Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals
Abstract
:1. Introduction
2. Mucoadhesion
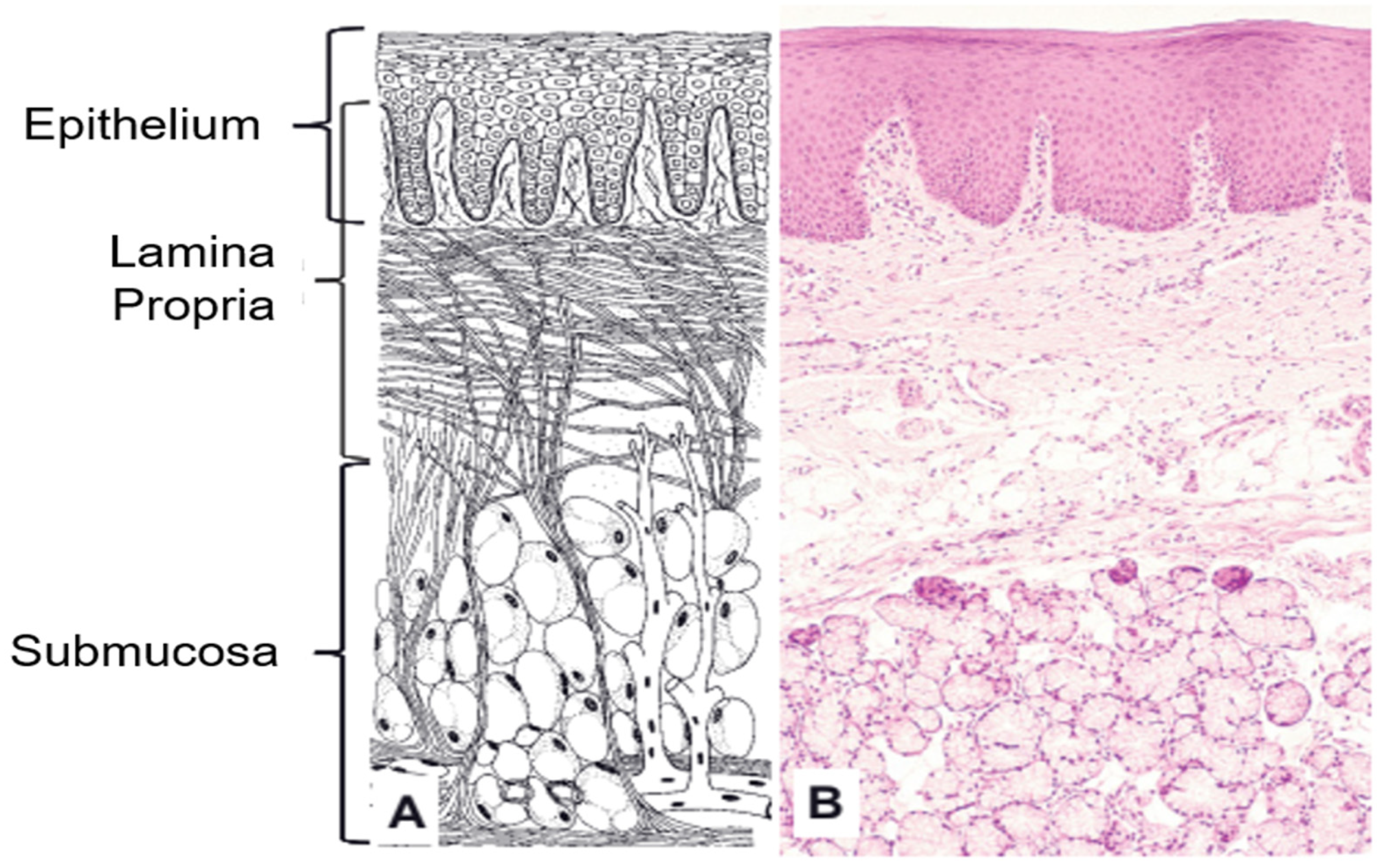
2.1. Oral Mucosa
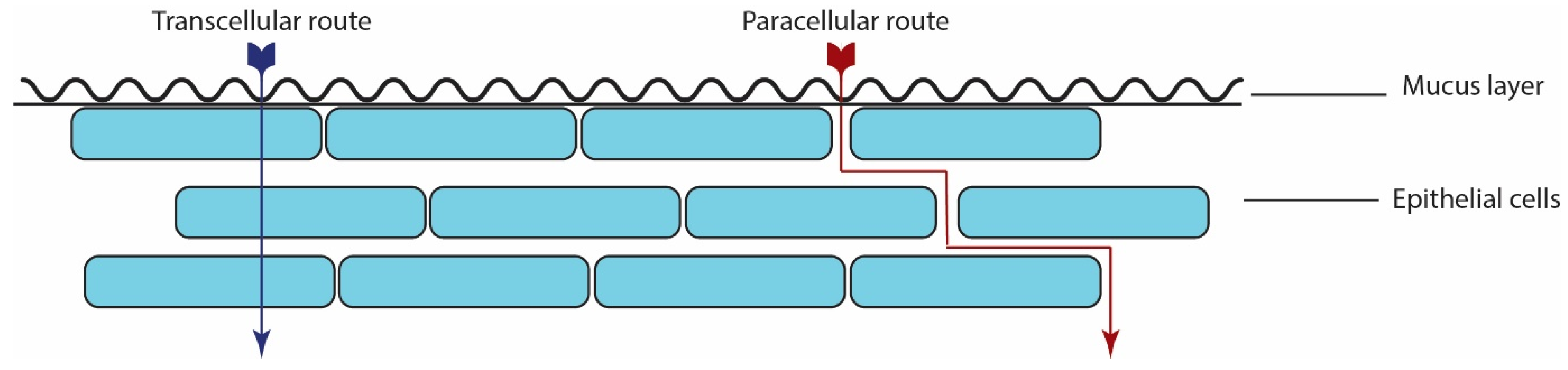
2.2. Absorption Mechanism
2.3. Mucoadhesion Theories
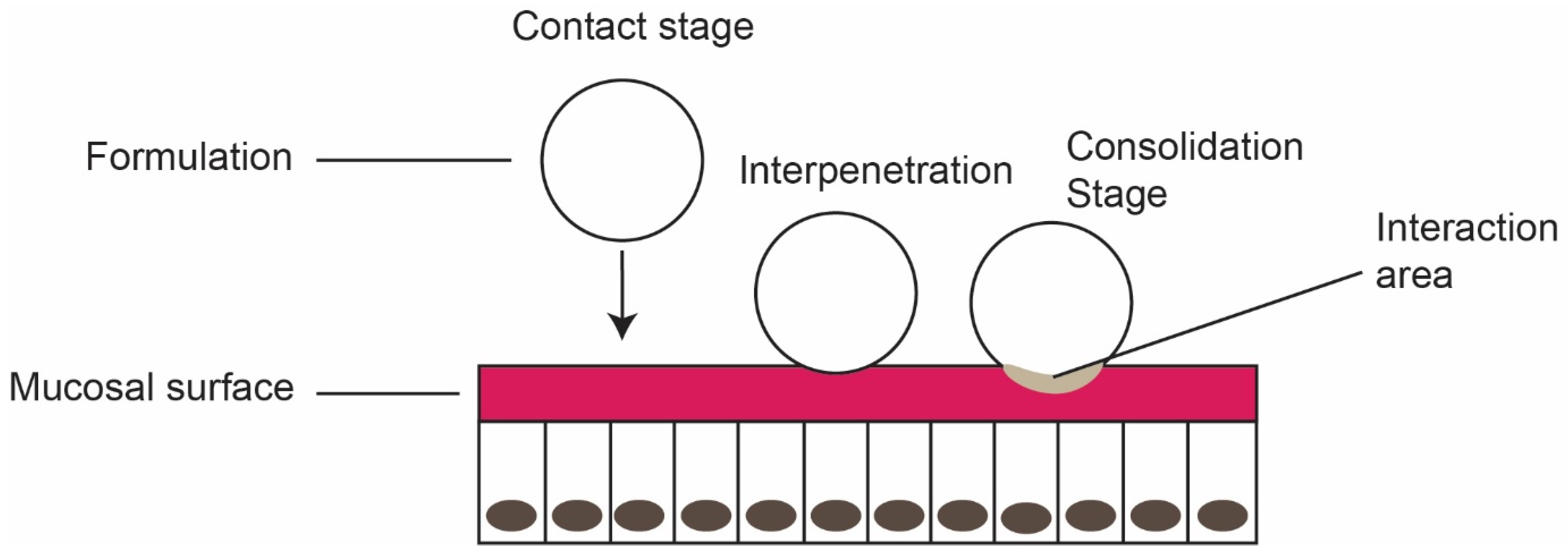
- Contact stage: The first stage in the mucoadhesion, initiating the contact between mucoadhesive formulation and the mucous membrane. Wetting and/or spreading of the material enhances the contact stage and increases the surface area.
- Interpenetration stage: Diffusion of mucoadhesive polymer into the mucus layer through spreading and deep contact with the mucus layer.
- Consolidation stage: Strengthening of mucoadhesive joints through mechanical and/or chemical interactions for prolonged adhesion (see Figure 3). Mechanical bonds are physical interactions relating to the penetration of mucoadhesive polymer into the mucus layer. Chemical bond includes strong primary bond and weak secondary bonds, based on the polymer structure.
- Mechanical interlocking: According to this theory, adhesion is by interlocking the adhesives into the rough surface. Such irregular surface offers a higher surface area available for interaction between the adhesive and mucus [25];
- Electronic theory: The formation of an electrical double layer at the adhesive-mucus interface due to electron difference between adhesive and mucus layer facilitates the attractive force [26];
- Diffusion theory: Also called interpenetration theory. Adhesive material penetrates in-depth into the mucus layer and creates a semi-permanent adhesive layer. The penetration depth of adhesive polymer depends on the molecular weight (polymer chain length) and diffusion coefficient. The adhesion will not be a simple two-dimensional surface phenomenon; it will be a three-dimensional process [27];
- Adsorption theory: Adhesive material sticks to the surface through hydrogen bonding, van der Waals, and hydrophobic interactions. Though they are secondary weak forces, the sheer number of interactions provides intense adhesive strength [26];
- Wetting theory: This theory applies to the liquid adhesives, considering the interfacial tensions to predict spreading and adhesion [23];
- Fracture theory: Above theories were developed based on the joining behavior of adhesive material with the mucus layer. Fracture theory defines the force required to detach after adhesion, and the fracture is assumed to occur at the mucoadhesive interface. Further, the fracture strength strongly depends on the length of the polymer chain and the degree of cross-linking [28].
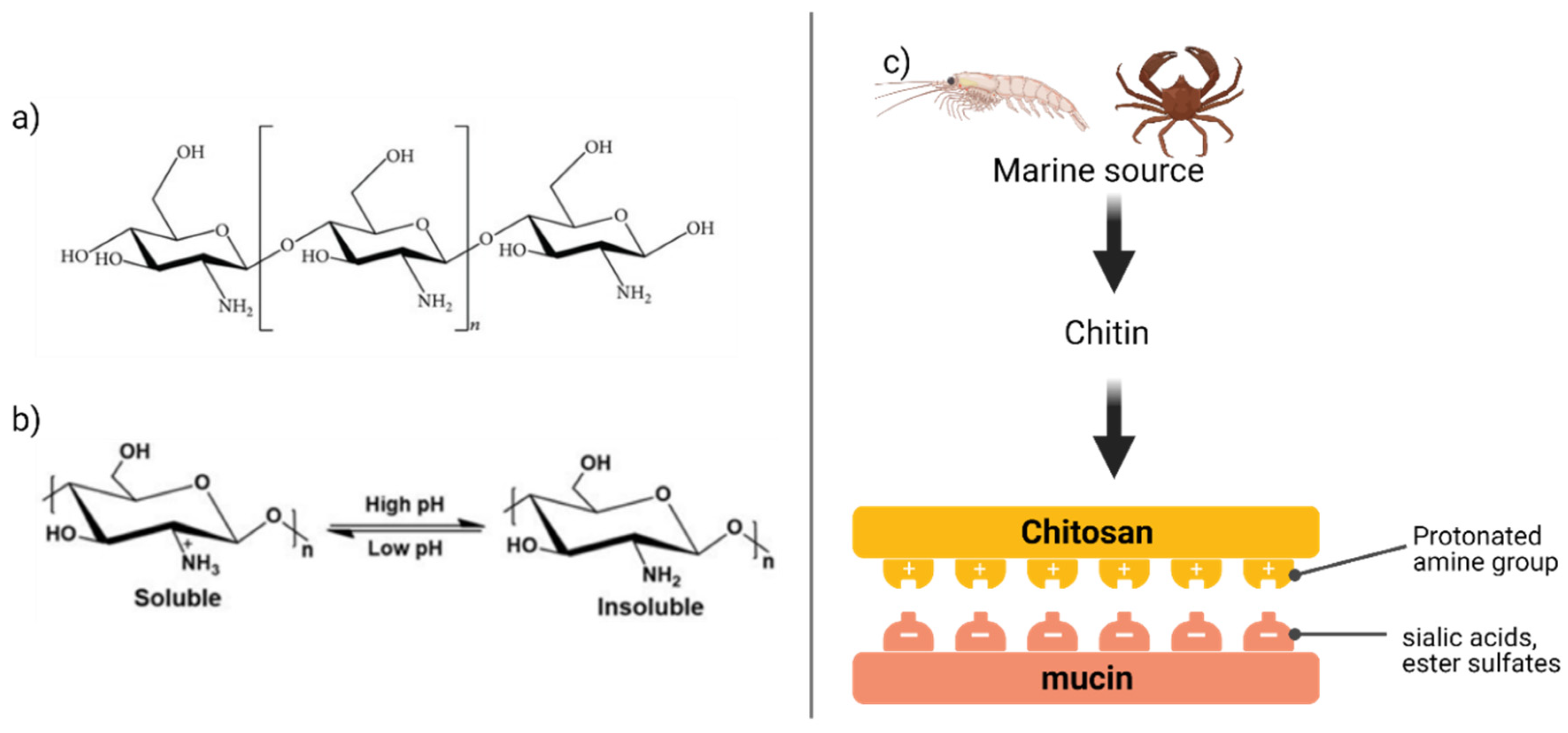
- Electrostatic interactions (also called as van der Waals interaction) appear if the charges (either positive or negative) are separated by a distance due to ionization or attachment of ionic species. Sialic acid and ester sulfates in the mucus layer provide a negative charge, which creates strong electrostatic interaction with the positively charged mucoadhesive polymers such as chitosan [29]. On the other hand, the negatively charged molecules (acrylates) can affix with mucins through the positively charged amino acids in the terminal domains;
- Hydrophobic interactions: The hydrophobic interactions can form between the naked protein core of mucin or lipids of mucus and the diffusion compounds created between the mucus and diffusing drugs. The hydrophobic interaction plays a key role in the tail-to-tail aggregation of mucins. For effective hydrophobic interaction to occur, it requires high energy and low sensitivity to the surrounding conditions. Thus, hydrophobic interaction is effective in gastric conditions where pH is very low and suppresses the electrostatic interactions [30].
3. Mucoadhesive Dosage Formulations
3.1. Buccal Tablets
3.2. Buccal Films
- Film: A thin layer or coating;
- Film for extended-release: Films releasing the embedded therapeutic compounds over an extended period and maintains the constant level in the blood or target tissue;
- Film, soluble (also called orodispersible films): Thin layer or coating, being dissolved when in contact with saliva.
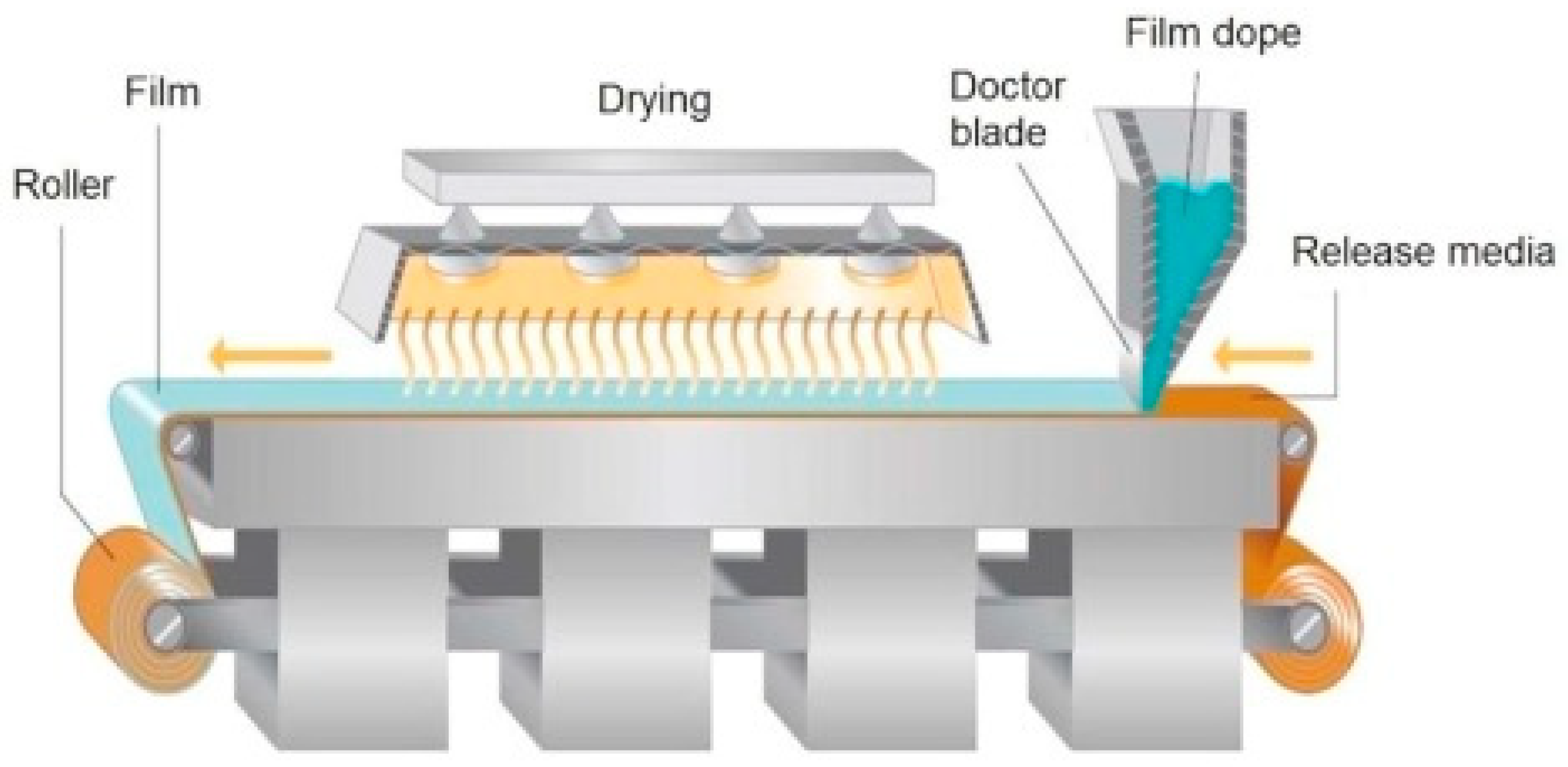
3.2.1. Solvent Casting Method
3.2.2. Liposomal Buccal Film
- Solvent casting is a multistep process, which brings variation in the final product for each batch;
- Air entrapment is a crucial flaw in this process, which leads to dose variation;
- Application of organic solvent during the production process, because solvent removal from the buccal film and subsequent disposal is a tedious process.
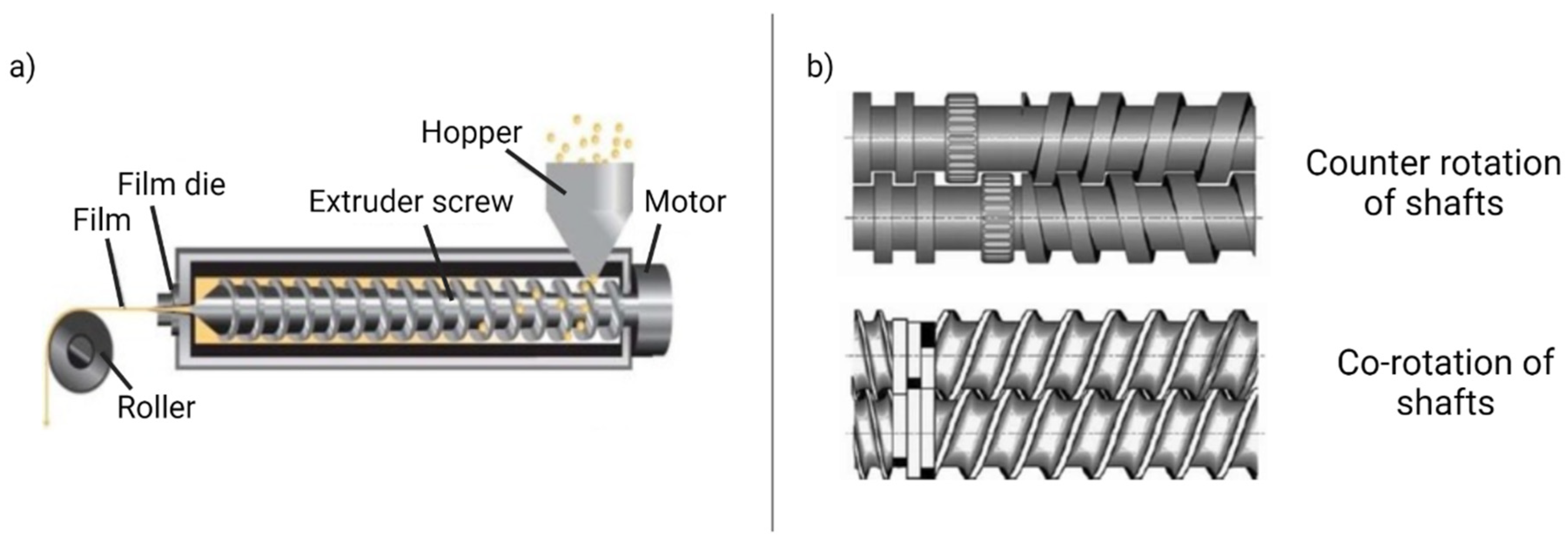
3.2.3. Hot-Melt Extrusion
- Feed the formulation ingredients and bioactive compounds to the extruder through a hopper;
- Mixing, grinding, and kneading;
- The molten ingredient conveyed to the through the rotating screw;
- Extrusion through the die and mold the desired shape.
3.3. Printing Technology
3.3.1. Inkjet Printing (2D)
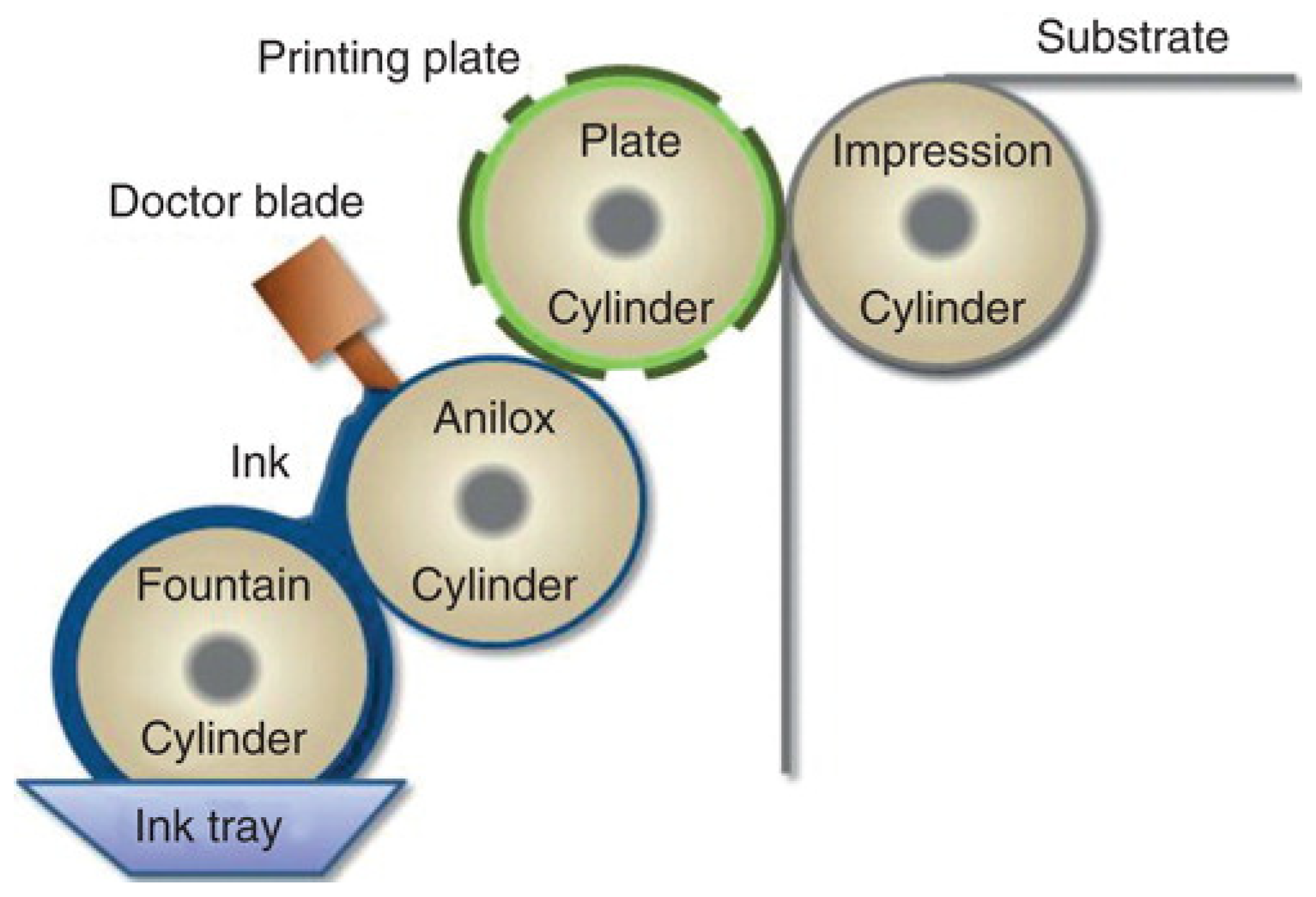
3.3.2. Flexographic Printer (2D)
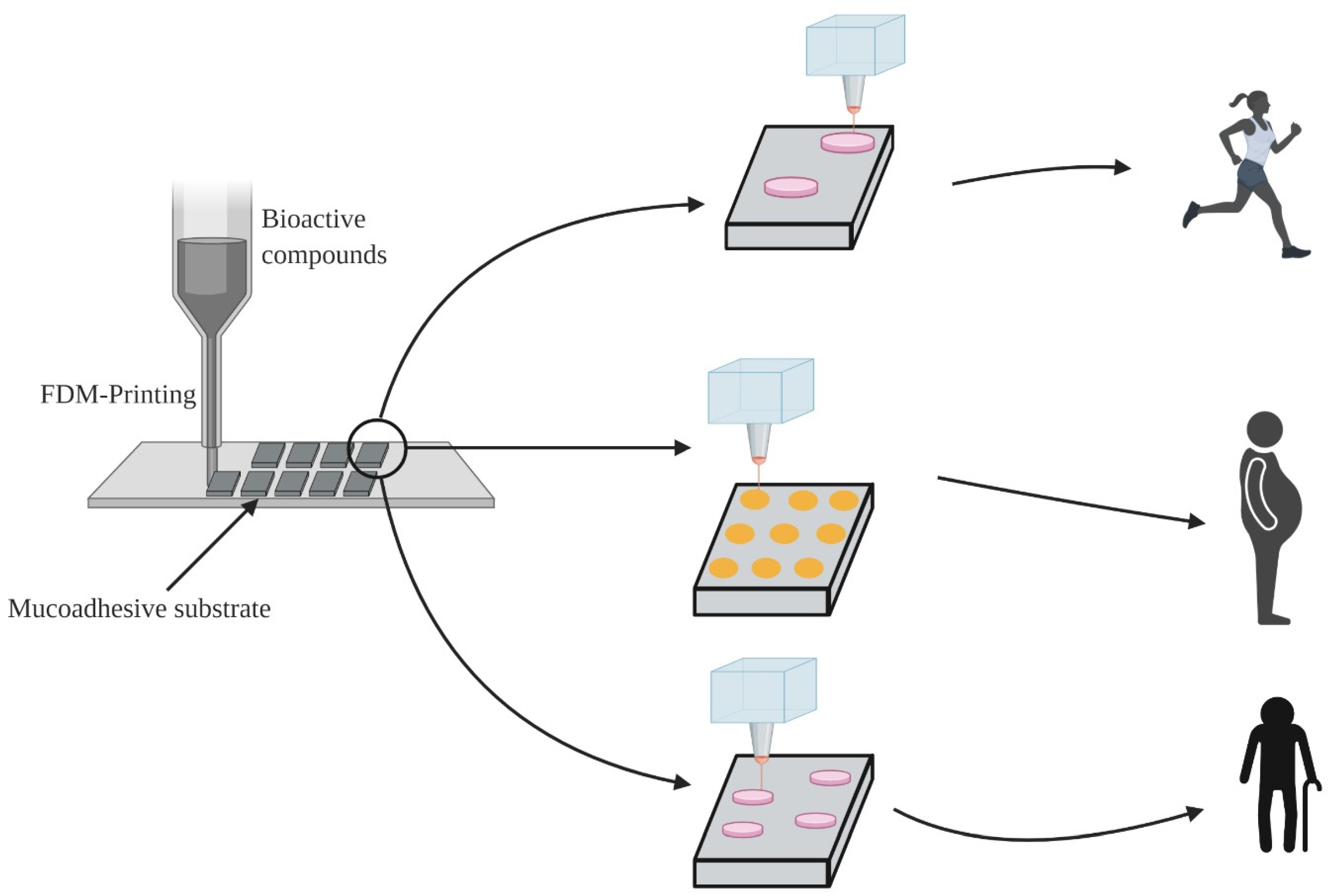
3.3.3. Fused Deposition Modeling (3D)
3.4. Buccal Wafers
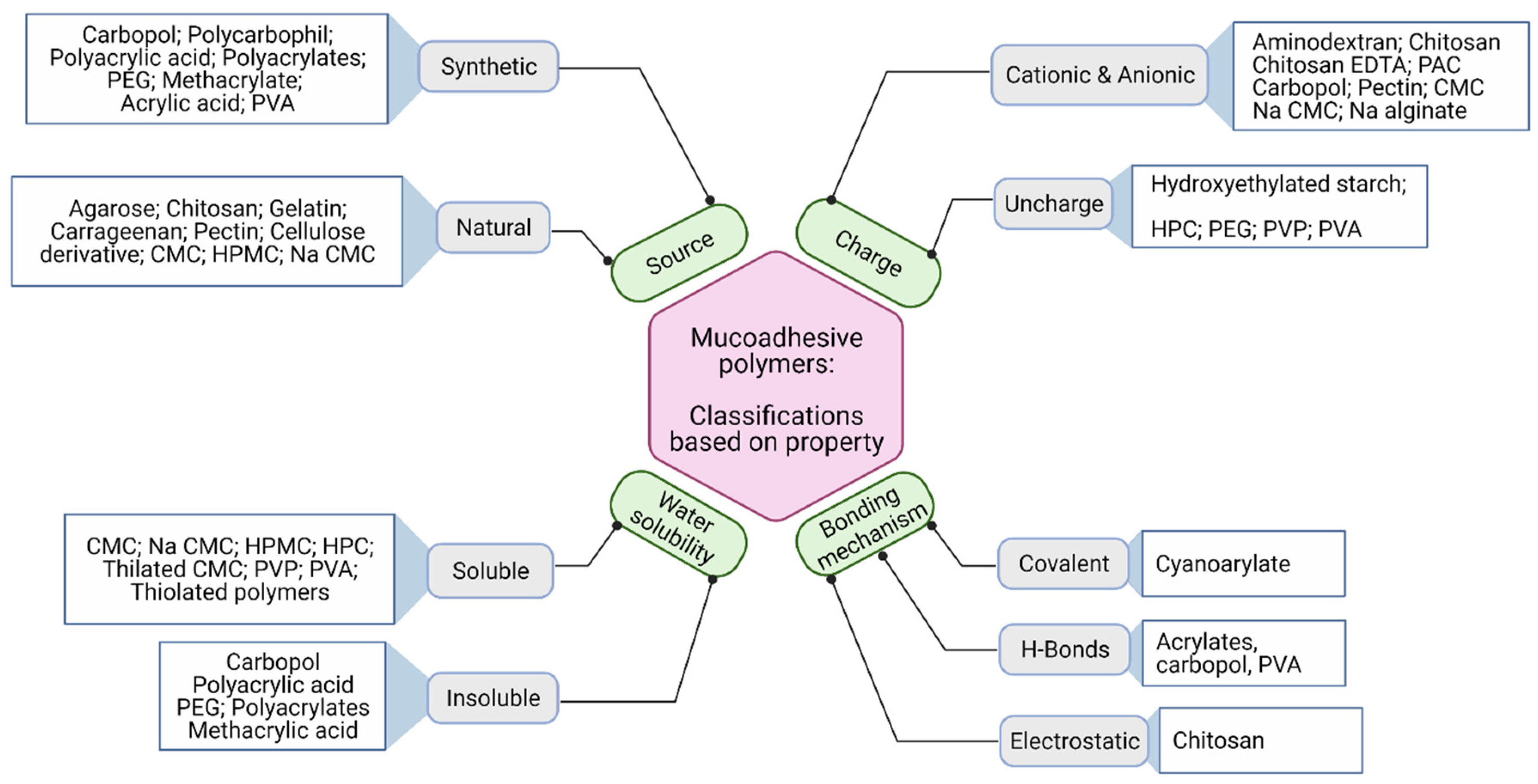
4. Mucoadhesive Polymers
- ⮚
- Strong hydrogen bonding groups, e.g., carboxyl, hydroxyl, amino- and sulfate groups;
- ⮚
- Strong anionic or cationic functional groups;
- ⮚
- Possess high molecular weight;
- ⮚
- Surface tension to induce spreading into mucus layer;
- ⮚
- High chain flexibility;
- ⮚
- Capacity to load bioactive compounds;
- ⮚
- Swell upon hydration;
- ⮚
- Interact with mucus for adequate adhesion;
- ⮚
- Provide controlled release of bioactives from the formulation;
- ⮚
- Should be biologically degradable.
4.1. Starch Buccal Films
4.2. Chitosan
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Divya, A.; Jaglan, S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci. Technol. 2016, 54, 114–126. [Google Scholar]
- Singh, M.; Singh, N.; Chandrasekaran, B.; Deb, P.K. Nanomaterials in nutraceuticals applications. In Integrative Nanomedicine for New Therapies; Krishnan, A., Chuturgoon, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 405–435. [Google Scholar]
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed. Res. 2014, 28, 81–97. [Google Scholar] [CrossRef]
- Leung, S.-H.S.; Robinson, J.R. The contribution of anionic polymer structural features to mucoadhesion. J. Control. Release 1987, 5, 223–231. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ahmad, R.; Li, W.; Swain, M.; Li, Q. Biomechanics of oral mucosa. J. R. Soc. Interface 2015, 12, 20150325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy Chinna, P.; Chaitanya, K.S.C.; Madhusudan Rao, Y. A review on bioadhesive buccal drug delivery systems: Current status of formulation and evaluation methods. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 385–403. [Google Scholar]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Abdullah, A.; Wahlgren, M.; Pedersen, L.; Engblom, J. Will a water gradient in oral mucosa affect transbuccal drug absorption? J. Drug Deliv. Sci. Technol. 2018, 48, 338–345. [Google Scholar] [CrossRef]
- Pramanik, R.; Osailan, S.M.; Challacombe, S.J.; Urquhart, D.; Proctor, G.B. Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur. J. Oral Sci. 2010, 118, 245–253. [Google Scholar] [CrossRef]
- Galey, W.R.; Lonsdale, H.K.; Nacht, S. The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water. J. Investig. Dermatol. 1976, 67, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.; Robinson, J.R. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992, 81, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marxen, E.; Jacobsen, J.; Hyrup, B.; Janfelt, C. Permeability barriers for nicotine and mannitol in porcine buccal mucosa studied by high-resolution MALDI mass spectrometry imaging. Mol. Pharm. 2018, 15, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.P. Anatomy and physiology of the oral mucosa. In Oral Mucosal Drug Delivery and Therapy; Springer: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Sattar, M.; Sayed, O.M.; Lane, M.E. Oral transmucosal drug delivery—Current status and future prospects. Int. J. Pharm. 2014, 471, 498–506. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- He, S.; Østergaard, J.; Ashna, M.; Nielsen, C.U.; Jacobsen, J.; Mu, H. Microenvironmental pH modifying films for buccal delivery of saquinavir: Effects of organic acids on pH and drug release in vitro. Int. J. Pharm. 2020, 585, 119567. [Google Scholar] [CrossRef] [PubMed]
- NadMorsi, I.M.; Abdelbary, G.A.; Elshafeey, A.H.; Ahmed, M.A. Engineering of a novel optimized platform for sublingual delivery with novel characterization tools: In vitro evaluation and in vivo pharmacokinetics study in human. Drug Deliv. 2017, 24, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.K.W.; Xu, Y.; Worsley, A.; Wong, I.C.K. Oral transmucosal drug delivery for pediatric use. Adv. Drug Deliv. Rev. 2014, 73, 50–62. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C.M. Mucoadhesive polymers as enabling excipients for oral mucosal drug delivery. In Oral Mucosal Drug Delivery and Therapy; Springer: Cham, Switzerland, 2015; pp. 53–88. [Google Scholar]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Shinkar, D.M.; Dhake, A.S.; Setty, C.M. Drug delivery from the oral cavity: A focus on mucoadhesive buccal drug delivery systems. PDA J. Pharm. Sci. Technol. 2012, 66, 466–500. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y. Nanoscale technology of mucoadhesive interactions. Adv. Drug Deliv. Rev. 2004, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.J.; Gent, A.N. Effect of interfacial bonding on the strength of adhesion of elastomers. I. Self-Adhesion. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1619–1633. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Yakubov, G.E.; Singleton, S.; Williamson, A. Methods for assessing mucoadhesion: The experience of an integrative approach. In Mucoadhesive Materials and Drug Delivery Systems; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 197–232. [Google Scholar]
- Radha, B.; Nagrajan, R.K. A detailed review on oral mucosal drug delivery system. Int. J. Pharm. Sci. Res. 2012, 3, 659. [Google Scholar]
- Rathbone, M.J.; Drummond, B.K.; Tucker, I.G. The oral cavity as a site for systemic drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 1–22. [Google Scholar] [CrossRef]
- Teferra, T.F. Engineering properties of food materials. In Handbook of Farm, Dairy and Food Machinery Engineering, 3rd ed.; Myer Kutz, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–89. [Google Scholar]
- Gowthamarajan, K.; Jawahar, N.; Wake, P.; Jain, K.; Sood, S. Development of buccal tablets for curcumin using Anacardium occidentale gum. Carbohydr. Polym. 2012, 88, 1177–1183. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Abdelkader, H.; Elrehany, M.; Mansour, H.F. Vitamin B12 buccoadhesive tablets: Auspicious non-invasive substitute for intra muscular injection: Formulation, in vitro and in vivo appraisal. Drug Dev. Ind. Pharm. 2019, 45, 244–251. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; Mendes, P.R.D. Rheological characterization of Carbopol® dispersions in water and in water/glycerol solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, A.; Akbari, J.; Boorboor, M.; Nekoukar, Z.; Eslami, G. Evaluation of zinc sulfate mucoadhesive formulation on recurrent aphthous stomatitis: A randomized double-blind, placebo-controlled clinical trial. BMC Oral Health 2020, 20, 212. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H. Clinical evaluation of an oral mucoadhesive film containing chitosan for the treatment of recurrent aphthous stomatitis: A randomized, double-blind study. J. Dermatol. Treat. 2020, 31, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-Mangostin hydrogel film based chitosan—Alginate for recurrent aphthous stomatitis. Appl. Sci. 2019, 9, 5235. [Google Scholar] [CrossRef] [Green Version]
- Haghpanah, P.; Moghadamnia, A.A.; Zarghami, A.; Motallebnejad, M. Muco-Bioadhesive containing ginger officinal e extract in the management of recurrent aphthous stomatitis: A randomized clinical study. Casp. J. Intern. Med. 2015, 6, 3–8. [Google Scholar]
- Chandrashekar, A.; Annigeri, R.G.; Va, U.; Thimmasetty, J. A clinicobiochemical evaluation of curcumin as gel and as buccal mucoadhesive patches in the management of oral submucous fibrosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Conte, C.; d’Angelo, I.; Miro, A.; Ungaro, F.; Quaglia, F. Mucoadhesive zein/beta-cyclodextrin nanoparticles for the buccal delivery of curcumin. Int. J. Pharm. 2020, 586, 119587. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Siedle, I.; Boni, F.I.; Chorilli, M.; Müller, I.; Cury, B.S.F. Mucoadhesive films based on gellan gum/pectin blends as potential platform for buccal drug delivery. Pharm. Dev. Technol. 2020, 25, 159–167. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.; Braga, G.; Oliveira, É.L.; da Silva, J.B.; Rosseto, H.C.; Hoshino, L.V.D.; Baesso, M.L.; Caetano, W.; Murdoch, C.; Colley, H.E.; et al. Design of a nanostructured mucoadhesive system containing curcumin for buccal application: From physicochemical to biological aspects. Beilstein J. Nanotechnol. 2019, 10, 2304–2328. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Shi, W.; Aldrich, A.L.; Kielian, T.; Carlson, M.A.; Sun, R.; Qin, X.; Duan, B. Large-Scale and rapid preparation of nanofibrous meshes and their application for drug-loaded multilayer mucoadhesive patch fabrication for mouth ulcer treatment. ACS Appl. Mater. Interfaces 2019, 11, 28740–28751. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-Coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Mudassir, A.; Sadarani, B.; Majumdar, A. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. J. Drug Deliv. Sci. Technol. 2018, 44, 278–288. [Google Scholar] [CrossRef]
- Tedesco, M.P.; Monaco-Lourenço, C.A.; Carvalho, R.A. Characterization of oral disintegrating film of peanut skin extract—Potential route for buccal delivery of phenolic compounds. Int. J. Biol. Macromol. 2017, 97, 418–425. [Google Scholar] [CrossRef]
- Borges, J.G.; Garcia, V.A.d.S.; Osiro, D.; de Carvalho, R.A. Printing ethanol pomegranate extract in films by inkjet technology. Ind. Crop. Prod. 2019, 140, 111643. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Sarhan, H.A.; Abdelkader, H.; Mansour, H.F. Vitamin B12–loaded buccoadhesive films as a noninvasive supplement in vitamin B12 deficiency: In vitro evaluation and in vivo comparative study with intramuscular injection. J. Pharm. Sci. 2017, 106, 1849–1858. [Google Scholar] [CrossRef]
- El-Say, K.M.; Ahmed, T.A.; Ahmed, O.A.A.; Hosny, K.M.; Abd-Allah, F.I. Self-Nanoemulsifying lyophilized tablets for flash oral transmucosal delivery of vitamin K: Development and clinical evaluation. J. Pharm. Sci. 2017, 106, 2447–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriadis, G.; Monou, P.K.; Andriotis, E.; Mitsouli, E.; Moutafidou, N.; Markopoulou, C.; Bouropoulos, N.; Fatouros, D. Development and characterization of inkjet printed edible films for buccal delivery of B-complex vitamins. Pharmaceuticals 2020, 13, 203. [Google Scholar] [CrossRef]
- Loyd, A.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bhagurkar, M.A.; Darji, M.; Lakhani, P.; Thipsay, P.; Bandari, S.; Repka, M.A. Effects of formulation composition on the characteristics of mucoadhesive films prepared by hot-melt extrusion technology. J. Pharm. Pharmacol. 2018, 71, 293–305. [Google Scholar] [CrossRef]
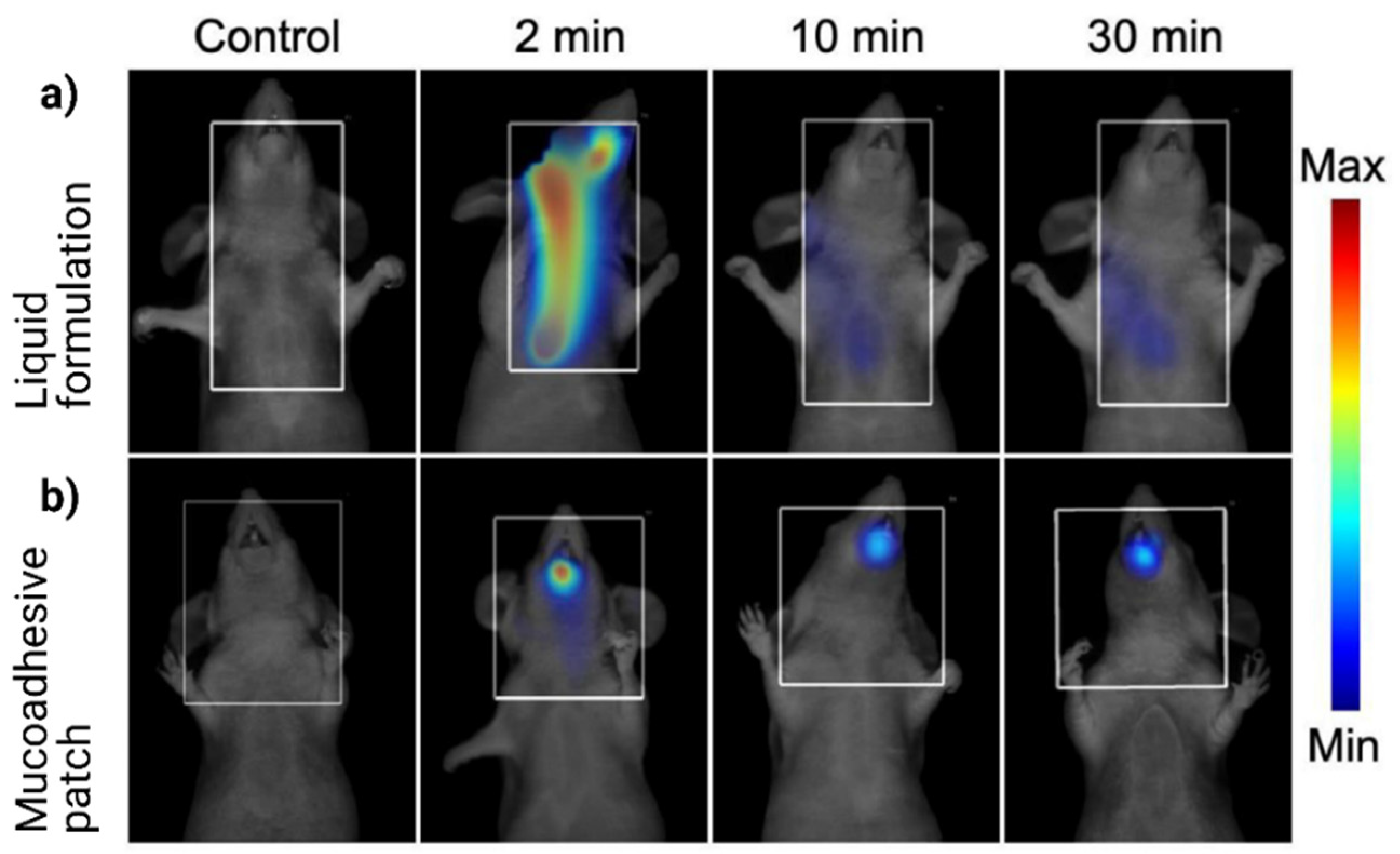
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 2017, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Abd el Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utkarshini, A.; Feridooni, T.; Agu, R.U. Novel mucoadhesive polymers for nasal drug delivery. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Pimparade, M.B.; Vo, A.; Maurya, A.S.; Bae, J.; Morott, J.T.; Feng, X.; Kim, D.W.; Kulkarni, V.I.; Tiwari, R.; Vanaja, K.; et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur. J. Pharm. Biopharm. 2017, 119, 81–90. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Buanz, A.B.M.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-Jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Preis, M.; Breitkreutz, J.; Sandler, N. Perspective: Concepts of printing technologies for oral film formulations. Int. J. Pharm. 2015, 494, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Rayleigh, L. On the instability of jets. Proc. Lond. Math. Soc. 1878, 1, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Edinger, M.; Jacobsen, J.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Analytical aspects of printed oral dosage forms. Int. J. Pharm. 2018, 553, 97–108. [Google Scholar] [CrossRef]
- Montenegro-Nicolini, M.; Reyes, P.E.; Jara, M.O.; Vuddanda, P.R.; Neira-Carrillo, A.; Butto, N.; Velaga, S.; Morales, J.O. The effect of inkjet printing over polymeric films as potential buccal biologics delivery systems. AAPS PharmSciTech 2018, 19, 3376–3387. [Google Scholar] [CrossRef]
- Pinheiro, M.J.; Freitas, S.; Miranda, E.A.; Filho, P.D.P. Solubility of lysozyme in aqueous solution containing ethanol or acetone: Unexpected dependence on the initial protein concentration. Fluid Phase Equilibria 2016, 429, 9–13. [Google Scholar] [CrossRef]
- Kolakovic, R.; Viitala, T.; Ihalainen, P.; Genina, N.; Peltonen, J.; Sandler, N. Printing technologies in fabrication of drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 1711–1723. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Azzollini, D. Variables affecting the printability of foods: Preliminary tests on cereal-based products. Innov. Food Sci. Emerg. Technol. 2016, 38, 281–291. [Google Scholar] [CrossRef]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D printing in food processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Andreadis, D.A.; Tzetzis, D.; Ritzoulis, C.; Bouropoulos, N.; Kanellopoulou, D.; Andriotis, E.G.; Tsibouklis, J.; Fatouros, D.G. Inkjet printing of a thermolabile model drug onto FDM-printed substrates: Formulation and evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1253–1264. [Google Scholar] [CrossRef]
- Costa, J.S.R.; Cruvinel, K.D.O.; Oliveira-Nascimento, L. A mini-review on drug delivery through wafer technology: Formulation and manufacturing of buccal and oral lyophilizates. J. Adv. Res. 2019, 20, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Auffret, A.D.; Humphrey, M.J.; Stevens, H.N.; Eccleston, G.M. In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using paracetamol as a model soluble drug. Int. J. Pharm. 2009, 378, 66–72. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M.; Stevens, H.N. Comparison of the in vitro release characteristics of mucosal freeze-dried wafers and solvent-cast films containing an insoluble drug. Drug Dev. Ind. Pharm. 2012, 38, 47–54. [Google Scholar] [CrossRef]
- Betini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Szabó, B.; Kállai, N.; Tóth, G.; Hetényi, G.; Zelkó, R. Drug release profiles and microstructural characterization of cast and freeze dried vitamin B12 buccal films by positron annihilation lifetime spectroscopy. J. Pharm. Biomed. Anal. 2014, 89, 83–87. [Google Scholar] [CrossRef]
- Scrivener, C.A.; Schantz, C.W. Penicillin: New methods for its use in dentistry. J. Am. Dent. Assoc. 1947, 35, 644–647. [Google Scholar] [CrossRef]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Puri, V.; Sharma, A.; Kumar, P.; Singh, I. Thiolation of biopolymers for developing drug delivery systems with enhanced mechanical and mucoadhesive properties: A review. Polymers 2020, 12, 1803. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 42, 274–283. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Sharma, R.; Agrawal, U.; Mody, N.; Vyas, S.P. Polymer nanotechnology based approaches in mucosal vaccine delivery: Challenges and opportunities. Biotechnol. Adv. 2015, 33, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Raskin, M.M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol. Adv. 2015, 33, 1380–1392. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Okonogi, S.; Khongkhunthian, S.; Jaturasitha, S. Development of mucoadhesive buccal films from rice for pharmaceutical delivery systems. Drug Discov. Ther. 2014, 8, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Miksusanti; Fithri, A.N.; Herlina; Wijaya, D.P.; Taher, T. Optimization of chitosan–tapioca starch composite as polymer in the formulation of gingival mucoadhesive patch film for delivery of gambier (Uncaria gambir Roxb) leaf extract. Int. J. Biol. Macromol. 2020, 144, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Soe, M.T.; Chitropas, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Thai glutinous rice starch modified by ball milling and its application as a mucoadhesive polymer. Carbohydr. Polym. 2020, 232. [Google Scholar] [CrossRef]
- Hsu, L.-W.; Lee, P.-L.; Chen, C.-T.; Mi, F.-L.; Juang, J.-H.; Hwang, S.-M.; Ho, Y.-C.; Sung, H.-W. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials 2012, 33, 6254–6263. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med Bull. 1999, 46, 183–196. [Google Scholar]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Xing, L.; Fan, Y.-T.; Zhou, T.-J.; Gong, J.-H.; Cui, L.-H.; Cho, K.-H.; Choi, Y.-J.; Jiang, H.-L.; Cho, C.-S. Chemical modification of chitosan for efficient vaccine delivery. Molecules 2018, 23, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Dekina, S.; Romanovska, I.; Ovsepyan, A.; Tkach, V.; Muratov, E. Gelatin/Carboxymethyl cellulose mucoadhesive films with lysozyme: Development and characterization. Carbohydr. Polym. 2016, 147, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Paris, A.-L.; Caridade, S.; Colomb, E.; Bellina, M.; Boucard, E.; Verrier, B.; Monge, C. Sublingual protein delivery by a mucoadhesive patch made of natural polymers. Acta Biomater. 2021. [Google Scholar] [CrossRef]
| Saliva/Mucosal Surface | Mucosal Surface Condition | Thickness of Mucosal Fluid (μm) | Protein Concentration (mg mL−1) |
|---|---|---|---|
| Unstimulated whole-mouth saliva | n/a | n/a | 3.07 ± 0.27 |
| Anterior hard palate | Wet | 9.6 ± 3.0 | 22.0 ± 5.5 |
| Buccal mucosa | Wet | 39.5 ± 7.4 | 7.1 ± 0.6 |
| Dry | 17.1 ± 3.4 | 19.6 ± 7.4 | |
| Anterior tongue | Wet | 54 ± 5.8 | 3.3 ± 0.7 |
| Dry | 12.3 ± 2.2 | 12.5 ± 2.6 | |
| Lower labial mucosa | Wet | 20.8 ± 2.5 | 22.2 ± 4.3 |
| Dry | 6.0 ± 0.6 | 41.3 ± 13.5 |
| Challenges | Description and Impact |
|---|---|
| Mucosal structure | It varies at different regions in the oral cavity, and the mucosal epithelial barrier act as a barrier |
| Saliva flow | The continuous secretion and flow of saliva may detach the formulation base |
| Variation | Mucosal surface vary person to person attributed to the tongue movements and variation in saliva secretion amount |
| Application region | Surface area available for the mucoadhesive formulation is very limited, which affects the loading capacity |
| Comfortability | The design of mucoadhesive formulation ensures easy installation/removal and accounts for the consumers’ comfortability |
| Sl. No | Bioactive Compound | Mucoadhesive Polymer | Mucoadhesive Formulation | Study Objective | Study Method | Research Findings | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Zinc sulfate | Carbopol 940 + sodium alginate | Tablets | Zinc sulfate for the treatment of recurrent aphthous stomatitis (RAS) | Human clinical trial conducted with 46 participants having RAS | Conducted clinical trial with mouth ulcer patients, and authors found that the zinc tablets can reduce the pain, diameter of ulcer wounds and its inflammation, and accelerates the recovery time of ulcer. | [37] |
| 2 | Chitosan | Polyurethane + chitosan | Films | Chitosan mucoadhesive film for the treatment of RAS | Human clinical trial. 72 participants were recruited for the study and conducted data analysis with 66 subjects | Chitosan film promoted the healing of RAS, and the pain score was significantly reduced from day 4 to day 6. Chitosan films shielded the ulcer from external stimuli and thereby reduced the related pain from the ulcer region. | [38] |
| 3 | α-mangostin | Chitosan + alginate | Hydrogel films | α-mangostin hydrogel film for the treatment of RAS | In vitro release and mucoadhesive study in mouse mucosa | Chitosan +alginate +α-mangostin hydrogel films were adhesive toward the mouse mucosa for 46.7 min, and showing burst release characteristics, which is necessary for the treatment of RAS. | [39] |
| 4. | Ginger extract | Tragacanth gum | Films | Ginger extract for the treatment of RAS | Clinical study in 15 patients | The mucoadhesive ginger formulation can relieve the pain of RAS patients; however, there is no statistical difference in the ulcer diameter, healing time with placebo. | [40] |
| 5. | Curcumin | Hydroxypropyl methylcellulose + glycerin | Patches of 2 × 3 cm containing 2% curcumin | Curcumin for the treatment of oral submucous fibrosis (OMSF). OSMF is a chronic inflammatory, and immune-mediated disease occurs commonly by chewing arecanuts | Forty patients with OMSF in two groups. One group of patients was administered with curcumin gel and another group was administered with curcumin mucoadhesive patches | All patients were relieved from a burning sensation in the oral cavity, and the patients can open the mouth by 5.9 ± 2.00 mm. The curcumin patches were easy to apply and provided a non-invasive mode of treatment for OSMF. | [41] |
| 6. | Curcumin | Zein + beta-cyclodextrin | Curcumin loaded nanoparticles | Develop Mucoadhesive zein NPs for curcumin buccal delivery | Ex vivo study. Mucoadhesive properties were conducted with buccal mucosa from freshly killed pigs | Curcumin permeation study revealed the highest curcumin permeation for zein cyclodextrin mucoadhesive formulation than the curcumin nanoparticles. | [42] |
| 7 | Curcumin | Gellan gum + Pectin | Films | The efficiency of gellan gum and pectin mucoadhesive formulation for delivering curcumin | In vitro release kinetics and disintegration | The mucoadhesive film was not disintegrated after 24 h exposed to simulated saliva. That means the film can remain in the target site for a prolonged time and release the therapeutic compounds. In vitro release study showed that there was an initial burst release till the first 10 min of exposure and then the release rate lowered up to 60 min of the test. Fast swelling of the film and rapid liquid uptake attributed to initial burst release behavior. | [43] |
| 8 | Curcumin | Poloxamer 407 + Carbopol 974P | Films | Develop nanostructured curcumin incorporated in films to target oral squamous cell carcinoma | In vitro release study, and ex vivo mucosal permeation in the porcine oral mucosa | A complete release of curcumin after 8 h exposure in the in vitro simulated condition, which makes it a suitable formulation for a buccal application. | [44] |
| 9 | Curcumin | Poly (L-lactic acid) | Patch | Develop mucoadhesive patch containing curcumin nanofibers using electrospinning techniques | In vitro release study and ex vivo adhesive properties in porcine buccal mucosa | Curcumin patch showed the least adhesion force of 0.14 ± 0.01 N, attributed to the non-mucoadhesive characteristics of polymer PLLA. | [45] |
| 10 | Resveratrol | Chitosan + Zein | Nanoparticles | Effectiveness of chitosan-coated zein nanoparticles for the oral delivery of resveratrol | In vitro mucoadhesion study in mucin solutions | Particle diameter was increasing with increasing mucin concentration as more mucin adsorbs on the nanoparticle surface. Thus, chitosan-coated nanoparticles were showing a higher increment in size than the uncoated particles. | [46] |
| 11 | Resveratrol | Hydroxypropyl cellulose + ethyl cellulose | Films | Optimize the polymer concentration on the mucoadhesive strength, swelling, and in vitro release | In vitro release study and ex vivo permeation study with goat buccal mucosa | Ex vivo permeation study revealed that there was a restriction in resveratrol permeation due to the low wetting and hydration of polymer matrix. In addition, the low aqueous solubility of resveratrol limited the release and penetration characteristics. | [47] |
| 12 | Peanut skin extract | Gelatin + hydroxypropyl methylcellulose | Films | Develop polyphenol enriched films using the casting technique | In vitro release technique | Mucoadhesive films followed the initial burst release of phenolic content about 1.2 mg gallic acid equivalent, attributed to the hydrophilic polymer, hydroxypropyl methylcellulose. | [48] |
| 13 | Pomegranate fruit extract | Carboxymethyl cellulose | Films | Produce multilayered oral films using printing techniques incorporating phenols | In vitro release technique | The release of polyphenols from the films showed a Fickian diffusion pattern, and the phenolic compounds were stable for 196 days at room temperature. | [49] |
| 14 | Vitamin B-12 | Chitosan + Polyvinyl alcohol | Films | Develop vitamin B-12 mucoadhesive hydrogel films | In vivo pharmacokinetic study with rabbits | Compared the release of vitamin B12 from the buccal films and commercial Neuroton I.M. injection. The area under the curve (AUC0–8h) showed a 1.5-fold increase in bioavailability from the buccal film compared with the I.M. injection. | [50] |
| 15 | Vitamin B12 | Hydroxypropyl methyl cellulose + Carbopol + chitosan | Tablets | Develop buccal tablets of vitamin B12 and improve the oral bioavailability | In vivo pharmacokinetic study with rabbits | Rabbits injected with vitamin B12 (intramuscular) showed rapid release till 15 min at a maximum concentration of 109.29 ± 9.39 pg/mL and gradually decreased to 43.23 ± 2.034 pg/mL at 30 min. Rabbits administered with buccal tablets were released vitamin B12 in a sustained release manner and showed a 2.7-fold increase in bioavailability compared to the I.M. injection. | [35] |
| 16 | Vitamin K | Labrasol + Transcutol | Self-nano emulsifying lyophilized tablets | Improve the oral bioavailability of vitamin K | Human clinical trial. A group of volunteers administered buccal tablets and another group with intramuscular injection | Pharmacokinetic study in human volunteers revealed the buccal tablets enhanced vitamin K absorption and relative bioavailability. Interestingly, there was no significant difference in the vitamin K in systemic circulation for the two groups of volunteers. | [51] |
| 17 | B-complex vitamins: thiamine hydrochloride (THCl) and nicotinic acid (NA) | Propylene glycol | Films | Develop vitamin B-complex buccal films by inkjet printing technique | In vitro technique | Both vitamins are released within 10 minutes from the buccal film. For increasing vitamin content, there was an increase in permeation across the cellulosic membrane. | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, P. Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals. Foods 2021, 10, 1362. https://doi.org/10.3390/foods10061362
Subramanian P. Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals. Foods. 2021; 10(6):1362. https://doi.org/10.3390/foods10061362
Chicago/Turabian StyleSubramanian, Parthasarathi. 2021. "Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals" Foods 10, no. 6: 1362. https://doi.org/10.3390/foods10061362
APA StyleSubramanian, P. (2021). Mucoadhesive Delivery System: A Smart Way to Improve Bioavailability of Nutraceuticals. Foods, 10(6), 1362. https://doi.org/10.3390/foods10061362







