Production and Characterization of a Novel Low-Sugar Beverage from Red Jujube Fruits and Bamboo Shoots Fermented with Selected Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Raw Materials
2.2. Strains Screening
2.3. Screening of Fermentation Conditions for L. plantarum TUST-232
2.4. Changes in Sugar Content
2.5. Changes in Organic Acid Content
2.6. GC–MS Analysis of Volatile Compounds
2.7. Electronic Nose Analysis
2.8. Antioxidant Activity and the Contents of Total Phenolic, Total Flavonoid, and Dietary Fibers
2.9. Color Measurement
2.10. Statistical Analyses
3. Results
3.1. Selection of Starter Culture and Fermentation Conditions
3.1.1. Selection of Starter Culture
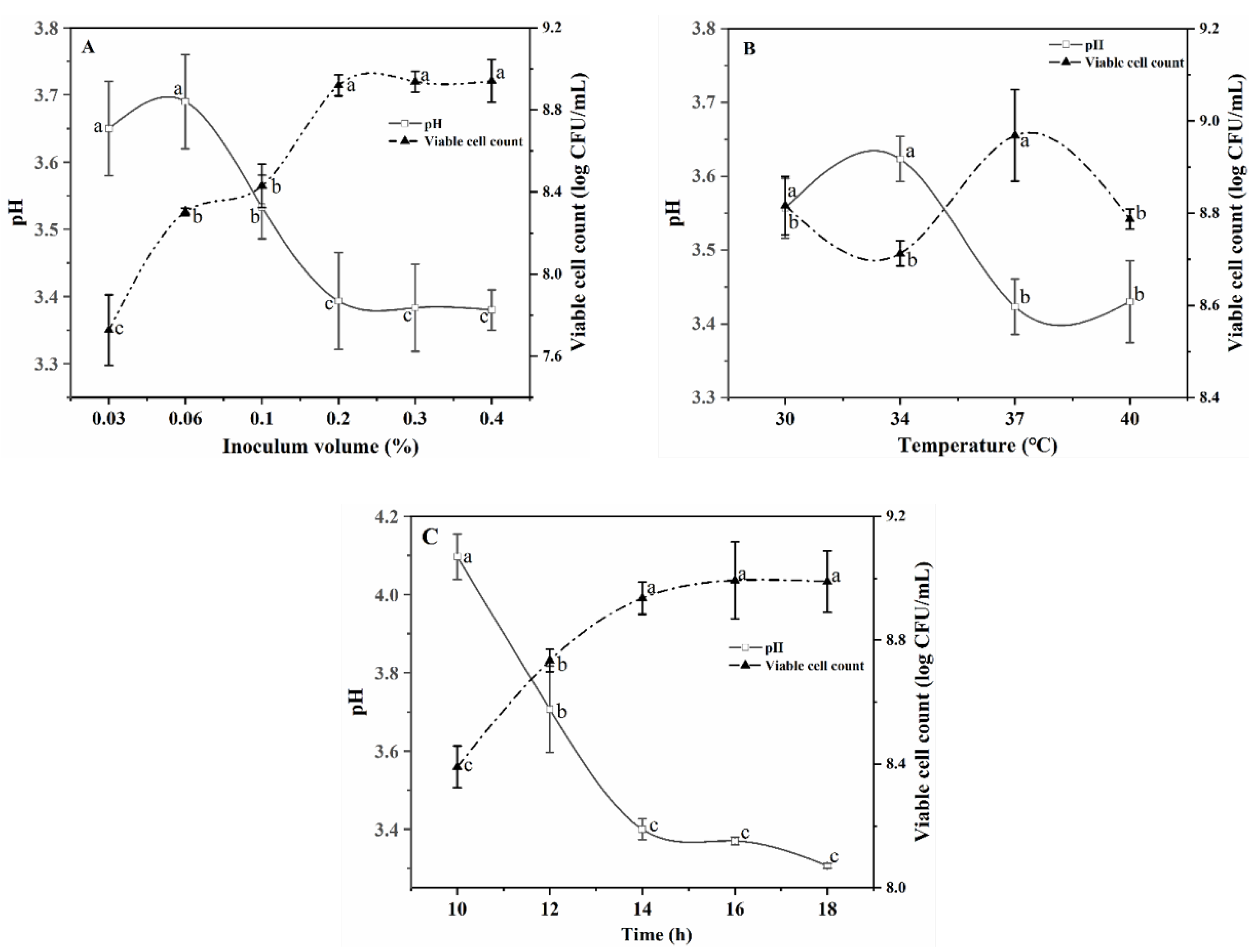
3.1.2. Selection of Fermentation Conditions
3.1.3. Growth Curve of L. plantarum TUST-232
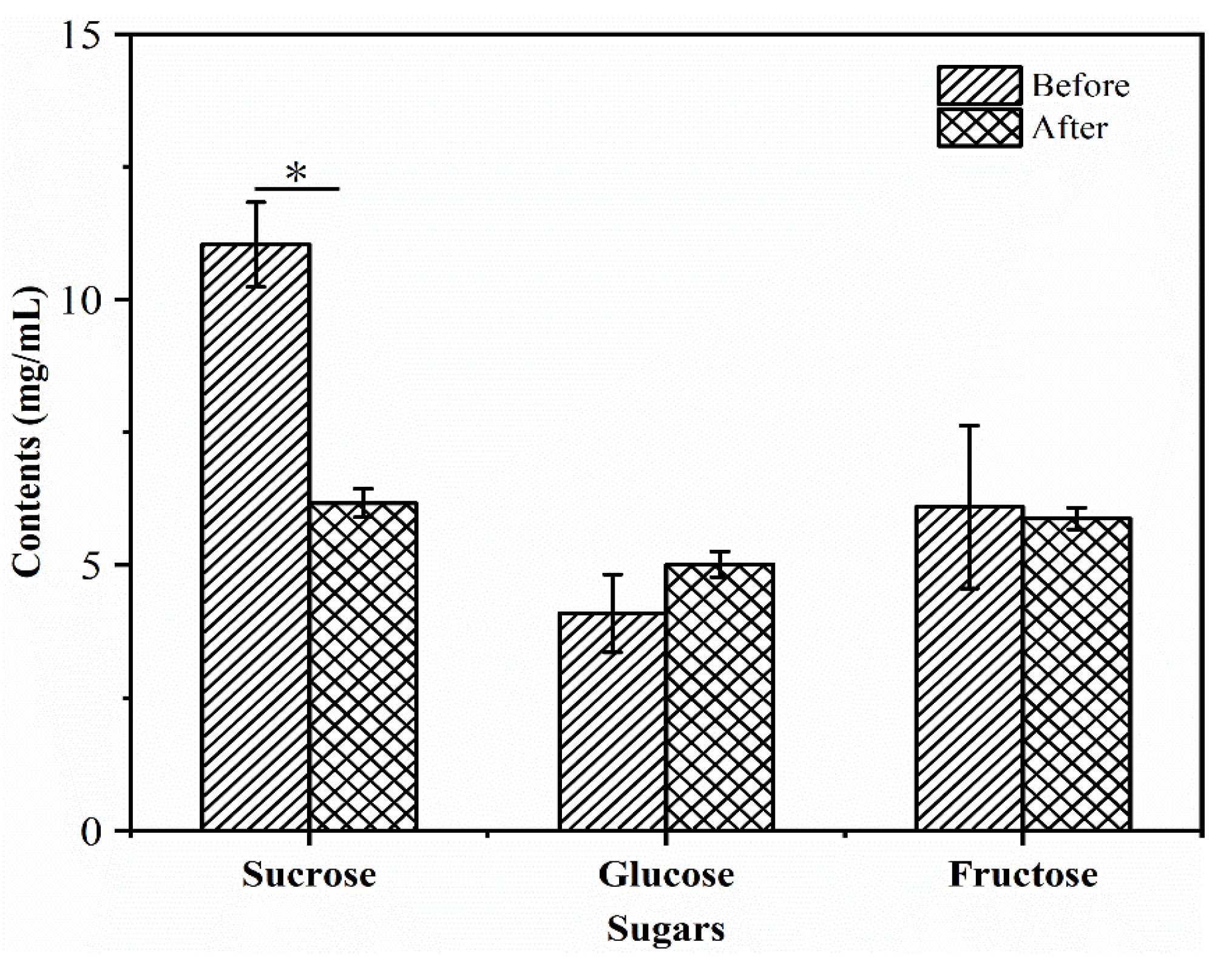
3.2. Changes in Sugar Content
3.3. Changes in Organic Acid Content
3.4. Changes in Volatile Compounds
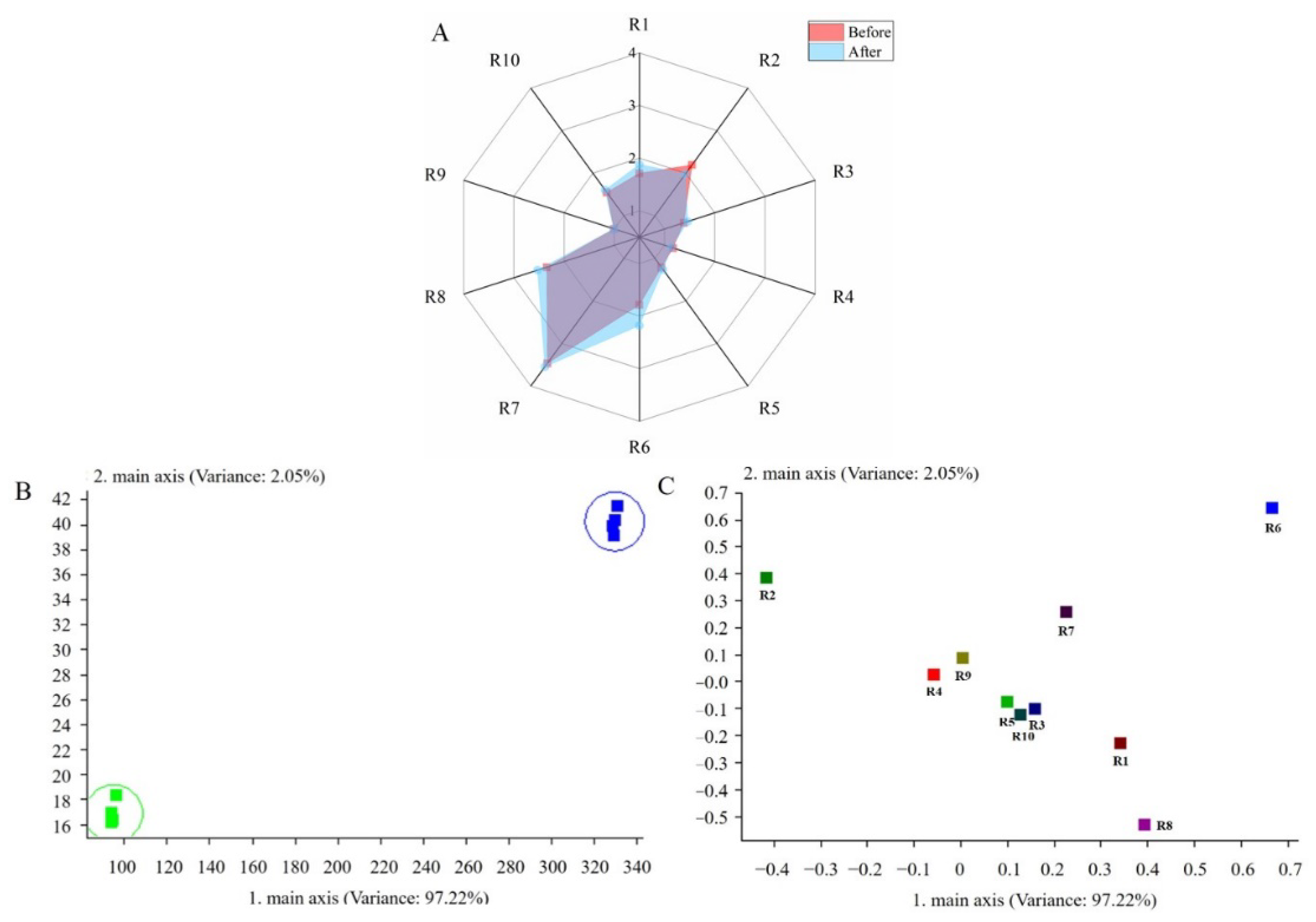
3.5. Changes in Aroma Profile
3.6. Changes of Antioxidant Activity and the Contents of Total Phenolic, Total Flavonoid, and Dietary Fibers
3.7. Changes in Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S. Bamboo: A prospective ingredient for Functional food and Nutraceuticals. Jpn. Bamboo J. 2017, 30, 82–99. [Google Scholar]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Frank, S.M.; Webster, J.; McKenzie, B.; Geldsetzer, P.; Manne-Goehler, J.; Andall-Brereton, G.; Houehanou, C.; Houinato, D.; Gurung, M.S.; Bicaba, B.W.; et al. Consumption of Fruits and Vegetables Among Individuals 15 Years and Older in 28 Low- and Middle-Income Countries. J. Nutr. 2019, 149, 1252–1259. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- Husain, M.M.; Kumar, A.; Mukhtar, H.; Krishnamurti, C.R. Xenobiotic biotransformation in wild birds: Activity, induction, characterization and comparison with rat and mouse microsomal enzymes. Xenobiotica 1981, 11, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Ojha, P.; Karki, T.B. Functional quality evaluation and shelf life study of synbiotic yacon juice. Food Sci. Nutr. 2020, 8, 1546–1553. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Shieh, M.-J.; Shang, H.-F.; Liao, F.-H.; Zhu, J.-S.; Chien, Y.-W. Lactobacillus fermentum improved intestinal bacteria flora by reducing Clostridium perfringens. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2011, 6, e59–e63. [Google Scholar] [CrossRef][Green Version]
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. Adv. Food Nutr. Res. 2019, 87, 147–185. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.; Ramos, C.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.P.; Patel, H. Sugandha Enzymatic added extraction and clarification of fruit juices–A review. Crit. Rev. Food Sci. Nutr. 2016, 57, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Dimitrovski, D.; Velickova, E.; Dimitrovska, M.; Langerholc, T.; Winkelhausen, E. Synbiotic functional drink from Jerusalem artichoke juice fermented by probiotic Lactobacillus plantarum PCS26. J. Food Sci. Technol. 2015, 53, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a Potential Probiotic, Lactobacillus plantarum L7, for the Preparation of a Rice-Based Fermented Beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Moghaddam, A.D.; Garavand, F.; Razavi, S.H.; Talatappe, H.D. Production of saffron-based probiotic beverage by lactic acid bacteria. J. Food Meas. Charact. 2018, 12, 2708–2717. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Feitosa, W.S.C.; Abreu, V.K.G.; Lemos, T.D.O.; Gomes, W.F.; Narain, N.; Rodrigues, S. Impact of fermentation conditions on the quality and sensory properties of a probiotic cupuassu (Theobroma grandiflorum) beverage. Food Res. Int. 2017, 100, 603–611. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Quan, J.; Zheng, Q.; Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Buratti, S.; Civelli, R.; Guidetti, R. Monitoring of fresh-cut Valerianella locusta Laterr. shelf life by electronic nose and VIS–NIR spectroscopy. Talanta 2014, 120, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-S.; Gao, B.; Li, X.-L.; Li, W.-N.; Qiao, Z.-A.; Han, L. Chemical Composition and Antimicrobial and Antioxidant Activities of Essential Oil of Sunflower (Helianthus annuus L.) Receptacle. Molecules 2020, 25, 5244. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. In vitro antioxidant and angiotensin-converting enzyme inhibitory activity of fermented milk with different culture combinations. J. Dairy Sci. 2020, 103, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Georgetti, S.; Vicentini, F.; Yokoyama, C.; Borin, M.; Spadaro, A.; Fonseca, M. Enhancedin vitroandin vivoantioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different β-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef]
- Klopotek, Y.; Otto, K.; Böhm, V. Processing Strawberries to Different Products Alters Contents of Vitamin C, Total Phenolics, Total Anthocyanins, and Antioxidant Capacity. J. Agric. Food Chem. 2005, 53, 5640–5646. [Google Scholar] [CrossRef]
- Letaief, H.; Zemni, H.; Mliki, A.; Chebil, S. Composition of Citrus sinensis (L.) Osbeck cv «Maltaise demi-sanguine» juice. A comparison between organic and conventional farming. Food Chem. 2016, 194, 290–295. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC Official Method 991.43 Total, Soluble, and Insoluble Dietary Fiber in Foods Enzymatic–Gravimetric Method, MES–TRIS Buffer First Action 1991 Final Action 1994; Association of Official Analytical Chemists: Washington, DC, USA, 1994. [Google Scholar]
- Raja, K.K.; Hari, P.; Chin, M.Q.K.; Singbal, K.; Fareez, I.M. Color Stability of a New Rice Husk Composite in Comparison with Conventional Composites after Exposure to Commonly Consumed Beverages in Malaysia. Int. J. Dent. 2019, 2019, 9753431–9753437. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Guerrero-Beltrán, J.Á. Antioxidant and physicochemical characteristics of unfermented and fermented pomegranate (Punica granatum L.) beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules 2017, 22, 1633. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; He, X.; Tang, Y.; Li, J.; Ling, D.; Li, C.-B.; Li, L.; Zheng, F.; Sheng, J.; et al. Fermentation process optimization and chemical constituent analysis on longan (Dimocarpus longan Lour.) wine. Food Chem. 2018, 256, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y.; Wang, Y.; Cao, B.; Zhang, F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019, 277, 238–245. [Google Scholar] [CrossRef]
- Nongdam, P.; Tikendra, L. The Nutritional Facts of Bamboo Shoots and Their Usage as Important Traditional Foods of Northeast India. Int. Sch. Res. Not. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Paz, P.C.; Janny, R.J.; Håkansson, Å. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef]
- Holzapfel, W. Use of starter cultures in fermentation on a household scale. Food Control. 1997, 8, 241–258. [Google Scholar] [CrossRef]
- Phiri, S.; Schoustra, S.E.; Heuvel, J.V.D.; Smid, E.J.; Shindano, J.; Linnemann, A. Fermented cereal-based Munkoyo beverage: Processing practices, microbial diversity and aroma compounds. PLoS ONE 2019, 14, e0223501. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef]
- Lu, Y.; Putra, S.D.; Liu, S.-Q. A novel non-dairy beverage from durian pulp fermented with selected probiotics and yeast. Int. J. Food Microbiol. 2018, 265, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter culture fermentation of Chinese sauerkraut: Growth, acidification and metabolic analyses. Food Control. 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef]
- Jin, X.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Comparative Evaluation of the Antioxidant Capacities and Organic Acid and Volatile Contents of Mango Slurries Fermented with Six Different Probiotic Microorganisms. J. Food Sci. 2018, 83, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.; Zeng, L.; Tang, J.; Xiang, W. Characterization of γ-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2020, 129, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Oh, J.; Hurh, B.-S.; Jeong, G.-H.; Shin, Y.-K.; Kim, Y.-S. Volatile Compounds Produced byLactobacillus paracaseiDuring Oat Fermentation. J. Food Sci. 2016, 81, C2915–C2922. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of lactic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Ferrada, B.M.B.; Pozo-Bayón, M.; Ángeles; Semorile, L.; Tymczyszyn, E.E. Changes in the volatile profile of Pinot noir wines caused by Patagonian Lactobacillus plantarum and Oenococcus oeni strains. Food Res. Int. 2018, 106, 22–28. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Sun, S.-H.; Zhang, Q.-D.; Su, Y.; Zong, Y.-L.; Xie, J.-P. Comparison of Liquid–Liquid Extraction, Simultaneous Distillation Extraction, Ultrasound-Assisted Solvent Extraction, and Headspace Solid-Phase Microextraction for the Determination of Volatile Compounds in Jujube Extract by Gas Chromatography/Mass Spectrometry. Anal. Lett. 2014, 47, 654–674. [Google Scholar] [CrossRef]
- Oliveira Érica, R.; Caliari, M.; Júnior, M.S.S.; Oliveira, A.R.; Duarte, R.C.M.; Boas, E.V.D.B.V. Assessment of chemical and sensory quality of sugarcane alcoholic fermented beverage. J. Food Sci. Technol. 2017, 55, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Caven-Quantrill, D.J.; Buglass, A.J. Comparison of micro-scale simultaneous distillation–extraction and stir bar sorptive extraction for the determination of volatile organic constituents of grape juice. J. Chromatogr. A 2006, 1117, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Wang, W.; Zheng, F.; Chen, F. Comparison of volatile compositions of 15 different varieties of Chinese jujube (Ziziphus jujuba Mill.). J. Food Sci. Technol. 2019, 56, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhu, P.; Zhu, Y.; Zeng, Y.; Yang, J.; Sun, Y. The development of low-calorie sugar and functional jujube food using biological transformation and fermentation coupling technology. Food Sci. Nutr. 2019, 7, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.-H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Ávila, G.C.G.M.; Montañez, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Sidari, R.; Martorana, A.; Zappia, C.; Mincione, A.; Giuffrè, A.M. Persistence and Effect of a Multistrain Starter Culture on Antioxidant and Rheological Properties of Novel Wheat Sourdoughs and Bread. Foods 2020, 9, 1258. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic Beverage From Pineapple Juice Fermented With Lactobacillus and Bifidobacterium Strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Van Der Kamp, J.W.; Harris, P.J. Editorial for the Special Issue “Dietary Fibre: New Insights on Biochemistry and Health Benefits. ” Int. J. Mol. Sci. 2018, 19, 3556. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary Fiber Content and Associated Antioxidant Compounds in Roselle Flower (Hibiscus sabdariffa L.) Beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef]


| Strain | 24 h | 48 h | ||
|---|---|---|---|---|
| pH | Viable Cell Count (Log CFU/mL) | pH | Viable Cell Count (Log CFU/mL) | |
| TUST-BS | 4.17 ± 0.08 b | 3.41 ± 0.11 c | 3.51 ± 0.05 b | 7.83 ± 0.11 c |
| TUST-232 | 3.43 ± 0.19 a | 8.90 ± 0.10 a | 3.29 ± 0.06 a | 8.69 ± 0.19 a |
| TUST-392 | 4.7 ± 0.16 c | 3.10 ± 0.29 c | 3.79 ± 0.12 c | 4.74 ± 0.08 d |
| TUST-354 | 3.99 ± 0.12 b | 4.64 ± 0.14 b | 3.31 ± 0.11 a | 8.42 ± 0.08 b |
| Number | Name | Retention Time (min) | Relative Content (%) | p-Value | |
|---|---|---|---|---|---|
| Before | After | ||||
| Aldehyde | |||||
| A1 | 3-methylbutyraldehyde | 2.756 | 0.41 | - | |
| A2 | valeraldehyde | 3.822 | 0.51 | - | |
| A3 | hexanal | 6.125 | 6.16 | 2.38 | * |
| A4 | heptanal | 8.827 | 3.35 | - | |
| A5 | 5-methylhexanal | 8.417 | - | 0.19 | |
| A6 | hexen-2-al | 9.989 | 2.25 | 0.16 | * |
| A7 | octanal | 11.926 | 3.14 | 2.81 | |
| A8 | (2Z)-2-heptenal | 13.105 | 11.23 | 9.23 | * |
| A9 | nonanal | 14.971 | 3.20 | 3.06 | |
| A10 | 2-octenal | 16.089 | 9.97 | 14.80 | * |
| A11 | furfural | 17.106 | 0.91 | - | |
| A12 | decanal | 18.367 | - | 0.67 | |
| A13 | lauric aldehyde | 18.467 | - | 0.34 | |
| A14 | benzaldehyde | 18.658 | 3.95 | 1.26 | * |
| A15 | 2-nonenal | 19.656 | 0.98 | 1.25 | |
| A16 | 2-decenal | 22.399 | 3.26 | 3.15 | |
| A17 | 2,4-nonadienal | 23.775 | 0.12 | 0.27 | |
| A18 | 2-undecenal | 24.929 | 1.25 | 2.41 | * |
| A19 | 2-trans, 4-trans-decadienal | 24.916 | - | 0.27 | |
| A20 | 5-hydroxymethyl-2-furaldehyde | 38.962 | 0.56 | 0.22 | |
| Olefin | |||||
| B1 | 2-methyl-6-methyl-2-methylene | 15.227 | - | 0.91 | |
| B2 | 3-ethyl-2-methyl-1,3-hexadien | 15.659 | 0.08 | 0.25 | |
| B3 | 2,4-dimethyl-1,3-pentadiene | 17.160 | - | 0.58 | |
| B4 | 3,5,5-trimethyl-2-Hexene | 17.558 | 0.10 | - | |
| B5 | 2-methyl-1,5-hexadiene | 20.783 | - | 0.11 | |
| Ketone | |||||
| C1 | 3-hydroxy-4-hexanone | 9.416 | 0.29 | 1.75 | * |
| C2 | 3-octanone | 10.917 | 0.13 | - | |
| C3 | 1-octen-3-one | 12.379 | 5.08 | 7.07 | * |
| C4 | 6-methyl-5-hepten-2-one | 13.457 | 0.47 | 0.89 | * |
| C5 | 2-nonanone | 14.883 | 0.11 | 0.37 | * |
| C6 | 2-octanone | 20.917 | - | 0.35 | |
| C7 | 6-methyl-2-heptanone | 23.618 | 0.33 | - | |
| C8 | l-fenchone | 26.238 | 0.56 | - | |
| C9 | nerylacetone | 26.913 | - | 0.59 | |
| C10 | 6,10-dimethyl-5,9-undecadien-2-one | 27.172 | 0.27 | - | |
| C11 | 4,5-dimethyl-2-cyclohexen-1-one | 30.164 | 0.48 | - | |
| C12 | 3,6-dimethyl-5-octen-2-one | 31.557 | - | 0.05 | |
| C13 | 6,10-dimethyl-2-undecanone | 33.092 | 0.40 | 0.46 | |
| Alcohol | |||||
| D1 | 3-octyn-2-ol | 7.558 | 0.12 | - | |
| D2 | 3,5,5-trimethyl-1-hexanol | 7.857 | - | 0.16 | |
| D3 | amyl alcohol | 11.201 | 0.08 | - | |
| D4 | hexyl alcohol | 14.007 | 0.57 | 0.67 | |
| D5 | 3,5-octadien-2-ol | 15.500 | 0.16 | 0.15 | |
| D6 | 1-octen-3-ol | 16.623 | 2.97 | 1.55 | * |
| D7 | heptyl alcohol | 16.775 | - | 0.33 | |
| D8 | 1-octanol | 19.821 | 0.72 | 0.71 | |
| D9 | (E)-2-octen-1-ol | 21.215 | 1.59 | 0.92 | * |
| D10 | menthol | 21.755 | - | 0.38 | |
| D11 | nonyl alcohol | 22.198 | - | 1.21 | |
| D12 | nerolidol | 26.048 | - | 1.30 | |
| D13 | benzyldehyde | 27.112 | - | 0.58 | |
| D14 | farnesol | 28.528 | - | 0.17 | |
| D15 | (2E,6E)-2,6-dimethyl-2,6-octadiene-1,8-diol | 29.730 | - | 0.09 | |
| D16 | pentaethylene glycol | 43.650 | 1.48 | - | |
| Ester | |||||
| E1 | methyl benzoate | 21.458 | 0.72 | 0.35 | * |
| E2 | geranyl phenylacetate | 21.907 | 1.51 | - | |
| E3 | methyl Laurate | 26.125 | 0.52 | 0.39 | |
| E4 | (Z)-3-decen-1-yl acetate | 26.308 | - | 0.27 | |
| E5 | 10-undecen-1-yl acetate | 30.056 | - | 0.46 | |
| E6 | decyl propionate | 35.042 | - | 0.30 | |
| E7 | benzyl salicylate | 42.976 | - | 0.24 | |
| Acid | |||||
| F1 | acetic acid | 16.974 | 0.67 | 0.91 | * |
| F2 | valeric acid | 24.010 | - | 0.03 | |
| F3 | hexanoic acid | 26.500 | 0.77 | 1.80 | * |
| F4 | heptanoic acid | 28.836 | 0.86 | 1.47 | * |
| F5 | octanoic acid | 31.057 | 1.26 | 2.83 | * |
| F6 | nonanoic acid | 33.382 | 0.69 | 0.88 | |
| F7 | decanoic acid | 35.357 | 5.83 | 7.06 | * |
| F8 | undecanoic acid | 37.183 | 0.18 | 0.14 | |
| F9 | lauric acid | 38.739 | 6.37 | 8.72 | * |
| F10 | myristic acid | 41.607 | 2.99 | 5.37 | * |
| F11 | palmitic acid | 44.527 | 2.98 | 2.40 | |
| F12 | oleic acid | 45.251 | 1.94 | - | |
| Others | |||||
| G1 | 2-pentylfuran | 9.937 | 0.10 | 0.13 | |
| G2 | azulene | 24.312 | 0.39 | 0.07 | * |
| G3 | adenosine cyclophosphate | 29.060 | 0.08 | - | |
| G4 | 2-methoxy-4-vinylphenol | 37.310 | - | 0.35 | |
| Parameter | Before | After |
|---|---|---|
| Total antioxidant capacity (μmol Fe2+ equivalents/100 mL) | 84.369 ± 1.891 | 94.743 ± 1.698 * |
| Iron ion-reducing ability (absorbance) | 0.261 ± 0.009 | 0.315 ± 0.156 * |
| Hydroxyl radical-scavenging activity (%) | 4.045 ± 0.639 | 9.833 ± 1.165 * |
| Superoxide anion-scavenging ability (U/100 mL) | 4.774 ± 0.177 | 7.629 ± 0.292 * |
| Total phenolic (mg/L) | 29.633 ± 1.075 | 32.918 ± 1.193 * |
| Total flavonoid (mg/L) | 85.016 ± 4.321 | 95.810 ± 9.295 |
| Total dietary fiber (g/100 g) | 0.536 ± 0.024 | 0.570 ± 0.020 |
| Insoluble dietary fiber (g/100 g) | 0.472 ± 0.019 | 0.492 ± 0.021 |
| Soluble dietary fiber (g/100 g) | 0.059 ± 0.043 | 0.078 ± 0.017 |
| Sample | L* | a* | b* | ΔL* | Δa* | Δb* | ΔE*ab |
|---|---|---|---|---|---|---|---|
| Before | 38.47 ± 0.40 | 14.42 ± 0.19 | 45.00 ± 0.20 | - | - | - | - |
| After | 38.50 ± 0.19 | 14.32 ± 0.09 | 44.81 ± 0.12 | 0.03 ± 0.59 | −0.10 ± 0.28 | −0.19 ± 0.32 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.-M.; Du, T.; Li, P.; Du, X.-J.; Wang, S. Production and Characterization of a Novel Low-Sugar Beverage from Red Jujube Fruits and Bamboo Shoots Fermented with Selected Lactiplantibacillus plantarum. Foods 2021, 10, 1439. https://doi.org/10.3390/foods10071439
Zhao C-M, Du T, Li P, Du X-J, Wang S. Production and Characterization of a Novel Low-Sugar Beverage from Red Jujube Fruits and Bamboo Shoots Fermented with Selected Lactiplantibacillus plantarum. Foods. 2021; 10(7):1439. https://doi.org/10.3390/foods10071439
Chicago/Turabian StyleZhao, Chu-Min, Ting Du, Ping Li, Xin-Jun Du, and Shuo Wang. 2021. "Production and Characterization of a Novel Low-Sugar Beverage from Red Jujube Fruits and Bamboo Shoots Fermented with Selected Lactiplantibacillus plantarum" Foods 10, no. 7: 1439. https://doi.org/10.3390/foods10071439
APA StyleZhao, C.-M., Du, T., Li, P., Du, X.-J., & Wang, S. (2021). Production and Characterization of a Novel Low-Sugar Beverage from Red Jujube Fruits and Bamboo Shoots Fermented with Selected Lactiplantibacillus plantarum. Foods, 10(7), 1439. https://doi.org/10.3390/foods10071439







