Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Meat Samples
2.2. L. monocytogenes Culture Preparation
2.3. Preparation of Lactic Acid Solution
2.4. UV-C Irradiation
2.5. Experimental Design
2.6. Sample Treatments
2.7. Microbiological Analyses
2.8. Instrumental Color
2.9. Optimization and Model Validation
2.10. Evolution of L. monocytogenes, LAB, pH and Instrumental Color of LA/UV-C Treated Meat Vacuum Packed and Stored at 4 °C for 8 Weeks
3. Results
3.1. Central Composite Design and Response Surface
3.2. Optimization and Model Validation
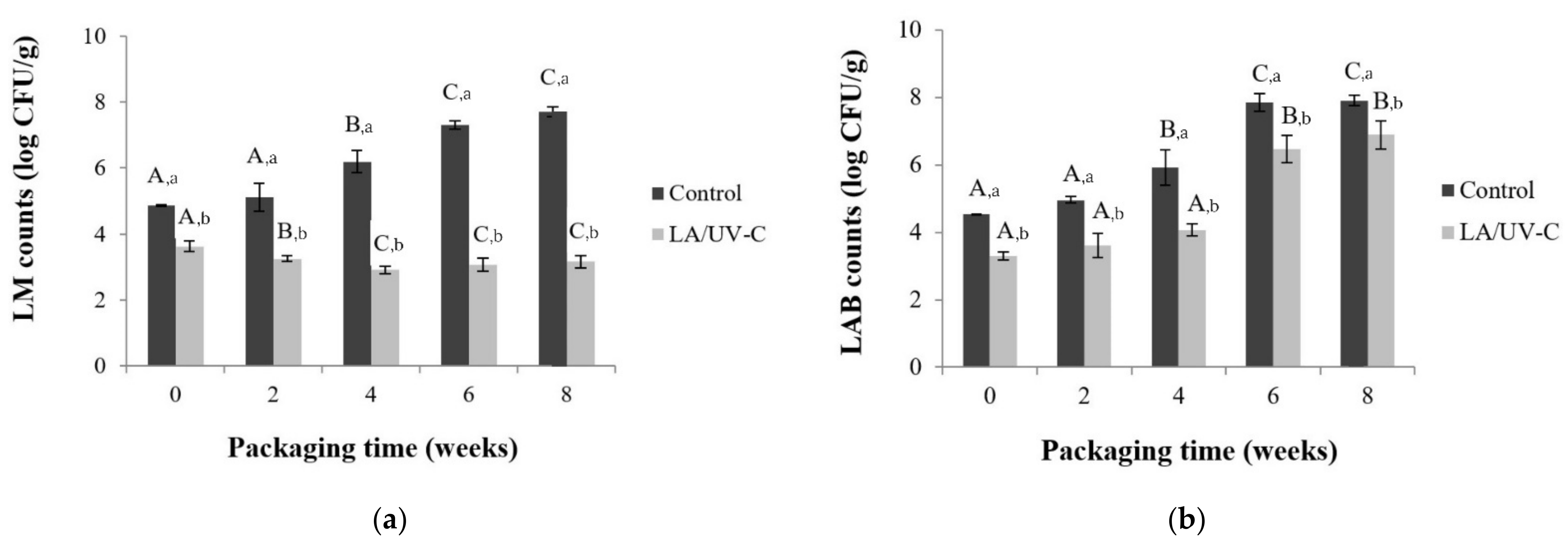
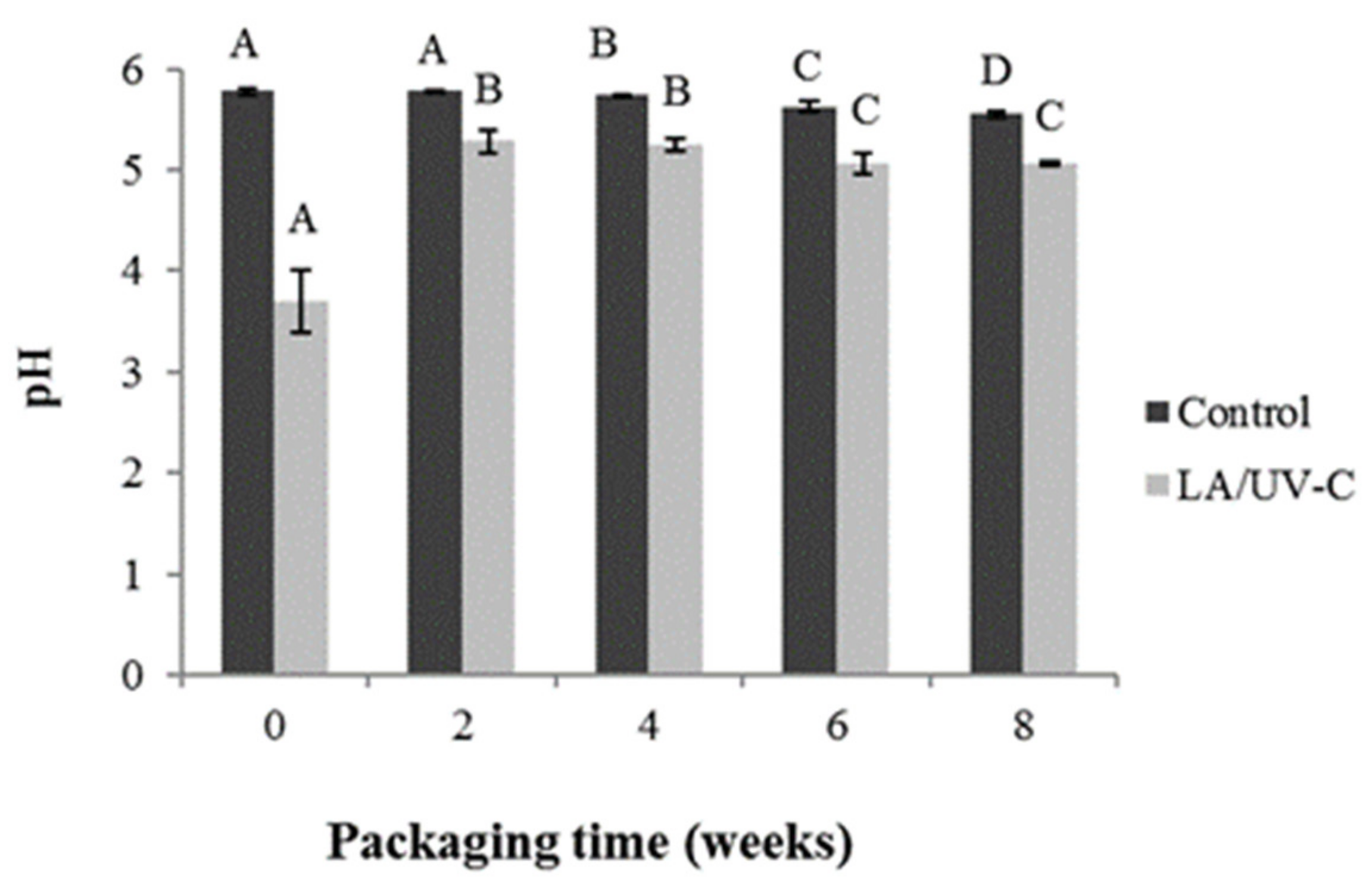
3.3. Evolution of L. monocytogenes, LAB, pH and Meat Color Treated with 2.6% of LA and 330 mJ/cm2 of UV-C Dose Vacuum Packed and Stored at 4 °C for 8 Weeks
3.3.1. L. monocytogenes and LAB Counts
3.3.2. pH Values
3.3.3. Color Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Labadie, J. Consequences of packaging on bacterial growth. Meat is an ecological niche. Meat Sci. 1999, 52, 299–305. [Google Scholar] [CrossRef]
- New and Established Carcass Decontamination Procedures Commonly Used in the Beef-Processing Industry. J. Food Prot. 1997, 60, 1146–1151. [CrossRef] [PubMed]
- Nychas, G.J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.; Klein, G.; Koutsoumanis, K.; Mclauchlin, J.; Müller-graf, C. Scientific Opinion on the evaluation of the safety and efficacy of lactic acid for the removal of microbial surface contamination of beef carcasses, cuts and trimmings. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Review: Advantages and limitations on processing foods by UV light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- FDA. Irradiation in the production, processing and handling of food: Ultraviolet radiation for the processing and treatment of food. Food Drugs 2000, 65, 71056–71058. [Google Scholar]
- Theron, M.M.; Lues, J.F.R. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- DeGeer, S.L.; Wang, L.; Hill, G.N.; Singh, M.; Bilgili, S.F.; Bratcher, C.L. Optimizing application parameters for lactic acid and sodium metasilicate against pathogens on fresh beef, pork and deli meats. Meat Sci. 2016, 118, 28–33. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and food processing environments. Front. Microbiol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Yoon, Y.; Stopforth, J.D.; Kendall, P.A.; Sofos, J.N. Heat and acid tolerance of Listeria monocytogenes after exposure to single and multiple sublethal stresses. Food Microbiol. 2008, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T.; Litopoulou-Tzanetaki, E. Existing and potential applications of ultraviolet light in the food industry—A critical review. J. Sci. Food Agric. 2010, 80, 637–645. [Google Scholar] [CrossRef]
- Sommers, C.H.; Cooke, P.H.; Fan, X.; Sites, J.E. Ultraviolet light (254 nm) inactivation of Listeria monocytogenes on frankfurters that contain potassium lactate and sodium diacetate. J. Food Sci. 2009, 74, M114–M119. [Google Scholar] [CrossRef] [PubMed]
- Gailunas, K.M.; Matak, K.E.; Boyer, R.R.; Alvarado, C.Z.; Williams, R.C.; Sumner, S.S. Use of UV light for the inactivation of Listeria monocytogenes and lactic acid bacteria species in recirculated chill brines. J. Food Prot. 2008, 71, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Kalchayanand, N.; Bosilevac, J.M.; King, D.A.; Wheeler, T.L. Evaluation of UVC radiation and a UVC-ozone combination as fresh beef interventions against Shiga toxin-producing Escherichia coli, salmonella, and listeria monocytogenes and their effects on beef quality. J. Food Prot. 2020, 83, 1520–1529. [Google Scholar] [CrossRef]
- Tarek, A.R.; Rasco, B.A.; Sablani, S.S. Ultraviolet-C Light Inactivation Kinetics of E. coli on Bologna Beef Packaged in Plastic Films. Food Bioprocess Technol. 2015, 8, 1267–1280. [Google Scholar] [CrossRef]
- Faustman, C.; Cassens, R.G. The Biochemical Basis for Discoloration in Fresh Meat: A Review. J. Muscle Foods 1990, 1, 217–243. [Google Scholar] [CrossRef]
- Zerby, H.N.; Belk, K.E.; Sofos, J.N.; McDowell, L.R.; Smith, G.C. Case life of seven retail products from beef cattle supplemented with alpha-tocopheryl acetate. J. Anim. Sci. 1999, 77, 2458–2463. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017, 129, 169–175. [Google Scholar] [CrossRef]
- Signorini, M.L.; Ponce-Alquicira, E.; Guerrero-Legarreta, I. Effect of lactic acid and lactic acid bacteria on growth of spoilage microorganisms in vacuum-packaged beef. J. Muscle Foods 2006, 17, 277–290. [Google Scholar] [CrossRef]
- García, A.L.; Brugnini, G.; Rodriguez, S.; Mir, A.; Carriquiry, J.; Rufo, C.; Briano, B. Vida útil de carne fresca de res envasada al vacío a 0 °C y +4 °C. Prod. Agropecu. Desarro. Sosten. 2015, 4, 27–45. [Google Scholar] [CrossRef]
- Small, A.H.; Jenson, I.; Kiermeier, A.; Sumner, J. Vacuum-packed beef primals with extremely long shelf life have unusual microbiological counts. J. Food Prot. 2012, 75, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Pennacchia, C.; Ercolini, D.; Villani, F. Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol. 2011, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Mombrú, Á.W.; Faccio, R.; Brugnini, G.; Miraballes, I.; Rufo, C.; Pardo, H. Optimization and characterization of nisin-loaded alginate-chitosan nanoparticles with antimicrobial activity in lean beef. LWT Food Sci. Technol. 2018, 91, 107–116. [Google Scholar] [CrossRef]
- Martinez-Silveira, A.; Villarreal, R.; Garmendia, G.; Rufo, C.; Vero, S. Process conditions for a rapid in situ transesterification for biodiesel production from oleaginous yeasts. Electron. J. Biotechnol. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Mutz, Y.S.; Castro, V.S.; Bernardes, P.C.; Rajkovic, A.; Conte-Junior, C.A. Optimization of UV-C light and lactic acid combined treatment in decontamination of sliced Brazilian dry-cured loin: Salmonella Typhimurium inactivation and physicochemical quality. Meat Sci. 2021, 172, 108308. [Google Scholar] [CrossRef]
- Brugnini, G.; Acquistapace, M.J.; Rodriguez, S.; Rufo, C. Efecto de la aplicación de ácido láctico sobre Listeria monocytogenes en carne envasada al vacío en Uruguay. Innotec 2018, 15, 7–14. [Google Scholar] [CrossRef]
- MacDougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–88. [Google Scholar] [CrossRef]
- McLeod, A.; Liland, K.H.; Haugen, J.E.; Sørheim, O.; Myhrer, K.S.; Holck, A.L. Chicken fillets subjected to UV-C and pulsed UV light: Reduction of pathogenic and spoilage bacteria, and changes in sensory quality. J. Food Saf. 2018, 38, 2421. [Google Scholar] [CrossRef]
- Ariyapitipun, T.; Mustapha, A.; Clarke, A.D. Survival of Listeria monocytogenes Scott A on Vacuum-Packaged Raw Beef Treated with polylactic Acid, Lactic Acid and Nisin. J. Food Prot. 2000, 63, 131–136. [Google Scholar] [CrossRef]
- Tsigarida, E.; Skandamis, P.; Nychas, G.J.E. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef]
- Dickson, J.S. Survival and Growth of Listeria Monoctyogenes on Beef Tissue Surfaces As Affected By Simulated Processing Conditions. J. Food Saf. 1989, 10, 165–174. [Google Scholar] [CrossRef]
- Duffy, G.; Walsh, D.; Sheridan, J.J.; Logue, C.M.; Harrington, D.; Blair, I.S.; McDowell, D.A. Behaviour of Listeria monocytogenes in the presence of Listeria innocua during storage of minced beef under vacuum or in air at 0 °C and 10 °C. Food Microbiol. 2000, 17, 571–578. [Google Scholar] [CrossRef]
- Salim, A.P.A.; Canto, A.C.; Costa-Lima, B.R.; Simoes, J.S.; Panzenhagen, P.H.; Costa, M.P.; Franco, R.M.; Silva, T.J.; Conte-Junior, C.A. Inhibitory effect of acid concentration, aging, and different packaging on Escherichia coli O157:H7 and on color stability of beef. J. Food Process. Preserv. 2017, 42, 1–8. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
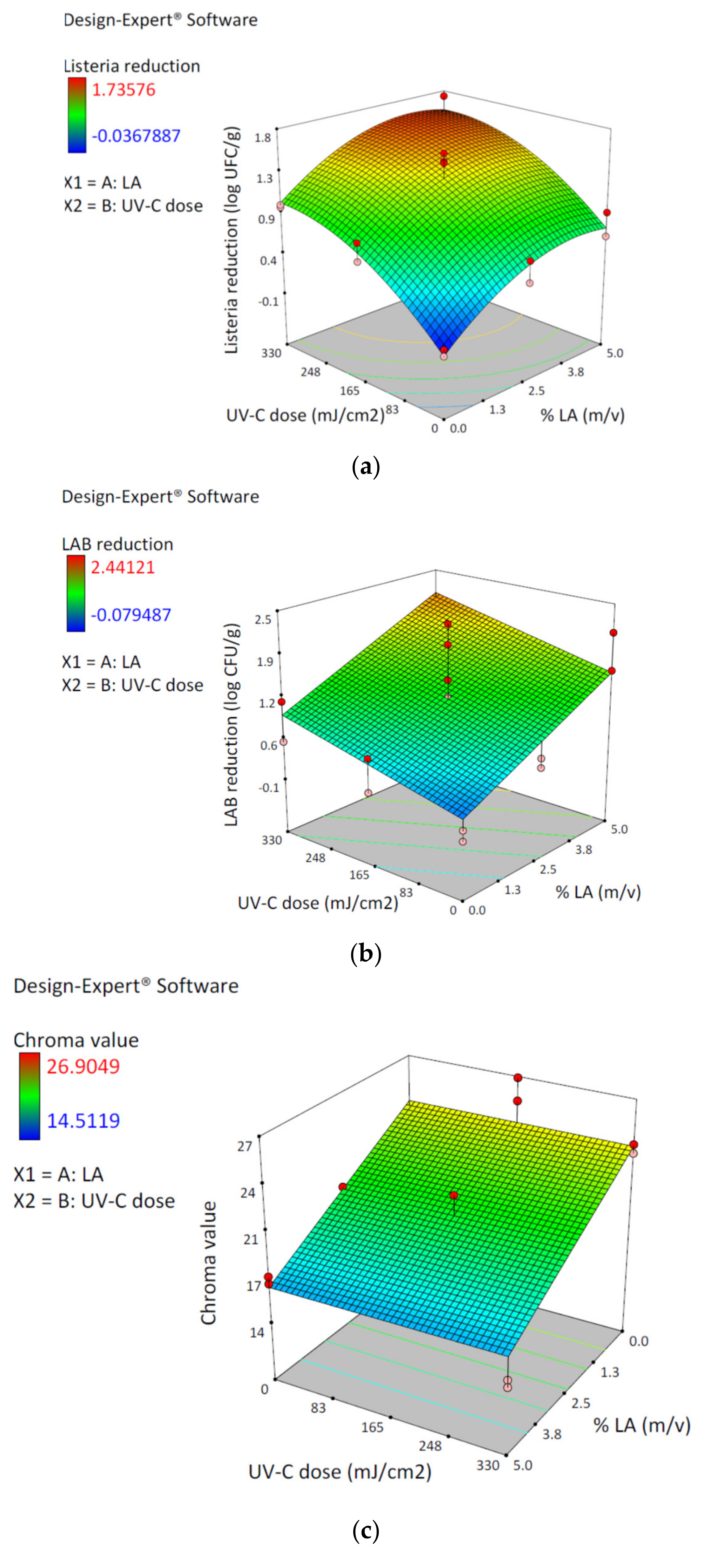
| Runs | % Lactic Acid (m/v) (X1) | UV-C Dose (mJ/cm2) (X2) | Reduction LM (Log CFU/g) (Y1) | Reduction LAB (Log CFU/g) (Y2) | Chroma Value (Y3) a |
|---|---|---|---|---|---|
| 1 | 2.5 | 398 | 1.59 | 2.44 | 21.48 |
| 2 | 0.0 | 165 | 0.62 | 0.66 | 26.90 |
| 3 | 2.5 | 0 | 0.38 | 0.45 | 19.14 |
| 4 | 2.5 | 0 | 0.63 | 0.59 | 20.25 |
| 5 | 2.5 | 165 | 1.20 | 1.13 | 18.69 |
| 6 | 5.0 | 330 | 1.74 | 1.14 | 15.02 |
| 7 | 5.0 | 330 | 1.34 | 1.70 | 14.51 |
| 8 | 5.0 | 0 | 0.58 | 1.48 | 17.34 |
| 9 | 2.5 | 165 | 1.53 | 1.11 | 18.73 |
| 10 | 0.0 | 0 | 0.04 | 0.08 | 22.57 |
| 11 | 2.5 | 165 | 1.43 | 2.25 | 21.61 |
| 12 | 2.5 | 398 | 1.45 | 1.67 | 20.61 |
| 13 | 0.0 | 165 | 0.82 | 0.15 | 25.24 |
| 14 | 6.0 | 165 | 1.49 | 2.24 | 16.60 |
| 15 | 0.0 | 0 | −0.04 | −0.08 | 22.77 |
| 16 | 5.0 | 0 | 0.85 | 2.06 | 16.82 |
| 17 | 2.5 | 165 | 1.08 | 1.39 | 18.69 |
| 18 | 6.0 | 165 | 0.96 | 1.63 | 17.43 |
| 19 | 0.0 | 398 | 0.92 | 0.49 | 23.82 |
| 20 | 0.0 | 398 | 0.94 | 1.12 | 23.20 |
| 21 | 2.5 | 165 | 1.42 | 1.94 | 18.73 |
| Response | Model | Significance | R2 | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|---|---|
| Y1 | Quadratic | <0.0001 | 0.9038 | 0.8718 | 0.8111 | 16.646 |
| Y2 | Linear | <0.0001 | 0.5774 | 0.5304 | 0.4329 | 9.563 |
| Y3 | Linear | <0.0001 | 0.8002 | 0.7780 | 0.7314 | 14.571 |
| Time (Weeks) | Control | LA/UV-C | |
|---|---|---|---|
| 0 | Lightness (L*) | 47.69 ± 2.94 a | 46.71 ± 3.51 a |
| Redness (a*) | 20.21 ± 2.85 a | 15.43 ± 1.92 b | |
| Yellowness (b*) | 13.07 ± 0.77 a | 13.36 ± 0.63 a | |
| Chroma (C*) | 24.10 ± 2.48 a | 20.44 ± 1.56 a | |
| 8 | Lightness (L*) | 46.94 ± 2.71 a | 40.95 ± 1.81 b |
| Redness (a*) | 13.53 ± 1.63 a | 9.92 ± 0.69 b | |
| Yellowness (b*) | 11.08 ± 0.27 a | 11.62 ± 0.56 a | |
| Chroma (C*) | 17.52 ± 1.14 a | 15.28 ± 0.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brugnini, G.; Rodríguez, S.; Rodríguez, J.; Rufo, C. Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef. Foods 2021, 10, 1217. https://doi.org/10.3390/foods10061217
Brugnini G, Rodríguez S, Rodríguez J, Rufo C. Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef. Foods. 2021; 10(6):1217. https://doi.org/10.3390/foods10061217
Chicago/Turabian StyleBrugnini, Giannina, Soledad Rodríguez, Jesica Rodríguez, and Caterina Rufo. 2021. "Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef" Foods 10, no. 6: 1217. https://doi.org/10.3390/foods10061217
APA StyleBrugnini, G., Rodríguez, S., Rodríguez, J., & Rufo, C. (2021). Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef. Foods, 10(6), 1217. https://doi.org/10.3390/foods10061217








