Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Raw Material
2.2. Superheated Steam Treatment of Whole Wheat Flour
2.3. Preparation and Storage Experiment of Dried Whole Wheat Noodles
2.4. Determination of Lipase, Lipoxygenase and Peroxidase Activity
2.4.1. Determination of Lipase Activity
2.4.2. Determination of Lipoxygenase Activity
2.4.3. Determination of Peroxidase Activity
2.5. Extraction of Lipids from Dried Whole Wheat Noodles
2.6. Determination of Fatty Acid Composition
2.7. Determination of Tocopherols and Tocotrienols Content
2.8. Characterization of Volatile Compounds
2.9. Determination of Fatty Acid Value
2.10. Statistical Analysis
3. Results and Discussion
3.1. Changes of Enzyme Activity in Dried Whole Wheat Noodles during Storage
3.2. Changes of Fatty Acid Value in Dried Whole Wheat Noodles Storage
3.3. Changes of Fatty Acid Composition in Dried Whole Wheat Noodles during Storage
3.4. Changes of Tocopherols and Tocotrienols Content in Dried Whole Wheat Noodles during Storage
3.5. Changes of Volatile Compounds in Dried Whole Wheat Noodles during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.N.; Wu, S.H.; Zhu, K.X. Effect of superheated steam treatment on quality characteristics of whole wheat flour and storage stability of semi-dried whole wheat noodle. Food Chem. 2020, 322, 126738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Sang, S.M. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Hou, G.G. Whole wheat noodle: Processing, quality improvement, and nutritional and health benefits. Cereal Chem. 2019, 96, 23–33. [Google Scholar] [CrossRef]
- Luthria, D.L.; Lu, Y.J.; John, K.M.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Lampi, A.M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and tocotrienols in wheat genotypes in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Mufari, J.R.; Gorostegui, H.; Miranda, P.; Bergesse, A.E.; Calandri, E. Oxidative Stability and Characterization of Quinoa Oil Extracted from Wholemeal and Germ Flours. J. Am. Oil Chem. Soc. 2020, 97, 12308. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Yu, X.L.; Wang, Z.H.; Zhang, Y.Q.; Wadood, S.A.; Wei, Y.M. Study on the water state and distribution of Chinese dried noodles during the drying process. J. Food Eng. 2018, 233, 81–87. [Google Scholar] [CrossRef]
- Doblado-Maldonado, A.F.; Pike, O.A.; Sweley, J.C.; Rose, D.J. Key issues and challenges in whole wheat flour milling and storage. J. Cereal Sci. 2012, 56, 119–126. [Google Scholar] [CrossRef]
- Poudel, R.; Rose, D.J. Changes in enzymatic activities and functionality of whole wheat flour due to steaming of wheat kernels. Food Chem. 2018, 263, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT-Food Sci. Technol. 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Lei, L.; Wen, W.; Zhang, R.; Wei, Z.; Deng, Y.; Xiao, J.; Zhang, M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017, 214, 1–8. [Google Scholar] [CrossRef]
- Bhat, N.A.; Wani, I.A.; Hamdani, A.M.; Gani, A.; Masoodi, F.A. Physicochemical properties of whole wheat flour as affected by gamma irradiation. LWT-Food Sci. Technol. 2016, 71, 175–183. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Li, Z. Superheated steam treatment on wheat bran: Enzymes inactivation and nutritional attributes retention. LWT-Food Sci. Technol. 2018, 91, 446–452. [Google Scholar] [CrossRef]
- Wu, J.; McClements, D.J.; Chen, J.; Liu, W.; Luo, S.; Liu, C. Improvement in storage stability of lightly milled rice using superheated steam processing. J. Cereal Sci. 2016, 71, 130–137. [Google Scholar] [CrossRef]
- Alfy, A.; Kiran, B.V.; Jeevitha, G.C.; Hebbar, H.U. Recent developments in superheated steam processing of foods-A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2191–2208. [Google Scholar] [CrossRef]
- Head, D.; Cenkowski, S.; Arntfield, S.; Henderson, K. Storage stability of oat groats processed commercially and with superheated steam. LWT-Food Sci. Technol. 2011, 44, 261–268. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.Y.; Xu, X.F.; Guo, B.Z.; Yang, R.; Chen, J.; Zhong, Y.J.; Luo, S.J.; Xu, J.G.; Wu, J.Y. Changes in Granular Swelling and Rheological Properties of Food Crop Starches Modified by Superheated Steam. Starch-Starke 2019, 71, 1800132. [Google Scholar] [CrossRef]
- Hu, Y.M.; Nie, W.; Hu, X.Z.; Li, Z.G. Microbial decontamination of wheat grain with superheated steam. Food Control 2016, 62, 264–269. [Google Scholar] [CrossRef]
- Sehrawat, R.; Nema, P.K.; Kaur, B.P. Effect of superheated steam drying on properties of foodstuffs and kinetic modeling. Innov. Food Sci. Emerg. Technol. 2016, 34, 285–301. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. American Association of Cereal Chemists Approved Methods, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Cai, J.; Xie, Y.; Song, B.; Wang, Y.; Zhang, Z.; Feng, Y. Fervidobacterium changbaicum Lip1: Identification, cloning, and characterization of the thermophilic lipase as a new member of bacterial lipase family V. Appl. Microbiol. Biotechnol. 2011, 89, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Cato, L.; Halmos, A.L.; Small, D.M. Measurement of lipoxygenase in Australian white wheat flour: The effect of lipoxygenase on the quality properties of white salted noodles. J. Sci. Food Agr. 2010, 86, 1670–1678. [Google Scholar] [CrossRef]
- Sessa, D.J.; Anderson, R.L. Soybean peroxidases: Purification and some properties. J. Agric. Food Chem. 1981, 29, 960–965. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Pulkkinen, M.; Wang, Y.J.; Lampi, A.M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT-Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Yao, L.; Schaich, K.M. Accelerated solvent extraction improves efficiency of lipid removal from dry pet food while limiting lipid oxidation. J. Am. Oil Chem. Soc. 2014, 92, 141–151. [Google Scholar] [CrossRef]
- Shin, T.S.; Godber, J.S. Changes of endogenous antioxidants and fatty acid composition in irradiated rice bran during storage. J. Agric. Food Chem. 1996, 44, 567–573. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Summo, C.; Trani, A.; Caponio, F. An effort to improve the shelf life of breakfast cereals using natural mixed tocopherols. J. Cereal Sci. 2008, 47, 322–330. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, S.; Qiu, C.; Xu, Y. Effect of microwaves on rice quality. J. Stored Prod. Res. 2007, 43, 496–502. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, J.; Liu, W.; Liu, C.M.; Zhong, Y.J.; Luo, D.W.; Li, Z.Q.; Huang, Z.L. Selective peroxidase inactivation of lightly milled rice by superheated steam. J. Cereal Sci. 2014, 60, 623–630. [Google Scholar] [CrossRef]
- Akyol, C.; Alpas, H.; Bayindirli, A. Inactivation of peroxidase and lipoxygenase in carrots, green beans, and green peas by combination of high hydrostatic pressure and mild heat treatment. Eur. Food Res. Technol. 2006, 224, 171–176. [Google Scholar] [CrossRef]
- Lusena, C.V.; McFarlane, W.D. Studies on the processing of wheat germ. Can. J. Res. 1945, 23, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhou, S.L.; Miao, W.J.; Gao, C.; Cai, M.J.; Dong, Y. Study on the stabilization effect of continuous microwave on wheat germ. J. Food Eng. 2013, 117, 1–7. [Google Scholar] [CrossRef]
- Kapranchikov, V.S.; Zherebtsov, N.A.; Popova, T.N. Purification and Characterization of Lipase from Wheat (Triticum aestivum L.) Germ. Appl. Biochem. Micro. 2004, 40, 84–88. [Google Scholar] [CrossRef]
- Wallace, J.M.; Wheeler, E.L. Two lipoxygenase isoenzymes and an activator in wheat germ. Phytochemistry 1979, 18, 389–393. [Google Scholar] [CrossRef]
- Tome, D.; Nicolas, J.; Drapron, R. Influence of water activity on the reaction catalyzed by polyphenoloxidase (E.C.1.14.18.1.) from mushrooms in organic liquid media. LWT Lebensmitt Wissensch Technol. 1978, 11, 38–41. [Google Scholar]
- Lai, H.M.; Hwang, S.C. Water status of cooked white salted noodles evaluated by MRI. Food Res. Int. 2004, 37, 957–966. [Google Scholar] [CrossRef]
- Xu, B.; Miao, W.J.; Hu, Q.S.; Gao, C.; Dong, Y. A modified colorimetric method for determining the activity of wheat germ lipase in low-aqueous media. Qual. Assur. Saf. Crops 2013, 5, 113–118. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Theorell, H.; Holman, R.T.; Akeson, A. A note on the preparation of crystalline soy bean lipoxidase. Arch. Biochem. 1947, 14, 250–252. [Google Scholar] [PubMed]
- Rose, D.J.; Ogden, L.V.; Dunn, M.L.; Pike, O.A. Enhanced Lipid Stability in Whole Wheat Flour by Lipase Inactivation and Antioxidant Retention. Cereal Chem. 2008, 85, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Aibara, S.; Ismail, I.A.; Yamashita, H.; Ohta, H.; Sekiyama, F.; Morita, Y. Changes in rice bran lipids and free amino acids during storage. Agric. Biol. Chem. 1986, 50, 665–673. [Google Scholar] [CrossRef]
- Obadi, M.; Zhu, K.X.; Peng, W.; Noman, A.; Mohammed, K.; Zhou, H.M. Characterization of oil extracted from whole grain flour treated with ozone gas. J. Cereal Sci. 2018, 79, 527–533. [Google Scholar] [CrossRef]
- Schaich, K.M.; Shahidi, F.; Zhong, Y.; Eskin, N.A.M. Lipid Oxidation. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 419–478. [Google Scholar]
- Lampi, A.M.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimecheva, I.P. Flaxseed oil stabilization using natural and synthetic antioxidants: Flaxseed oil stabilization using antioxidants. Eur. J. Lipid Sci. Tech. 2017, 119, 1700079. [Google Scholar] [CrossRef]
- Aguiar, C.M.; Sampaio, S.C.; Santos, K.A.; Silva, E.A.; Piana, P.; Richart, A.; Reis, R.R. Total fatty acid content, antioxidant composition, antioxidant activity, and content of oil from crambe seeds cultivated with phosphorus. Eur. J. Lipid Sci. Tech. 2017, 119, 1700043. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Andersson, R. A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J. Am. Oil Chem. Soc. 1997, 74, 375–380. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Lee, M.J.; Kim, S.S. Quality predictive models for whole flour of immature wheat during storage and consumer acceptance on its baked product. LWT-Food Sci. Technol. 2017, 83, 42–49. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME-GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, Y.; Pan, Q.F.; Fan, M.C.; Wang, L.; Qian, H.F. Analysis of the key aroma volatile compounds in rice bran during storage and processing via HS-SPME GC/MS. J. Cereal Sci. 2021, 99, 103178. [Google Scholar] [CrossRef]
- Ho, C.T.; Chen, Q. Lipids in food flavors: An overview. ACS Symp. Ser. 1994, 558, 2–14. [Google Scholar]
- Heimann, W.; Dresen, P.; Klaiber, V. Formation and decomposition of linoleic acid hydroperoxides in cereals quantitative determination of the reaction products. Z. Lebensm. Unters. Forsch. 1973, 153, 1–5. [Google Scholar] [CrossRef]
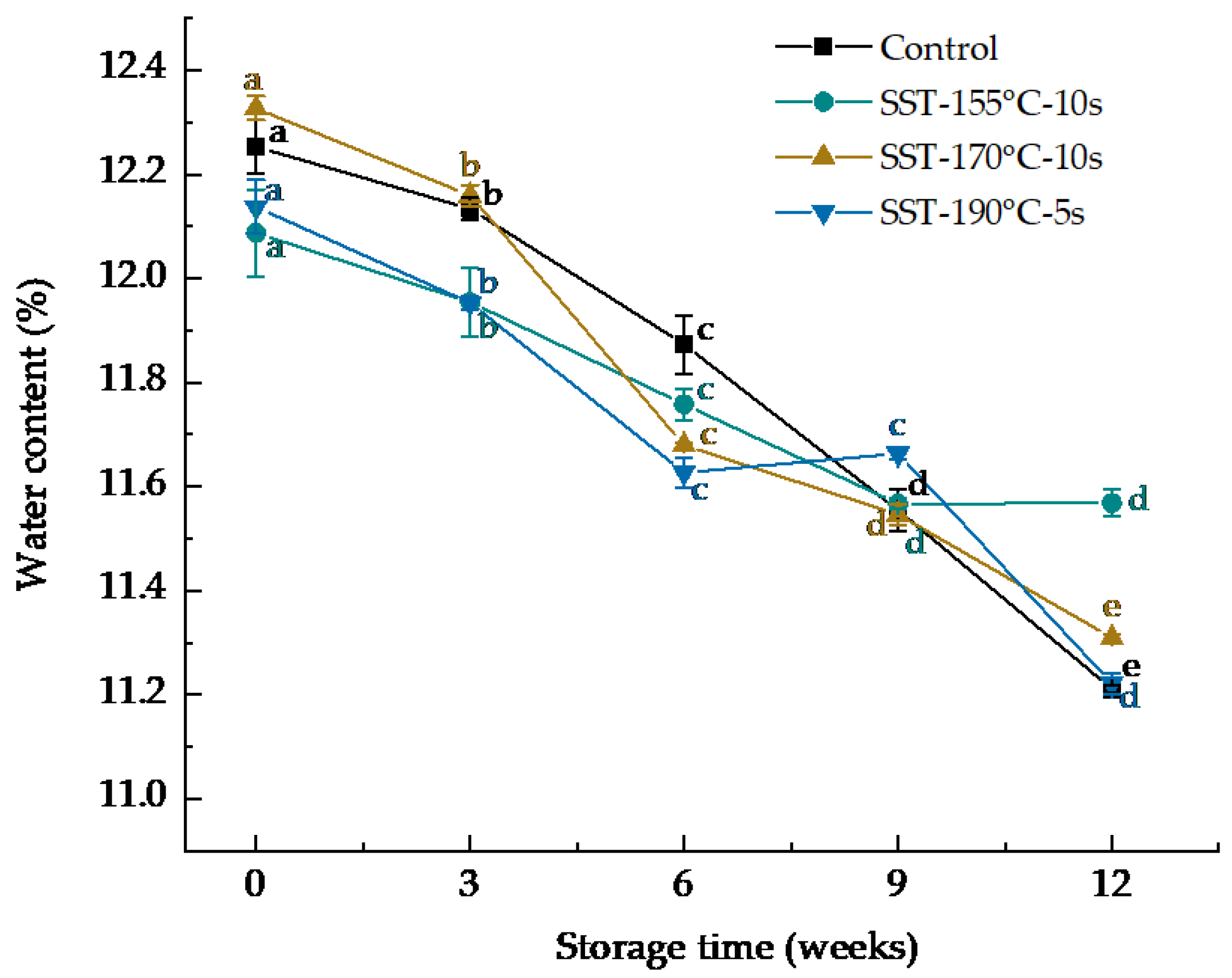
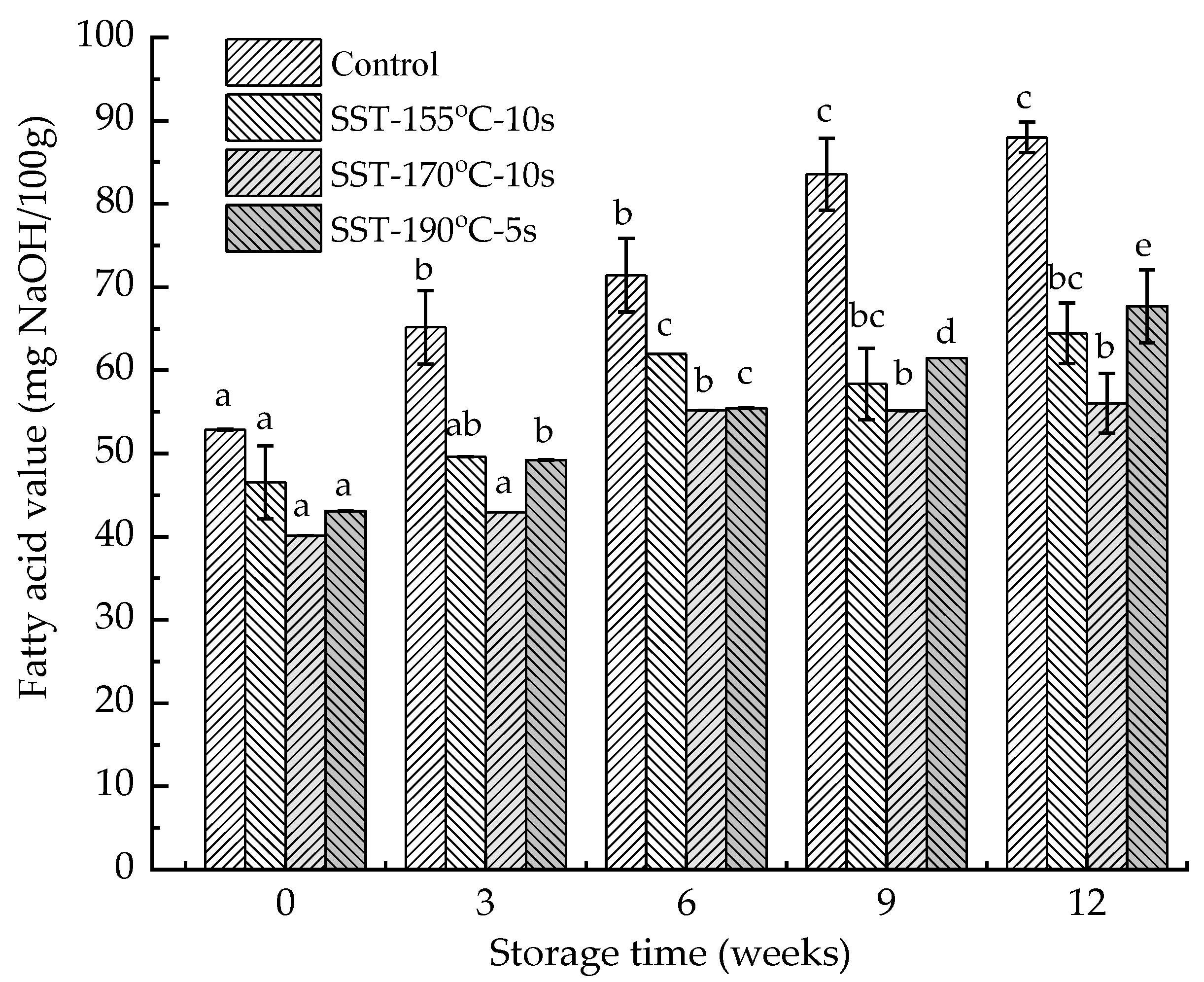

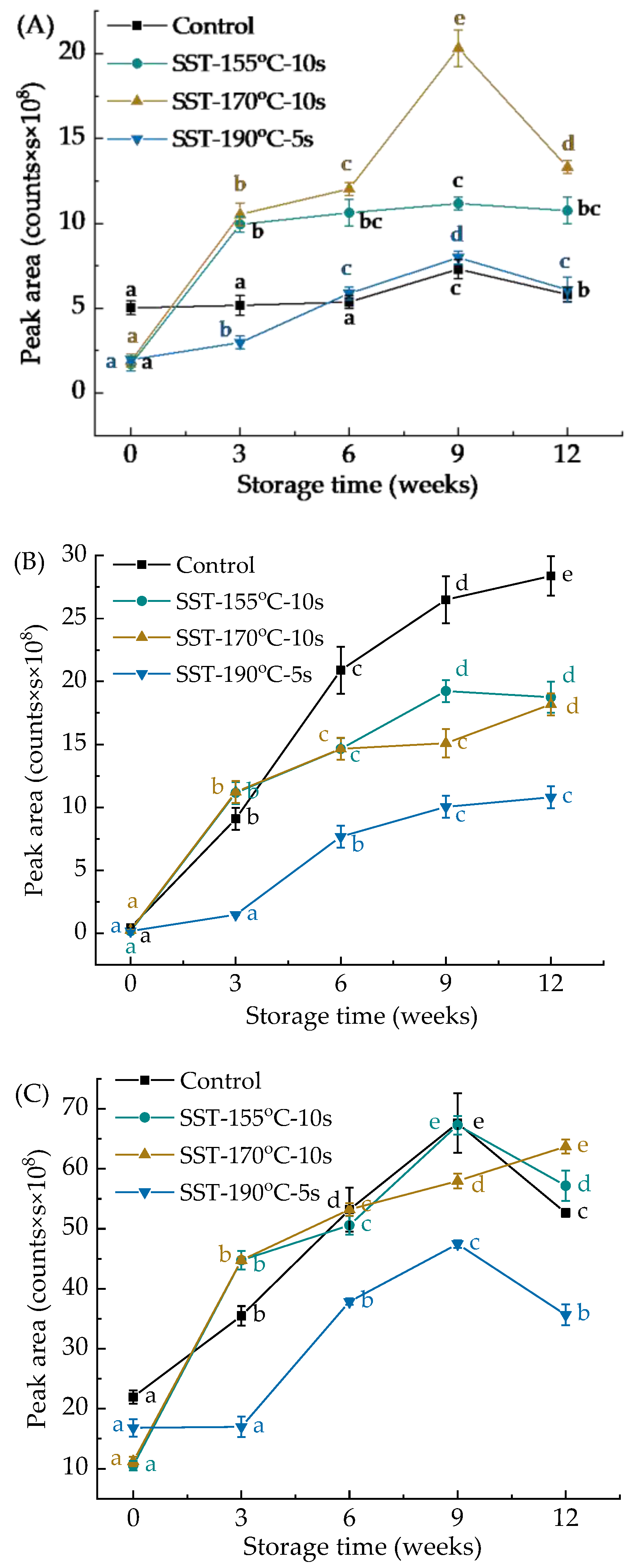
| Storage Time (Weeks) | Lipase Activity (U/g·min−1) | |||
|---|---|---|---|---|
| Control | SST-155 °C-10 s | SST-170 °C-10 s | SST-190 °C-5 s | |
| 0 | 46.60 ± 2.88 a,* | - | - | - |
| 3 | 34.11 ± 2.88 b | - | - | - |
| 6 | 28.70 ± 1.29 b,c | - | - | - |
| 9 | 24.40 ± 0.96 c,d | - | - | - |
| 12 | 21.21 ± 1.76 d | - | - | - |
| Storage Time (Weeks) | Lipoxygenase Activity (U/g·min−1) | |||
|---|---|---|---|---|
| Control | SST-155 °C-10 s | SST-170 °C-10 s | SST-190 °C-5 s | |
| 0 | 574.43 ± 35.32 a,* | 186.18 ± 20.76 | 176.17 ± 20.76 | 164.84 ± 7.06 |
| 3 | 217.87 ± 2.83 b | - | - | - |
| 6 | 51.97 ± 3.46 c | - | - | - |
| 9 | 49.83 ± 0.00 c | - | - | - |
| 12 | 39.98 ± 0.00 c | - | - | - |
| Storage Time (Weeks) | Peroxidase Activity (U/g·min−1) | |||
|---|---|---|---|---|
| Control | SST-155 °C-10 s | SST-170 °C-10 s | SST-190 °C-5 s | |
| 0 | 1412.36 ± 66.66 a,* | - | - | - |
| 3 | 668.21 ± 43.90 b | - | - | - |
| 6 | 579.85 ± 61.01 b,c | - | - | - |
| 9 | 514.56 ± 18.61 c | - | - | - |
| 12 | 583.79 ± 29.07 b,c | - | - | - |
| Sample/Storage Time (Weeks) | Fatty Acid Composition (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Palmitic Acid (C16:0) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | Linolenic Acid (C18:3) | Total SFAs | Total UFAs | Total FFAs | |
| Control | ||||||||
| 0 | 2.24 ± 0.01 A,a,* | 0.65 ± 0.00 A,a | 2.62 ± 0.00 B,a | 5.90 ± 0.01 C,a | 1.31 ± 0.00 B,a | 2.89 ± 0.01 A,a | 9.83 ± 0.02 B,a | 12.72 ± 0.03 B,a |
| 3 | 2.17 ± 0.05 B,b,c | 0.63 ± 0.00 B,b | 2.60 ± 0.05 B,a | 5.72 ± 0.05 C,b | 1.31 ± 0.01 B,a | 2.80 ± 0.05 B,b | 9.63 ± 0.04 C,b | 12.43 ± 0.03 C,b |
| 6 | 2.21 ± 0.03 A,a,b | 0.64 ± 0.01 A,b | 2.58 ± 0.10 A,a | 5.14 ± 0.03 B,c | 1.31 ± 0.02 B,a | 2.84 ± 0.03 A,a,b | 9.04 ± 0.09 B,c | 11.88 ± 0.12 B,c |
| 9 | 2.15 ± 0.02 D,b,c | 0.53 ± 0.00 B,c | 2.45 ± 0.01 D,b | 5.13 ± 0.05 D,c | 1.09 ± 0.00 C,b | 2.68 ± 0.02 D,c | 8.67 ± 0.06 D,d | 11.34 ± 0.09 D,d |
| 12 | 2.13 ± 0.03 D,c | 0.52 ± 0.01 C,c | 2.42 ± 0.04 C,b | 5.01 ± 0.03 D,d | 1.07 ± 0.02 C,b | 2.66 ± 0.02 D,c | 8.50 ± 0.03 D,e | 11.16 ± 0.05 D,e |
| SST-155 °C-10 s | ||||||||
| 0 | 2.27 ± 0.02 A,a | 0.65 ± 0.00 A,a | 2.77 ± 0.01 A,a | 7.76 ± 0.06 A,B,a | 1.42 ± 0.01 A,a | 2.92 ± 0.02 A,a | 11.95 ± 0.07 A,a | 14.87 ± 0.10 A,a |
| 3 | 2.23 ± 0.05 B,a | 0.64 ± 0.00 B,b | 2.69 ± 0.06 A,B,b | 7.43 ± 0.22 A,B,a,b | 1.39 ± 0.01 A,b | 2.87 ± 0.06 B,a | 11.51 ± 0.28 A,B,b | 14.38 ± 0.34 B,a,b |
| 6 | 2.25 ± 0.09 A,a | 0.64 ± 0.01 A,a,b | 2.68 ± 0.07 A,b,c | 7.13 ± 0.36 A,B,b | 1.37 ± 0.02 A,B,b | 2.89 ± 0.10 A,a | 11.18 ± 0.45 A,B,b | 14.07 ± 0.55 A,B,b |
| 9 | 2.20 ± 0.03 C,a | 0.53 ± 0.00 B,c | 2.60 ± 0.05 C,c,d | 7.40 ± 0.13 C,b | 1.16 ± 0.01 B,c | 2.73 ± 0.04 C,b | 11.16 ± 0.17 C,b | 13.89 ± 0.21 C,b |
| 12 | 2.22 ± 0.00 B,a | 0.53 ± 0.00 B,c | 2.59 ± 0.00 B,d | 7.39 ± 0.04 B,b | 1.16 ± 0.00 B,c | 2.74 ± 0.00 B,b | 11.14 ± 0.04 B,b | 13.89 ± 0.04 B,b |
| SST-170 °C-10 s | ||||||||
| 0 | 2.32 ± 0.05 A,a | 0.65 ± 0.00 A,a | 2.83 ± 0.06 A,a | 7.33 ± 0.10 B,a,b | 1.43 ± 0.02 A,a | 2.97 ± 0.05 A,a | 11.58 ± 0.16 A,a,b | 14.55 ± 0.21 A,a |
| 3 | 2.27 ± 0.03 B,a,b | 0.63 ± 0.00 B,b | 2.66 ± 0.08 A,B,a,b | 7.31 ± 0.39 B,a,b | 1.39 ± 0.03 A,b | 2.90 ± 0.03 B,a,b | 11.35 ± 0.50 B,a,b | 14.26 ± 0.51 B,a,b |
| 6 | 2.28 ± 0.13 A,a,b | 0.65 ± 0.01 A,a | 2.69 ± 0.10 A,b | 7.17 ± 0.44 A,b | 1.37 ± 0.03 A,b | 2.93 ± 0.14 A,a,b | 11.24 ± 0.58 A,a,b | 14.16 ± 0.72 A,a,b |
| 9 | 2.31 ± 0.02 B,a | 0.53 ± 0.00 B,c | 2.68 ± 0.00 B,a,b | 7.78 ± 0.07 B,a | 1.19 ± 0.01 A,c | 2.84 ± 0.02 B,b | 11.65 ± 0.07 B,a | 14.49 ± 0.09 B,a |
| 12 | 2.18 ± 0.02 C,b | 0.53 ± 0.00 B,c | 2.56 ± 0.01 B,b | 7.24 ± 0.05 C,b | 1.16 ± 0.01 B,d | 2.71 ± 0.02 C,c | 10.96 ± 0.07 C,b | 13.67 ± 0.08 C,b |
| SST-190 °C-5 s | ||||||||
| 0 | 2.30 ± 0.09 A,b | 0.64 ± 0.01 A,a | 2.78 ± 0.12 A,b | 7.95 ± 0.53 A,a,b | 1.43 ± 0.03 A,a | 2.94 ± 0.11 A,c | 12.15 ± 0.68 A,a | 15.10 ± 0.79 A,a |
| 3 | 2.38 ± 0.07 A,b | 0.64 ± 0.01 A,a | 2.77 ± 0.07 A,b | 7.86 ± 0.25 A,a,b | 1.41 ± 0.01 A,a,b | 3.03 ± 0.08 A,b,c | 12.05 ± 0.33 A,a | 15.08 ± 0.41 A,a |
| 6 | 2.42 ± 0.01 A,b | 0.65 ± 0.00 A,a | 2.79 ± 0.01 A,b | 7.70 ± 0.01 A,a,b | 1.40 ± 0.01 A,b | 3.07 ± 0.01 A,b | 11.90 ± 0.00 A,a | 14.97 ± 0.01 A,a |
| 9 | 2.41 ± 0.01 A,b | 0.55 ± 0.01 A,b | 2.75 ± 0.01 A,b | 8.23 ± 0.06 A,a | 1.20 ± 0.00 A,d | 2.96 ± 0.01 A,b,c | 12.19 ± 0.05 A,a | 15.15 ± 0.07 A,a |
| 12 | 2.48 ± 0.00 A,a | 0.55 ± 0.00 A,b | 3.05 ± 0.02 A,a | 7.51 ± 0.02 A,b | 1.26 ± 0.01 A,c | 3.03 ± 0.00 A,a | 11.82 ± 0.01 A,a | 14.85 ± 0.01 A,a |
| Sample | Storage Time (Weeks) | Tocopherols and Tocotrienols (μg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | α-Tocotrienol | β-Tocopherol | β-Tocotrienol | γ-Tocotrienol | δ-Tocotrienol | Tocopherols | Tocotrienols | Tocols | ||
| 0 | 3.11 ± 0.12 | 0.59 ± 0.03 | 0.53 ± 0.05 | 1.89 ± 0.22 | 1.24 ± 0.10 | 0.37 ± 0.00 | 3.64 ± 0.08 B,a,* | 4.09 ± 0.35 B,a | 7.73 ± 0.28 B,a | |
| 3 | 1.92 ± 0.18 | 0.40 ± 0.01 | 0.54 ± 0.03 | 1.53 ± 0.19 | 0.62 ± 0.13 | 0.15 ± 0.03 | 2.47 ± 0.21 B,b | 2.70 ± 0.11 C,b | 5.16 ± 0.32 C,b | |
| Control | 6 | 2.04 ± 0.08 | 0.43 ± 0.01 | 0.37 ± 0.01 | 1.80 ± 0.02 | 0.41 ± 0.01 | nd | 2.41 ± 0.09 D,b | 2.63 ± 0.01 D,b | 5.04 ± 0.10 D,b,c |
| 9 | 1.68 ± 0.27 | 0.48 ± 0.03 | 0.51 ± 0.04 | 1.48 ± 0.14 | 0.36 ± 0.03 | 0.23 ± 0.03 | 2.19 ± 0.25 C,b,c | 2.55 ± 0.12 C,b | 4.74 ± 0.13 C,c,d | |
| 12 | 1.25 ± 0.03 | 0.81 ± 0.01 | 0.62 ± 0.10 | 1.43 ± 0.08 | 0.43 ± 0.00 | nd | 1.86 ± 0.13C,c | 2.67 ± 0.07 C,b | 4.53 ± 0.15 C,d | |
| 0 | 2.77 ± 0.27 | 0.62 ± 0.20 | 0.98 ± 0.10 | 4.53 ± 0.68 | 1.45 ± 0.78 | 0.37 ± 0.04 | 3.75 ± 0.28B,a | 6.98 ± 1.68 A,a | 10.72 ± 1.71 A,a | |
| 3 | 1.52 ± 0.02 | 0.37 ± 0.01 | 0.68 ± 0.12 | 2.60 ± 0.62 | 0.53 ± 0.05 | 0.18 ± 0.02 | 2.20 ± 0.10 A,B,c | 3.68 ± 0.66 C,b | 5.87 ± 0.75 C,b | |
| SST- | 6 | 2.13 ± 0.06 | 1.03 ± 0.05 | 0.53 ± 0.08 | 1.51 ± 0.02 | 0.45 ± 0.01 | nd | 2.67 ± 0.05 C,b | 3.00 ± 0.02 C,b | 5.67 ± 0.06 C,b |
| 155 °C-10s | 9 | 2.17 ± 0.27 | 0.37 ± 0.04 | 0.34 ± 0.02 | 1.31 ± 0.05 | 0.27 ± 0.00 | 0.22 ± 0.02 | 2.50 ± 0.25 B,C,b | 2.16 ± 0.02 C,b | 4.66 ± 0.23 C,b |
| 12 | 1.73 ± 0.02 | 0.47 ± 0.00 | 0.63 ± 0.03 | 1.46 ± 0.08 | 0.32 ± 0.00 | nd | 2.36 ± 0.01 B,b,c | 2.25 ± 0.08 D,b | 4.61 ± 0.09 C,b | |
| 0 | 2.88 ± 0.19 | 0.48 ± 0.02 | 1.35 ± 0.12 | 6.46 ± 0.66 | 0.55 ± 0.06 | 0.33 ± 0.01 | 4.24 ± 0.26 A,a | 7.47 ± 0.77 A,a | 12.05 ± 0.78 A,a | |
| 3 | 1.34 ± 0.04 | 0.56 ± 0.16 | 0.74 ± 0.03 | 3.67 ± 0.55 | 1.13 ± 0.16 | 0.13 ± 0.00 | 2.08 ± 0.07 C,d | 5.48 ± 0.55 B,b | 7.56 ± 0.47 B,b | |
| SST- | 6 | 2.59 ± 0.08 | 1.02 ± 0.07 | 0.51 ± 0.12 | 1.74 ± 0.02 | 0.47 ± 0.02 | nd | 3.10 ± 0.18 B,b | 3.23 ± 0.06 B,c | 6.33 ± 0.15 B,c |
| 170 °C-10s | 9 | 2.23 ± 0.23 | 0.32 ± 0.03 | 0.54 ± 0.02 | 2.31 ± 0.02 | 0.30 ± 0.05 | 0.24 ± 0.01 | 2.77 ± 0.25 B,c | 3.17 ± 0.01 B,c | 5.94 ± 0.26 B,c |
| 12 | 1.78 ± 0.02 | 1.16 ± 0.10 | 0.66 ± 0.03 | 1.68 ± 0.05 | 0.47 ± 0.02 | nd | 2.44 ± 0.04 B,c,d | 3.31 ± 0.16 B,c | 5.75 ± 0.13 B,c | |
| 0 | 2.97 ± 0.10 | 0.53 ± 0.03 | 1.28 ± 0.03 | 5.94 ± 0.12 | 1.00 ± 0.19 | 0.36 ± 0.01 | 4.25 ± 0.07 A,a | 7.83 ± 0.16 A,b | 12.08 ± 0.18 A,a | |
| 3 | 1.66 ± 0.01 | 1.13 ± 0.01 | 1.56 ± 0.01 | 5.70 ± 0.03 | 1.33 ± 0.03 | 0.29 ± 0.01 | 3.22 ± 0.02 A,d | 8.45 ± 0.08 A,a | 11.67 ± 0.07 A,b | |
| SST- | 6 | 2.53 ± 0.04 | 0.99 ± 0.01 | 1.29 ± 0.05 | 5.95 ± 0.16 | 0.40 ± 0.04 | nd | 3.82 ± 0.09 A,b | 7.33 ± 0.15 A,c | 11.15 ± 0.22 A,c |
| 190 °C-5s | 9 | 2.40 ± 0.01 | 1.11 ± 0.04 | 1.17 ± 0.01 | 5.64 ± 0.01 | 0.26 ± 0.02 | 0.31 ± 0.02 | 3.57 ± 0.02 A,c | 7.33 ± 0.04 A,c | 10.90 ± 0.04 A,c,d |
| 12 | 1.75 ± 0.02 | 1.09 ± 0.02 | 1.92 ± 0.04 | 5.47 ± 0.03 | 0.54 ± 0.07 | nd | 3.67 ± 0.06 A,c | 7.09 ± 0.05 A,d | 10.76 ± 0.08 A,d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.-T.; Yang, Z.; Guo, X.-N.; Zhu, K.-X. Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage. Foods 2021, 10, 1348. https://doi.org/10.3390/foods10061348
Jia W-T, Yang Z, Guo X-N, Zhu K-X. Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage. Foods. 2021; 10(6):1348. https://doi.org/10.3390/foods10061348
Chicago/Turabian StyleJia, Wan-Ting, Zhen Yang, Xiao-Na Guo, and Ke-Xue Zhu. 2021. "Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage" Foods 10, no. 6: 1348. https://doi.org/10.3390/foods10061348
APA StyleJia, W.-T., Yang, Z., Guo, X.-N., & Zhu, K.-X. (2021). Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage. Foods, 10(6), 1348. https://doi.org/10.3390/foods10061348







