Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
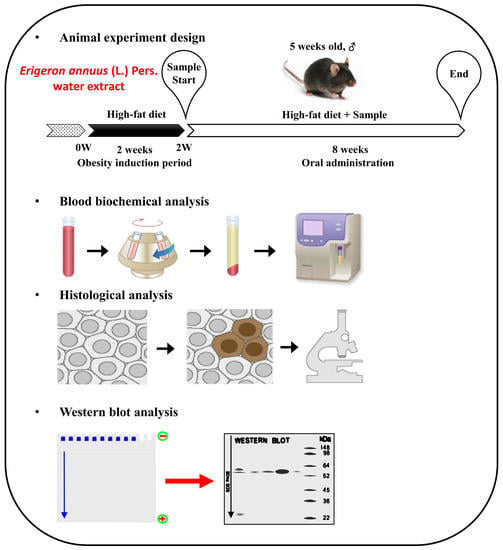
2.2. Experimental Animals
2.3. Measurement of Serum Biochemical Parameters
2.4. RNA Extraction and Real-Time PCR
2.5. Analysis of Protein Level
2.6. Adipose Histological Analysis
2.7. LC-ESI-MS and LC-ESI-MS/MS Analysis
2.8. Statistical Analysis
3. Results
3.1. EAW Prevents Body Weight Gain
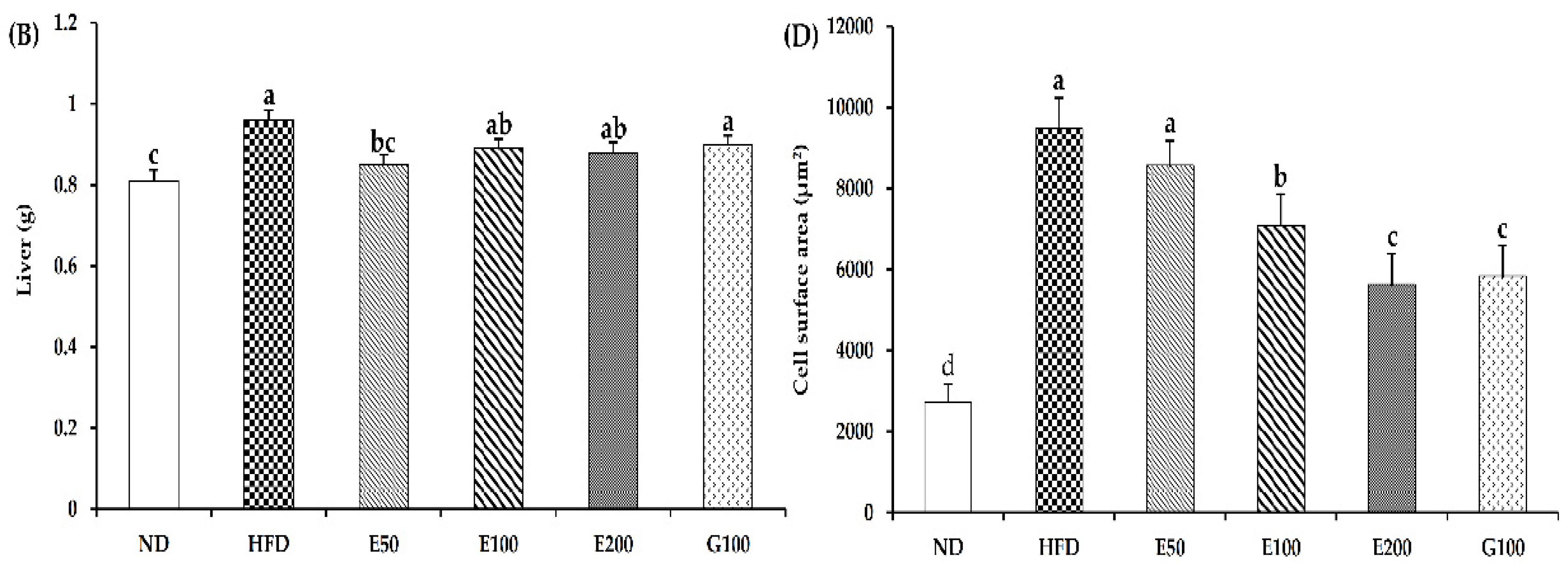
3.2. Effect of EAW on Epididymal Adipose Tissue and Liver Mass Gain
3.3. Effect of EAW on Serum Parameters
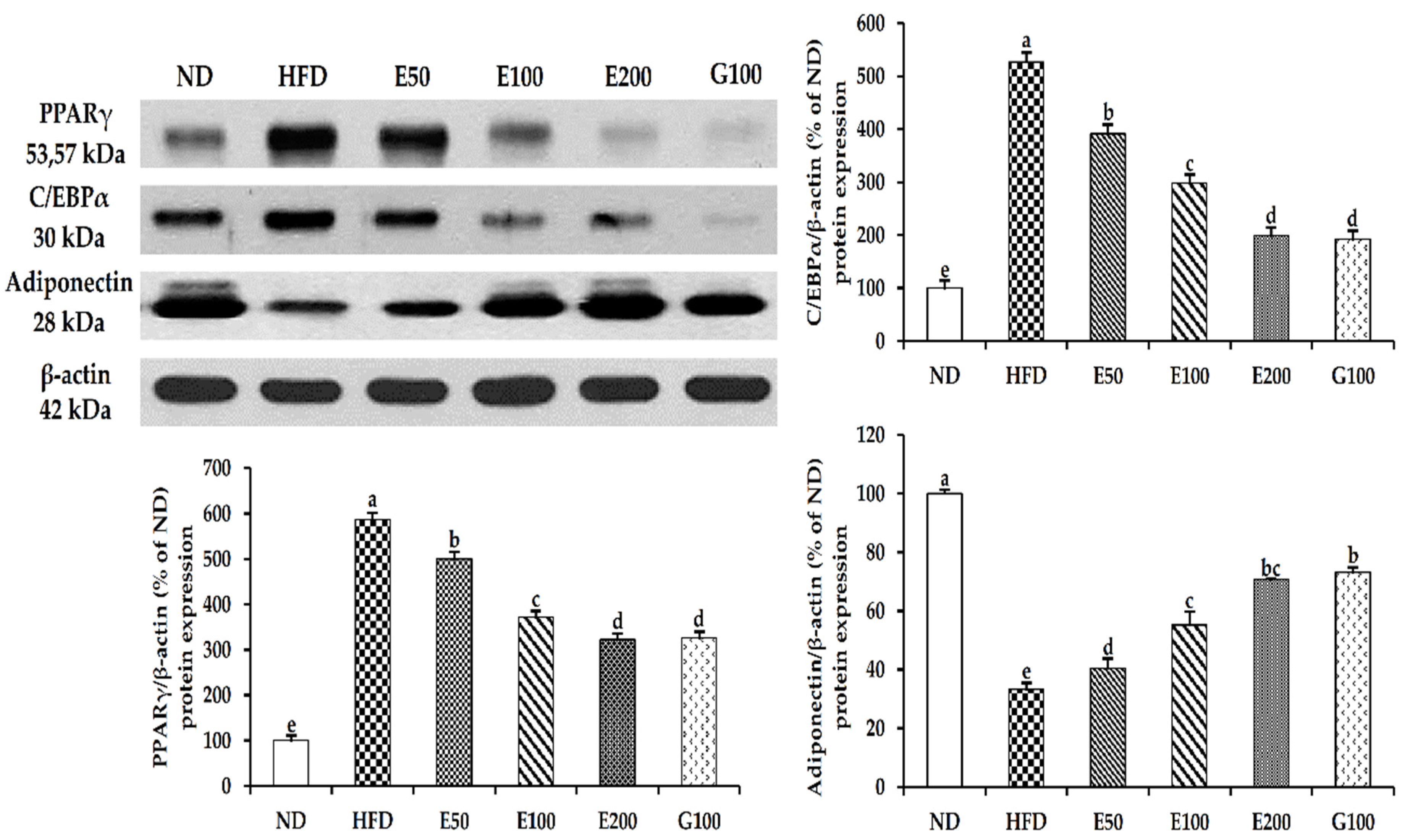
3.4. Effect of EAW on Adipogenic and Lipogenic Genes in Epididymal Adipose Tissue and Liver
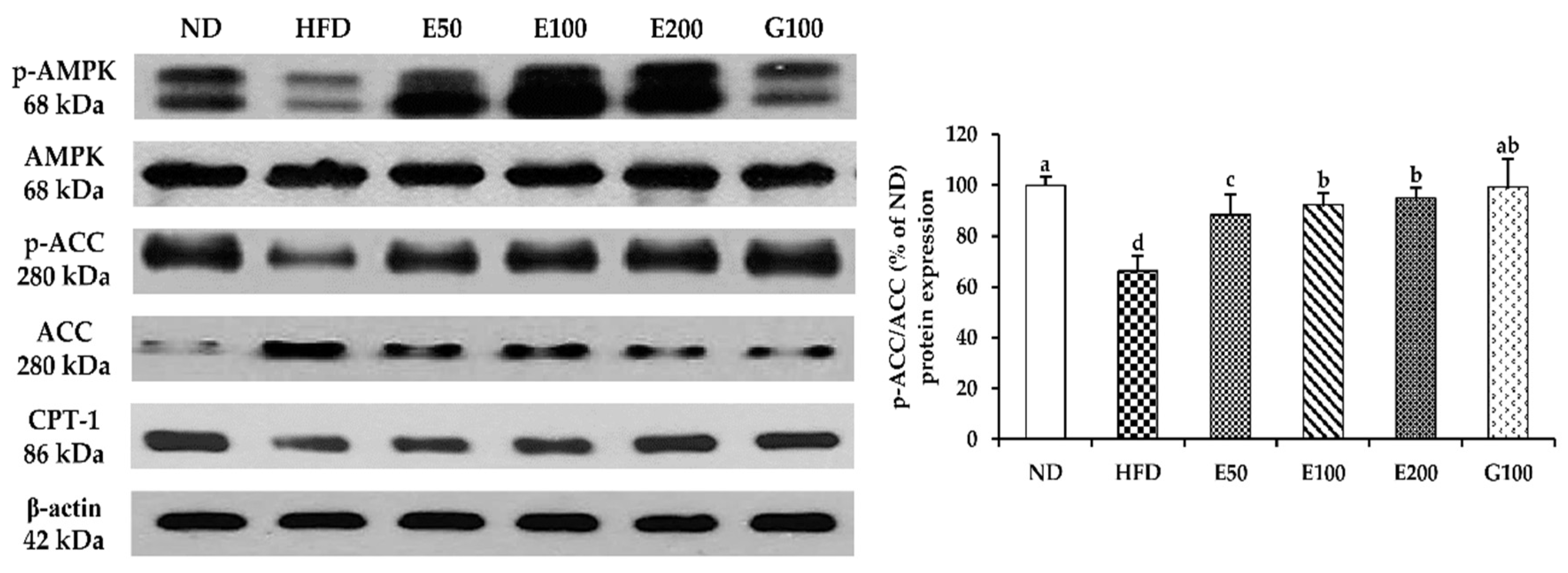
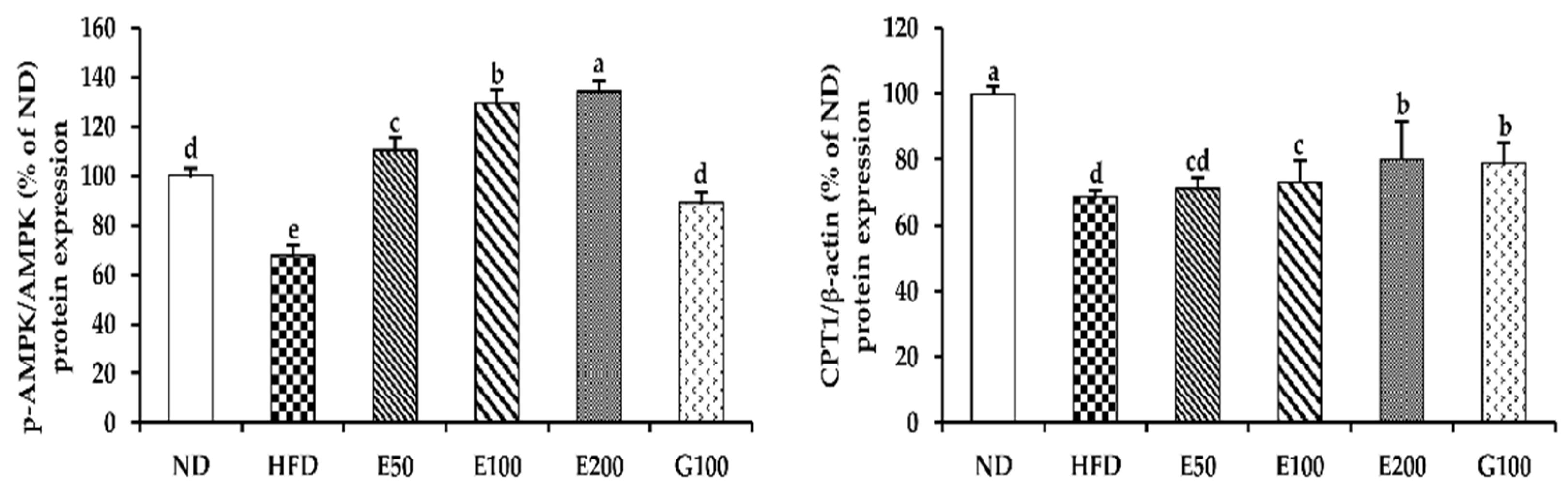
3.5. The AMPK Pathway of Activation by EAW in Epididymis Adipose Tissue
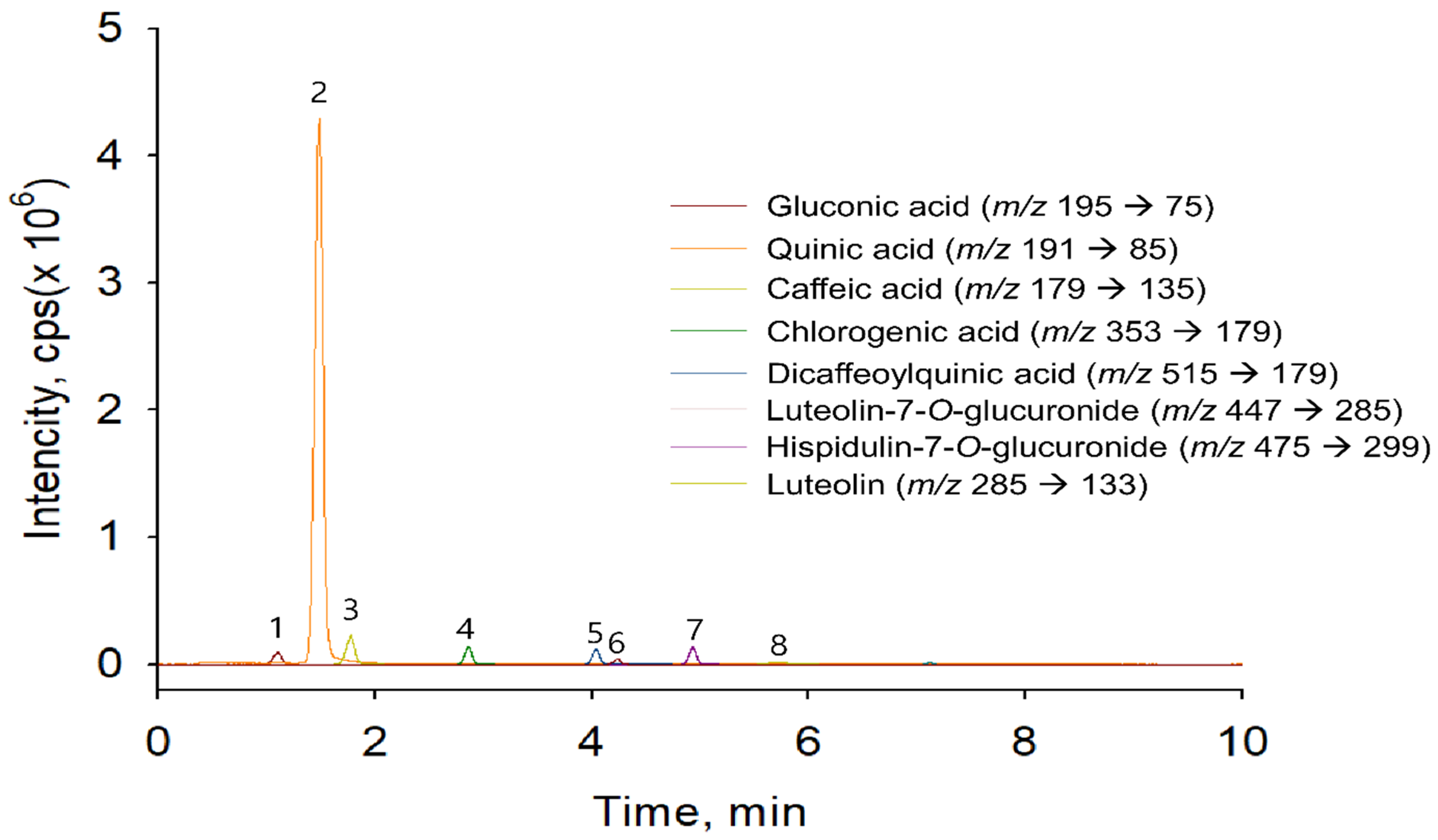
3.6. Identification of Phenolic Acids and Flavonoids in EAW
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal medicine for the treatment of obesity: An overview of scientific evidence from 2007 to 2017. Evid. Based Complement. Alternat. Med. 2017, 2017, 8943059. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ashford, M.L. AMPK: Regulating energy balance at the cellular and whole body levels. Physiology 2014, 29, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar]
- Jang, D.S.; Yoo, N.H.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.S. Constituents of the flowers of Erigeron annuus with inhibitory activity on the formation of advanced glycation end products (AGEs) and aldose reductase. Arch. Pharm. Res. 2008, 31, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Kalemba, D. Chemical Composition of the Essential Oils from the Roots of Erigeron acris L. and Erigeron annuus (L.) Pers. Molecules 2009, 14, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.H.; Jang, D.S.; Yoo, J.L.; Lee, Y.M.; Kim, Y.S.; Cho, J.-H.; Kim, J.S. Erigeroflavanone, a flavanone derivative from the flowers of Erigeron annuus with protein glycation and aldose reductase inhibitory activity. J. Nat. Prod. 2008, 71, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A. Study approach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, Y. Antioxidant properties of Erigeron annuus extract and its three phenolic constituents. Biotechnol. Bioprocess Eng. 2006, 11, 13–18. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Jeong, H.R.; Choi, G.N.; Kim, D.-O.; Lee, U.; Heo, H.J. Neuroprotective and anti-oxidant effects of caffeic acid isolated from Erigeron annuus leaf. Chin. Med. 2011, 6, 1–9. [Google Scholar] [CrossRef]
- Jo, M.J.; Lee, J.R.; Cho, I.J.; Kim, Y.W.; Kim, S.C. Roots of Erigeron annuus Attenuate Acute Inflammation as Mediated with the Inhibition of NF- kappa B-Associated Nitric Oxide and Prostaglandin E2 production. Evid-Based Compl. Alt. Med. 2013, 2013, 297427. [Google Scholar]
- Zheng, Y.; Lee, J.; Lee, E.-h.; In, G.; Kim, J.; Lee, M.-H.; Lee, O.-H.; Kang, I.-J. A Combination of Korean Red Ginseng Extract and Glycyrrhiza glabra L. Extract Enhances Their Individual Anti-Obesity Properties in 3T3-L1 Adipocytes and C57BL/6J Obese Mice. J. Med. Food 2020, 23, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lee, E.-H.; Lee, J.-H.; In, G.; Kim, J.; Lee, M.-H.; Lee, O.-H.; Kang, I.-J. Preclinical Research on a Mixture of Red Ginseng and Licorice Extracts in the Treatment and Prevention of Obesity. Nutrients 2020, 12, 2744. [Google Scholar] [CrossRef]
- Li, S.; Lin, Z.; Jiang, H.; Tong, L.; Wang, H.; Chen, S. Rapid identification and assignation of the active ingredients in fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. J. Chromatogr. Sci. 2016, 54, 1225–1237. [Google Scholar] [CrossRef]
- Felipe, D.F.; Brambilla, L.Z.; Porto, C.; Pilau, E.J.; Cortez, D.A. Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules 2014, 19, 15720–15734. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe J. Med. Sci. 2018, 64, E84–E92. [Google Scholar]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic acid promotes development of brown adipocyte-like phenotype in 3T3-L1 adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Randy, A.; Kim, M.; Nho, C.W. Ligularia fischeri and its constituent 3,4-dicaffeoylquinic acid improve obesity-induced nonalcoholic fatty liver disease by regulating lipid metabolism and activating AMPK. J. Funct. Foods 2016, 27, 1–16. [Google Scholar] [CrossRef]
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef]
- Xu, X.; So, J.-S.; Park, J.-G.; Lee, A.-H. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin. Liver Dis. 2013, 33, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fernand, V.E.; Henagan, T.M.; Shin, J.; Huypens, P.; Newman, S.; Gettys, T.W.; Chang, J.S. Regulation of brown and white adipocyte transcriptome by the transcriptional coactivator NT-PGC-1α. PLoS ONE 2016, 11, e0159990. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Yoon, H.E.; Shin, S.J.; Choi, B.S.; Kim, Y.-S.; Chang, Y.S.; Park, C.W. The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J. Am. Soc. Nephrol. 2018, 29, 1108–1127. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases Thematic Review Series: New Lipid and Lipoprotein Targets for the Treatment of Cardiometabolic Diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed]
- Zaha, V.G.; Young, L.H. AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Ha, J.-H.; Jang, J.; Chung, S.-I.; Yoon, Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int. J. Mol. Med. 2016, 37, 816–824. [Google Scholar] [CrossRef]
- Lindholm, C.R.; Ertel, R.L.; Bauwens, J.D.; Schmuck, E.G.; Mulligan, J.D.; Saupe, K.W. A high-fat diet decreases AMPK activity in multiple tissues in the absence of hyperglycemia or systemic inflammation in rats. J. Physiol. Biochem. 2013, 69, 165–175. [Google Scholar] [CrossRef]
- Bertrand, L.; Ginion, A.; Beauloye, C.; Hebert, A.D.; Guigas, B.; Hue, L.; Vanoverschelde, J.-L. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J. Physiol. Heart Circ. Physiol. Physiol. 2006, 291, H239–H250. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef]


| Gene Name | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| PPARγ | NM_011146 | 5’-CGCTGATGCACTGCCTATGA-3’ | 5’-AGAGGTCCACAGAGCTGATTCC-3’ |
| C/EBPα | BC058161 | 5’-CGCAAGAGCCGAGATAAAGC-3’ | 5’-CACGGCTCAGCTGTTCCA-3’ |
| aP2 | NM_024406 | 5’-CATGGCCAAGCCCAACAT-3’ | 5’-CGCCCAGTTTGAAGGAAATC-3’ |
| FAS | NM_007988 | 5’-CTGAGATCCCAGCACTTCTTGA-3’ | 5’-GCCTCCGAAGCCAAATGAG-3’ |
| PGC-1α | NM_008904 | 5’-TGTTCCCGATCACCATATTCC-3’ | 5’-GGTGTCTGTAGTGGCTTGATTC-3’ |
| Adiponectin | NM_009605 | 5’-ATCCACACGTGTACTCAC-3’ | 5’-AGCATGGTCTACTTCCAG-3’ |
| SREBP-1c | NM_011480.3 | 5’-GGACGAGCTGGCCTTCGGTGA-3’ | 5’-ATAGGGGGCGTCAAACAGGCCC-3’ |
| GAPDH | BC083080 | 5’-GTATGACTCCACTCACGGCAAA-3’ | 5’-GGTCTCGCTCCTGGAAGATG-3’ |
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| ND | HFD | E50 | E100 | E200 | G100 | |
| Initial body weight (g) | 24.06 ± 0.19 a | 24.16 ± 0.38 a | 24.08 ± 0.21 a | 24.09 ± 0.39 a | 24.09 ± 0.23 a | 24.11 ± 0.21 a |
| Final body weight (g) | 33.03 ± 1.21 d | 47.06 ± 2.25 a | 43.26 ± 1.51 b | 42.04 ± 1.78 b | 39.05 ± 1.51 c | 40.08 ± 1.89 c |
| Body weight gain (g) | 8.97 ± 1.47 d | 22.09 ± 2.29 a | 19.18 ± 0.63 b | 17.95 ± 1.54 b | 14.96 ± 1.49 c | 15.97 ± 1.74 c |
| Serum | Group | |||||
|---|---|---|---|---|---|---|
| ND | HFD | E50 | E100 | E200 | G100 | |
| TC (mg/dL) | 109.73 ± 4.01 c | 169.69 ± 2.212 a | 152.31 ± 9.89 ab | 152.23 ± 10.03 ab | 145.60 ± 5.165 b | 142.28 ± 1.235 b |
| TG (mg/dL) | 57.63 ± 3.77 c | 94.71 ± 4.80 a | 91.82 ± 3.41 a | 87.98 ± 3.22 ab | 75.66 ± 6.59 b | 76.42 ± 8.90 b |
| HDL-C (mg/dL) | 78.249 ± 1.24 b | 116.13 ± 3.21 a | 119.88 ± 3.32 a | 121.04 ± 4.12 a | 138.82 ± 2.99 a | 139.07 ± 3.11 a |
| LDL-C (mg/dL) | 10.06 ± 1.72 c | 31.24 ± 2.11 a | 31.14 ± 2.76 a | 29.87 ± 1.86 a | 19.39 ± 1.96 b | 20.31 ± 1.24 b |
| AST (U/L) | 127.43 ± 21.23 a | 119.04 ± 11.25 a | 119.64 ± 27.06 a | 127.22 ± 18.33 a | 95.19 ± 6.34 a | 95.16 ± 4.33 a |
| ALT (U/L) | 31.96 ± 3.45 a | 43.24 ± 2.11 a | 41.76 ± 3.25 a | 39.21 ± 2.31 a | 28.93 ± 1.66 a | 28.31 ± 1.32 a |
| GLU (mg/dL) | 146.38 ± 4.12 c | 178.13 ± 3.99 a | 165.09 ± 10.01 ab | 170.88 ± 9.74 ab | 150.65 ± 4.55 b | 155.34 ± 5.23 b |
| Peak | Retention Time (min) | [M − H]− | Mass Fragmentation | Compound Name | References |
|---|---|---|---|---|---|
| 1 | 1.11 | 195 | 177, 147, 129, 111, 99, 75 | Gluconic acid | Li et al. [19] Felipe et al. [20] |
| 2 | 1.52 | 191 | 173, 171, 127, 109, 93, 85, 65 | Quinic acid | Fathoni et al. [21] |
| 3 | 1.78 | 179 | 135, 107, 89, 59 | Caffeic acid | Li et al. [19] |
| 4 | 2.86 | 353 | 191, 179, 161, 135, 93, 85 | Chlorogenic acid | Li et al. [19] |
| 5 | 4.04 | 515 | 353, 85, 93, 135, 173, 179, 191, 161 | 3,4-Dicaffeoylquinic acid | Simirgiotis et al. [22] |
| 6 | 4.34 | 461 | 285, 133, 151, 175 | Luteolin-7-O-glucuronide | Li et al. [19] |
| 7 | 4.95 | 475 | 299, 284, 211, 227, 255 | Hispidulin-7-O-glucuronide | Li et al. [19] |
| 8 | 7.11 | 285 | 241, 217, 199, 175, 151, 133 | Luteolin | Fathoni et al. [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Choi, Y.-H.; Lee, J.-H.; Lee, S.-Y.; Kang, I.-J. Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids. Foods 2021, 10, 1266. https://doi.org/10.3390/foods10061266
Zheng Y, Choi Y-H, Lee J-H, Lee S-Y, Kang I-J. Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids. Foods. 2021; 10(6):1266. https://doi.org/10.3390/foods10061266
Chicago/Turabian StyleZheng, Yulong, Yoon-Hee Choi, Ji-Hyun Lee, So-Yeon Lee, and Il-Jun Kang. 2021. "Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids" Foods 10, no. 6: 1266. https://doi.org/10.3390/foods10061266
APA StyleZheng, Y., Choi, Y.-H., Lee, J.-H., Lee, S.-Y., & Kang, I.-J. (2021). Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids. Foods, 10(6), 1266. https://doi.org/10.3390/foods10061266