gayral, M.; Bakan, B.; Dalgalarrondo, M.; Elmorjani, K.; Delluc, C.; Brunet, S.; Linossier, L.; Morel, M.-H.; Marion, D. Lipid partitioning in maize (Zea mays L.) endosperm highlights relationships among starch lipids, amylose, and vitreousness. J. Agric. Food Chem. 2015, 63, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Waters, D.L.; Rose, T.J.; Bao, J.; King, G. Phospholipids in rice: Significance in grain quality and health benefits: A review. Food Chem. 2013, 139, 1133–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, W.; Azudin, M.N. Variation in the amylose and lipid contents and some physical properties of rice starches. J. Cereal Sci. 1987, 5, 35–44. [Google Scholar] [CrossRef]
- Putseys, J.; Lamberts, L.; Delcour, J. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Morrison, W.R.; Law, R.V.; Snape, C.E. Evidence for inclusion complexes of lipids with V-amylose in maize, rice and oat starches. J. Cereal Sci. 1993, 18, 107–109. [Google Scholar] [CrossRef]
- Hasjim, J.; Lee, S.-O.; Hendrich, S.; Setiawan, S.; Ai, Y.; Jane, J.-L. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010, 87, 257–262. [Google Scholar] [CrossRef]
- Godet, M.; Buléon, A.; Tran, V.; Colonna, P. Structural features of fatty acid-amylose complexes. Carbohydr. Polym. 1993, 21, 91–95. [Google Scholar] [CrossRef]
- Godet, M.; Tran, V.; Colonna, P.; Buleon, A.; Pezolet, M. Inclusion/exclusion of fatty acids in amylose complexes as a function of the fatty acid chain length. Int. J. Biol. Macromol. 1995, 17, 405–408. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. Effect of fatty acids on functional properties of normal wheat and waxy wheat starches: A structural basis. Food Chem. 2016, 190, 285–292. [Google Scholar] [CrossRef]
- Ai, Y.; Hasjim, J.; Jane, J.-L. Effects of lipids on enzymatic hydrolysis and physical properties of starch. Carbohydr. Polym. 2013, 92, 120–127. [Google Scholar] [CrossRef]
- Concepcion, J.C.T.; Calingacion, M.; Garson, M.J.; Fitzgerald, M.A. Lipidomics reveals associations between rice quality traits. Metabolomics 2020, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Gayral, M.; Fanuel, M.; Rogniaux, H.; Dalgalarrondo, M.; Elmorjani, K.; Bakan, B.; Marion, D. The spatiotemporal deposition of lysophosphatidylcholine within starch granules of maize endosperm and its relationships to the expression of genes involved in endoplasmic reticulum-amyloplast lipid trafficking and galactolipid synthesis. Plant Cell Physiol. 2019, 60, 139–151. [Google Scholar] [CrossRef]
- Long, W.; Wang, Y.; Zhu, S.; Jing, W.; Wang, Y.; Ren, Y.; Tian, Y.; Liu, S.; Liu, X.; Chen, L.; et al. FLOURY SHRUNKEN ENDOSPERM1 Connects Phospholipid Metabolism and Amyloplast Development in Rice. Plant Physiol. 2018, 177, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Gelders, G.G.; Goesaert, H.; Delcour, J.A. Amylose-lipid complexes as controlled lipid release agents during starch gelatinization and pasting. J. Agric. Food Chem. 2006, 54, 1493–1499. [Google Scholar] [CrossRef]
- Raphaelides, S.; Karkalas, J. Thermal dissociation of amylose-fatty acid complexes. Carbohydr. Res. 1988, 172, 65–82. [Google Scholar] [CrossRef]
- Kowblansky, M. Calorimetric investigation of inclusion complexes of amylose with long-chain aliphatic compounds containing different functional groups. Macromolecules 1985, 18, 1776–1779. [Google Scholar] [CrossRef]
- Karkalas, J.; Ma, S.; Morrison, W.R.; Pethrick, R.A. Some factors determining the thermal properties of amylose inclusion complexes with fatty acids. Carbohydr. Res. 1995, 268, 233–247. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Krog, N. Physical properties of amylose-monoglyceride complexes. J. Cereal Sci. 1985, 3, 239–248. [Google Scholar] [CrossRef]
- Yamada, T.; Kato, T.; Tamaki, S.; Teranishi, K.; Hisamatsu, M. Introduction of fatty acids to starch granules by ultra-high-pressure treatment. Starch Stärke 1998, 50, 484–486. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid Biosynthesis. Plant Cell 1995, 7, 957. [Google Scholar] [PubMed] [Green Version]
- Benning, C. A role for lipid trafficking in chloroplast biogenesis. Prog. Lipid Res. 2008, 47, 381–389. [Google Scholar] [CrossRef]
- Zaplin, E.S.; Liu, Q.; Li, Z.; Butardo, V.M.; Blanchard, C.L.; Rahman, S. Production of high oleic rice grains by suppressing the expression of the OsFAD2-1 gene. Funct. Plant Biol. 2013, 40, 996–1004. [Google Scholar] [CrossRef]
- Luo, J.; Jobling, S.A.; Millar, A.; Morell, M.K.; Li, Z. Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice 2015, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Tong, C.; Bao, J.; Waters, D.L.; Rose, T.J.; King, G.J. Determination of starch lysophospholipids in rice using liquid chromatography-mass spectrometry (LC-MS). J. Agric. Food Chem. 2014, 62, 6600–6607. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, D.; Du, X.; Wang, H.; Larroque, O.; Jenkins, C.L.D.; Jobling, S.A.; Morell, M.K. The barley amo1 locus is tightly linked to the starch synthase IIIa gene and negatively regulates expression of granule-bound starch synthetic genes. J. Exp. Bot. 2011, 62, 5217–5231. [Google Scholar] [CrossRef] [Green Version]
- Konik-Rose, C.M.; Moss, R.; Rahman, S.; Appels, R.; Stoddard, F.; McMaster, G. Evaluation of the 40 mg swelling test for measuring starch functionality. Starch Stärke 2001, 53, 14–20. [Google Scholar] [CrossRef]
- O’Shea, M.G.; Samuel, M.S.; Konik, C.M.; Morell, M.K. Fluorophore-assisted carbohydrate electrophoresis (FACE) of oligosaccharides: Efficiency of labelling and high-resolution separation. Carbohydr. Res. 1998, 307, 1–12. [Google Scholar] [CrossRef]
- He, H.; Zheng, B.; Wang, H.; Li, X.; Chen, L. Insights into the multi-scale structure and in vitro digestibility changes of rice starch-oleic acid/linoleic acid complex induced by heat-moisture treatment. Food Res. Int. 2020, 137, 109612. [Google Scholar] [CrossRef] [PubMed]
- Biliaderis, C.G.; Page, C.M.; Maurice, T.J.; Juliano, B.O. Thermal characterization of rice starches: A polymeric approach to phase transitions of granular starch. J. Agric. Food Chem. 1986, 34, 6–14. [Google Scholar] [CrossRef]
- Somerville, C.; Browse, J. Plant Lipids: Metabolism, Mutants, and Membranes. Science 1991, 252, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D. Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; Jouhet, J.; Block, M.A. Importance of phosphatidylcholine on the chloroplast surface. Prog. Lipid Res. 2017, 65, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, M.; Donovan, J.W.; Wong, R.Y. Phase Transitions of Amylose-Lipid Complexes in Starches: A Calorimetric Study. Starch Stärke 1980, 32, 265–270. [Google Scholar] [CrossRef]
- Ocloo, F.C.K.; Minnaar, A.; Emmambux, N.M. Effects of gamma irradiation and stearic acid, alone and in combination, on functional, structural, and molecular characteristics of high amylose maize starch. Starch Stärke 2014, 66, 624–635. [Google Scholar] [CrossRef] [Green Version]
| Genotype | C14:0 (%) | C16:0 (%) | C18:0 (%) | C18:1 (%) | C18:2 (%) |
|---|---|---|---|---|---|
| FAD2DR | 0.49 (0.02) ** | 20.23 (0.58) ** | 3.32 (0.25) | 48.07 (1.62) ** | 24.13 (2.16) ** |
| FAD2WT | 1.10 (0.10) | 22.90 (0.26) | 3.39 (0.15) | 27.26 (0.92) | 40.82 (0.91) |
| Sample | Total Starch (%) | Amylose (%) | Protein (%) |
|---|---|---|---|
| FAD2DR | 74.47 (1.28) ** | 14.63 (4.11) | 7.41 (0.81) * |
| FAD2WT | 72.28 (1.29) | 12.00 (3.16) | 9.41 (0.68) |
| Genotype | Peak 1 | Peak 2 | Swelling Power | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1To (°C) | 1Tp (°C) | 1Te (°C) | 1∆H (J/g) | 2To (°C) | 2Tp (°C) | 2Te (°C) | 2∆H (J/g) | ||
| FAD2DR | 55.45 (1.36) | 65.44 (2.08) | 83.41 (2.06) | 4.78 (0.48) | 100.07 (2.49) | 110.75 (1.32) ** | 118.42 (1. 89) | 0.49 (0.04) | 11.84 (0.25) ** |
| AD2WT | 55.36 (1.54) | 64.02 (0.52) | 81.88 (1.69) | 4.42 (0.51) | 100.26 (1.03) | 109.18 (0.86) | 116.97 (1.10) | 0.38 (0.08) | 13.57 (0.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Liu, L.; Konik-Rose, C.; Tian, L.; Singh, S.; Howitt, C.A.; Li, Z.; Liu, Q. Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties. Foods 2021, 10, 1169. https://doi.org/10.3390/foods10061169
Luo J, Liu L, Konik-Rose C, Tian L, Singh S, Howitt CA, Li Z, Liu Q. Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties. Foods. 2021; 10(6):1169. https://doi.org/10.3390/foods10061169
Chicago/Turabian StyleLuo, Jixun, Lei Liu, Christine Konik-Rose, Lijun Tian, Surinder Singh, Crispin A. Howitt, Zhongyi Li, and Qing Liu. 2021. "Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties" Foods 10, no. 6: 1169. https://doi.org/10.3390/foods10061169
APA StyleLuo, J., Liu, L., Konik-Rose, C., Tian, L., Singh, S., Howitt, C. A., Li, Z., & Liu, Q. (2021). Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties. Foods, 10(6), 1169. https://doi.org/10.3390/foods10061169








