Impact of Feeding Cover Crop Forage Containing Brassicas to Steers during Backgrounding on Palatability Attributes of Beef Strip Steaks
Abstract
1. Introduction
2. Materials and Methods
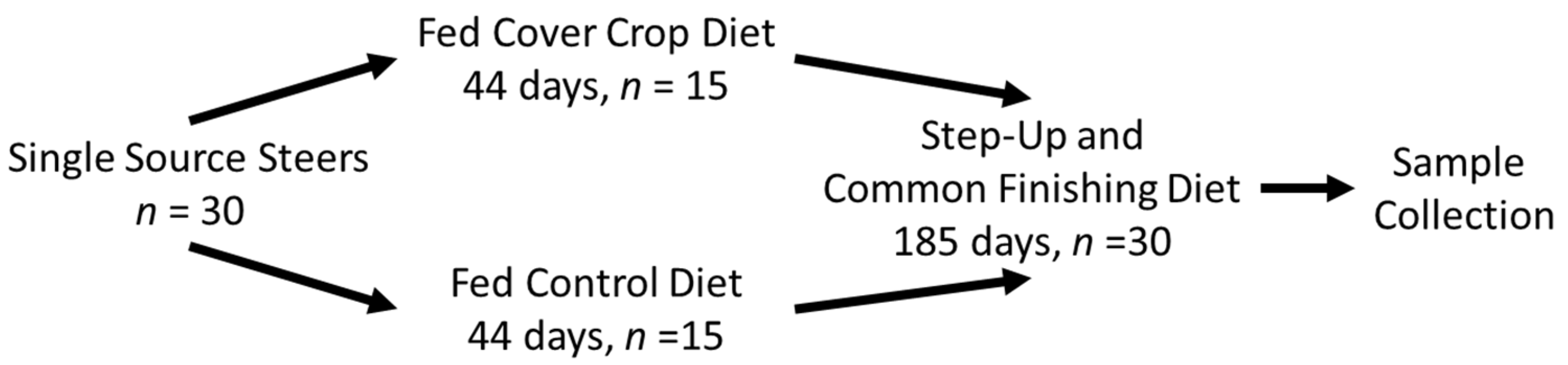
2.1. Animals and Experimental Diets
2.2. Finishing Phase, Harvest, and Product Collection
2.3. Strip Loin Fabrication
2.4. Cook Loss and Warner–Bratzler Shear Force
2.5. Protein Extraction
2.6. Western Blot Analysis
2.7. Trained Sensory Panel
2.8. Collagen Content Analysis
2.9. Statistical Analysis
3. Results
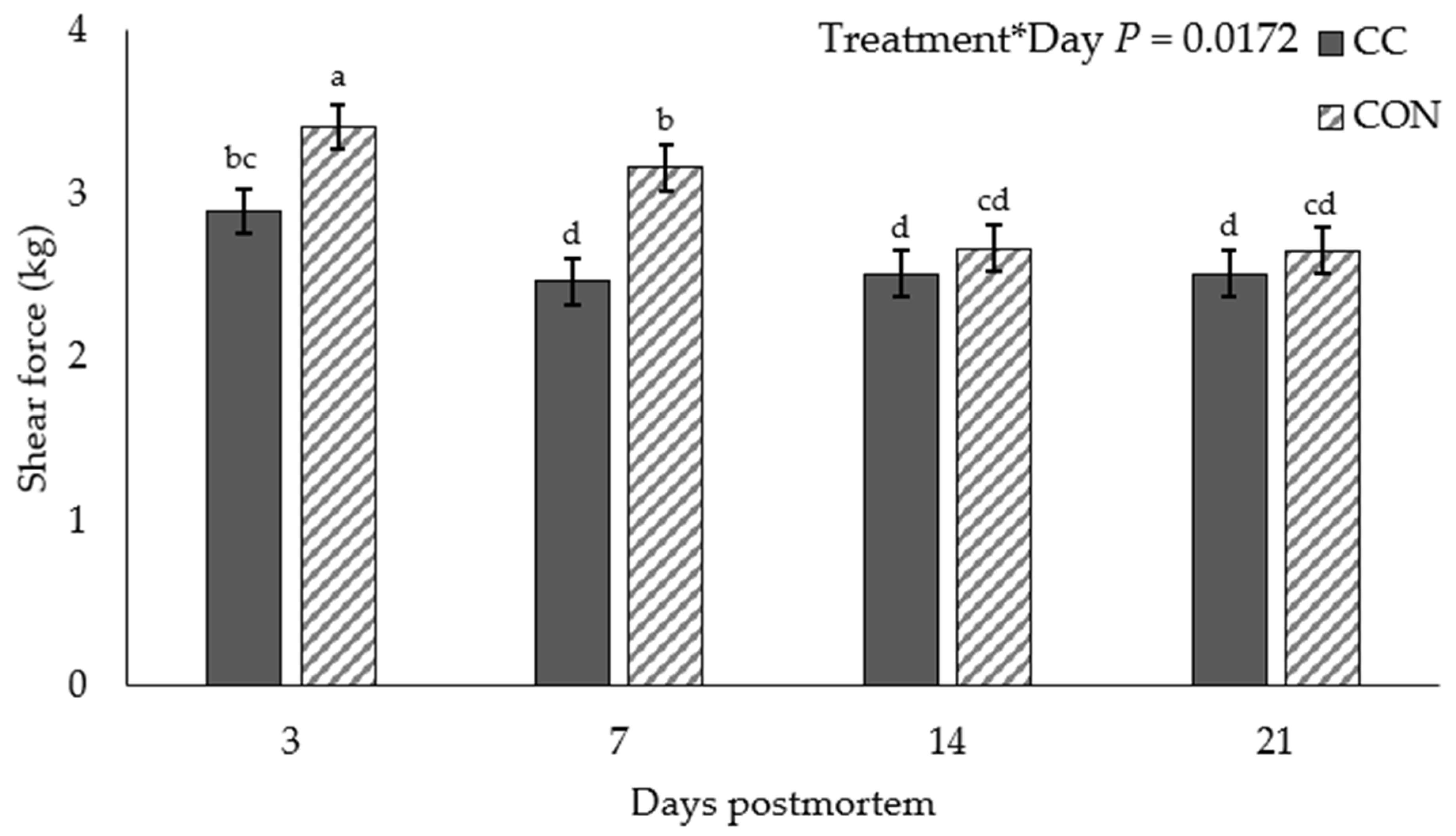
3.1. Warner–Bratzler Shear Force and Cook Loss
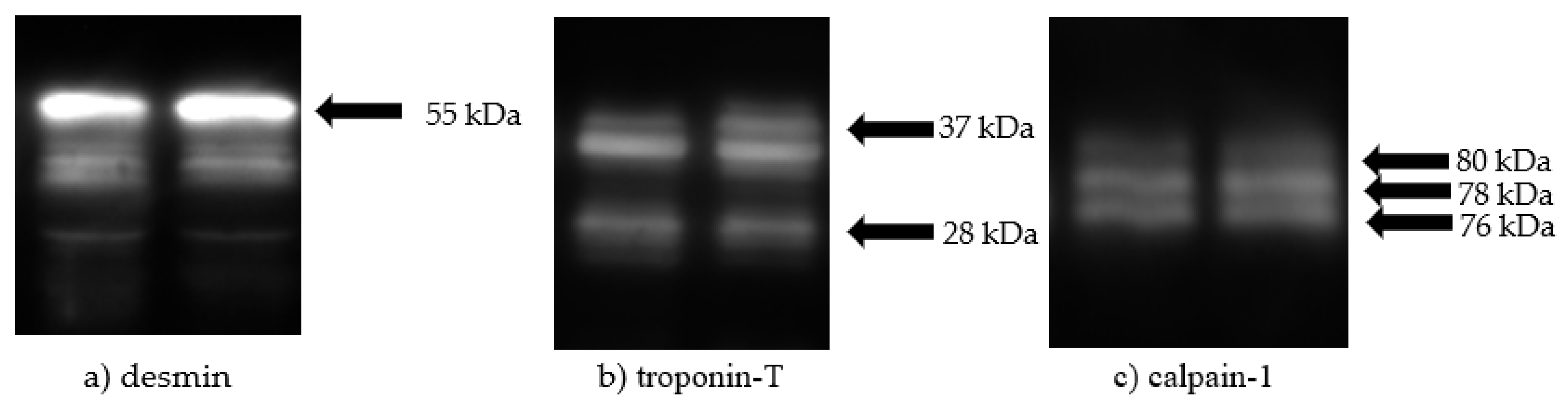
3.2. Western Blot Analysis of Desmin, Troponin-T, and Calpain-1
3.3. Sensory Analysis
3.4. Collagen Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swanek, S.S.; Morgan, J.B.; Owens, F.N.; Gill, D.R.; Strasia, C.A.; Dolezal, H.G.; Ray, F.K. Vitamin D3 Supplementation of Beef Steers Increases Longissimus Tenderness. J. Anim. Sci. 1999, 77, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.L.; Parrish, F.C., Jr.; Beitz, D.C.; Horst, R.L.; Huff-Lonergan, E.J.; Trenkle, A.H. The use of vitamin D3 to improve beef tenderness. J. Anim. Sci. 2000, 78, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Roeber, D.L.; Gill, R.K.; DiCostanzo, A. Meat quality responses to feeding distiller’s grains to finishing holstein steers. J. Anim. Sci. 2005, 83, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Harsh, B.N.; Dilger, A.C.; Boler, D.D.; Shike, D.W. Effects of a multielement trace mineral injection and vitamin E supplementation on performance, carcass characteristics, and color stability of strip steaks from feedlot heifers. J. Anim. Sci. 2018, 96, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Jäpelt, R.B.; Didion, T.; Smedsgaard, J.; Jakobsen, J. Seasonal variation of provitamin D2 and vitamin D2 in perennial ryegrass (Lolium perenne). J. Agric. Food Chem. 2011, 59, 10907–10912. [Google Scholar] [CrossRef] [PubMed]
- USDA. 2017 Census of Agriculture; National Agricultural Statistics Service: Washington, DC, USA, 2019. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_1_US/usv1.pdf (accessed on 10 June 2019).
- McCartney, D.; Fraser, J.; Ohama, A. Potential of warm-season annual forages and Brassica crops for grazing: A Canadian Review. Can. J. Anim. Sci. 2009, 89, 431–440. [Google Scholar] [CrossRef]
- Fehrman, C. Effects of Feeding Cattle Calcium Hydroxide Treated Corn Stover during Backgrounding on Carcass Characteristics and Beef Quality. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2016. [Google Scholar]
- NRC. Nutrient Requirements of Beef Cattle, 8th ed.; National Academy Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Melody, J.L.; Lonergan, S.M.; Rowe, L.J.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 2004, 84, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness of Meat; American Meat Science Association: Champaign, IL, USA, 2016. [Google Scholar]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC–MS analysis of free amino acids in biological fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Cross, H.R.; Carpenter, Z.L.; Smith, G.C. Effects of intramuscular collagen and elastin on bovine muscle tenderness. J. Food Sci. 1973, 38, 998–1003. [Google Scholar] [CrossRef]
- Shanks, B.C.; Wulf, D.M.; Maddock, R.J. Technical note: The effect of freezing on Warner-Bratzler shear force values of beef longissimus steaks across several postmortem aging periods. J. Anim. Sci. 2002, 80, 2122–2125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huff-Lonergan, E.; Mitsuhashi, T.; Beekman, D.D.; Parrish, F.C., Jr.; Olson, D.G.; Robson, R.M. Proteolysis of Specific Muscle Structural Proteins by Mu-Calpain at Low pH and Temperature Is Similar to Degradation in Postmortem Bovine Muscle. J. Anim. Sci. 1996, 74, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.L.; Kendall, T.L.; Thompson, V.F.; Goll, D.E. Changes in the Calpains and Calpastatin During Postmortem Storage of Bovine Muscle. J. Anim. Sci. 1998, 76, 2415–2434. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M. The role of Ca2+-dependent proteases (calpains) in postmortem proteolysis and meat tenderness. Biochimie 1992, 74, 239–245. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Kennick, W.H.; Elgasim, E.A.; Angelmier, A.F. Effects of Postmortem Storage on Muscle Protein Degradation: Analysis by SDS-Polyacrylamide Gel Electrophoresis. J. Food Sci. 1984, 49, 292–293. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.S.; Sonon, R.N., Jr.; Fontenot, J.P.; Clapham, W.M.; Scaglia, G. Effects of winter stocker growth rate and finishing system on: II. Ninth-tenth-eleventh-rib composition, muscle color, and palatability. J. Anim. Sci. 2007, 85, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Intramuscular connective tissue and its role in meat quality. Meat Sci. 2005, 70, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C.R.; Sullivan, G. Ranking of Beef Muscles for Tenderness; University of Nebraska: Lincoln, NE, USA, 2007; pp. 1–5. [Google Scholar]
- Archile-Contreras, A.C.; Cha, M.C.; Mandell, I.B.; Miller, S.P.; Purslow, P.P. Vitamins E and C may increase collagen turnover by intramuscular fibroblasts. Potential for improved meat quality. J. Agric. Food Chem. 2011, 59, 608–614. [Google Scholar] [CrossRef] [PubMed]
| Nutrient Composition 2 | CC | CON | CC | CON | Finishing |
|---|---|---|---|---|---|
| day 0–14 | day 15–44 | day 106–231 | |||
| Crude Protein | 13.31 | 17.02 | 13.06 | 16.10 | 13.73 |
| NEm, Mcal/cwt 3 | 60.15 | 71.62 | 60.11 | 72.35 | 86.03 |
| NEg, Mcal/cwt 4 | 34.34 | 44.93 | 34.25 | 45.26 | 58.14 |
| Variable | CC | CON | SEM 2 | p-Value |
|---|---|---|---|---|
| Cook loss, % | 17.56 | 18.78 | 0.528 | 0.114 |
| Tenderness 3 | 68.24 | 74.00 | 4.389 | 0.346 |
| Juiciness 3 | 97.28 | 92.22 | 4.552 | 0.428 |
| Flavor 3 | 80.85 | 86.16 | 3.054 | 0.204 |
| Insoluble collagen, mg/g | 1.95 | 2.02 | 0.041 | 0.111 |
| Heat soluble collagen, mg/g | 0.25 | 0.24 | 0.015 | 0.909 |
| Total collagen, mg/g | 2.20 | 2.26 | 0.049 | 0.189 |
| Variable | 3 | 7 | 14 | 21 | 21 | SEM 2 | p-Value |
|---|---|---|---|---|---|---|---|
| Cook loss, % | 17.86 b | 17.27 b | 18.21 ab | 19.36 a | 19.36 a | 0.579 | 0.042 |
| Intact desmin (55 kDa) 3 | 1.4994 a | 1.0025 b | 0.7607 c | 0.6053 d | 0.6053 d | 0.064 | <0.001 |
| Intact troponin-T (37 kDa) 3 | 1.3437 a | 0.9993 b | 0.6769 c | 0.5887 c | 0.5887 c | 0.045 | <0.001 |
| Degraded troponin-T (28 kDa) 3 | 0.6758 c | 1.0216 b | 1.0786 b | 1.2881 a | 1.2881 a | 0.074 | <0.001 |
| Inactive calpain-1 (80 kDa) 4, % | 13.46 | 7.45 | - | - | - | 0.899 | <0.001 |
| Active calpain-1 (78 kDa) 4, % | 34.21 | 30.03 | - | - | - | 0.735 | <0.001 |
| Previously active calpain-1 (76 kDa) 4, % | 52.22 | 62.47 | - | - | - | 1.373 | <0.001 |
| Variable | CC | CON | SEM 2 | p-Value |
|---|---|---|---|---|
| Intact desmin (55 kDa) 3 | 0.9262 | 1.0076 | 0.047 | 0.215 |
| Intact troponin-T (37 kDa) 3 | 0.8926 | 0.9117 | 0.032 | 0.673 |
| Degraded troponin-T (28 kDa) 3 | 1.0589 | 0.9781 | 0.052 | 0.314 |
| Inactive calpain-1 (80 kDa) 4, % | 9.99 | 10.92 | 0.899 | 0.470 |
| Active calpain-1 (78 kDa) 4, % | 31.71 | 32.52 | 0.735 | 0.444 |
| Previously active calpain-1 (76 kDa) 4, % | 58.24 | 56.44 | 1.373 | 0.361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, C.; Hite, L.; Wright, C.; Smart, A.; Dinh, T.; Blair, A.; Underwood, K.; Grubbs, J.K. Impact of Feeding Cover Crop Forage Containing Brassicas to Steers during Backgrounding on Palatability Attributes of Beef Strip Steaks. Foods 2021, 10, 1250. https://doi.org/10.3390/foods10061250
Bakker C, Hite L, Wright C, Smart A, Dinh T, Blair A, Underwood K, Grubbs JK. Impact of Feeding Cover Crop Forage Containing Brassicas to Steers during Backgrounding on Palatability Attributes of Beef Strip Steaks. Foods. 2021; 10(6):1250. https://doi.org/10.3390/foods10061250
Chicago/Turabian StyleBakker, Christina, Lydia Hite, Cody Wright, Alexander Smart, Thu Dinh, Amanda Blair, Keith Underwood, and J. Kyle Grubbs. 2021. "Impact of Feeding Cover Crop Forage Containing Brassicas to Steers during Backgrounding on Palatability Attributes of Beef Strip Steaks" Foods 10, no. 6: 1250. https://doi.org/10.3390/foods10061250
APA StyleBakker, C., Hite, L., Wright, C., Smart, A., Dinh, T., Blair, A., Underwood, K., & Grubbs, J. K. (2021). Impact of Feeding Cover Crop Forage Containing Brassicas to Steers during Backgrounding on Palatability Attributes of Beef Strip Steaks. Foods, 10(6), 1250. https://doi.org/10.3390/foods10061250







