Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enzyme Assays
2.3. Preparation and Pretreatment of Sea Bass Skin Using Pulsed Electric Field (PEF)
2.4. Defatting of PEF-Treated Skin
2.5. HC Preparation
2.6. Size Distribution
2.7. Impact of HC on Proliferation and Differentiation of MC3T3-E1 Preosteoblast Cells
2.7.1. Cell Culture
2.7.2. Cell Proliferation Assays
2.7.3. Alkaline Phosphatase Activity (AP-A)
2.7.4. Determination of Mineralization in MC3T3-E1 Cells
2.7.5. Western Blot Analysis
2.7.6. Effect of HC Combined with Inhibitor on AP-A and Mineralization in Cells
2.8. Statistical Analysis
3. Results
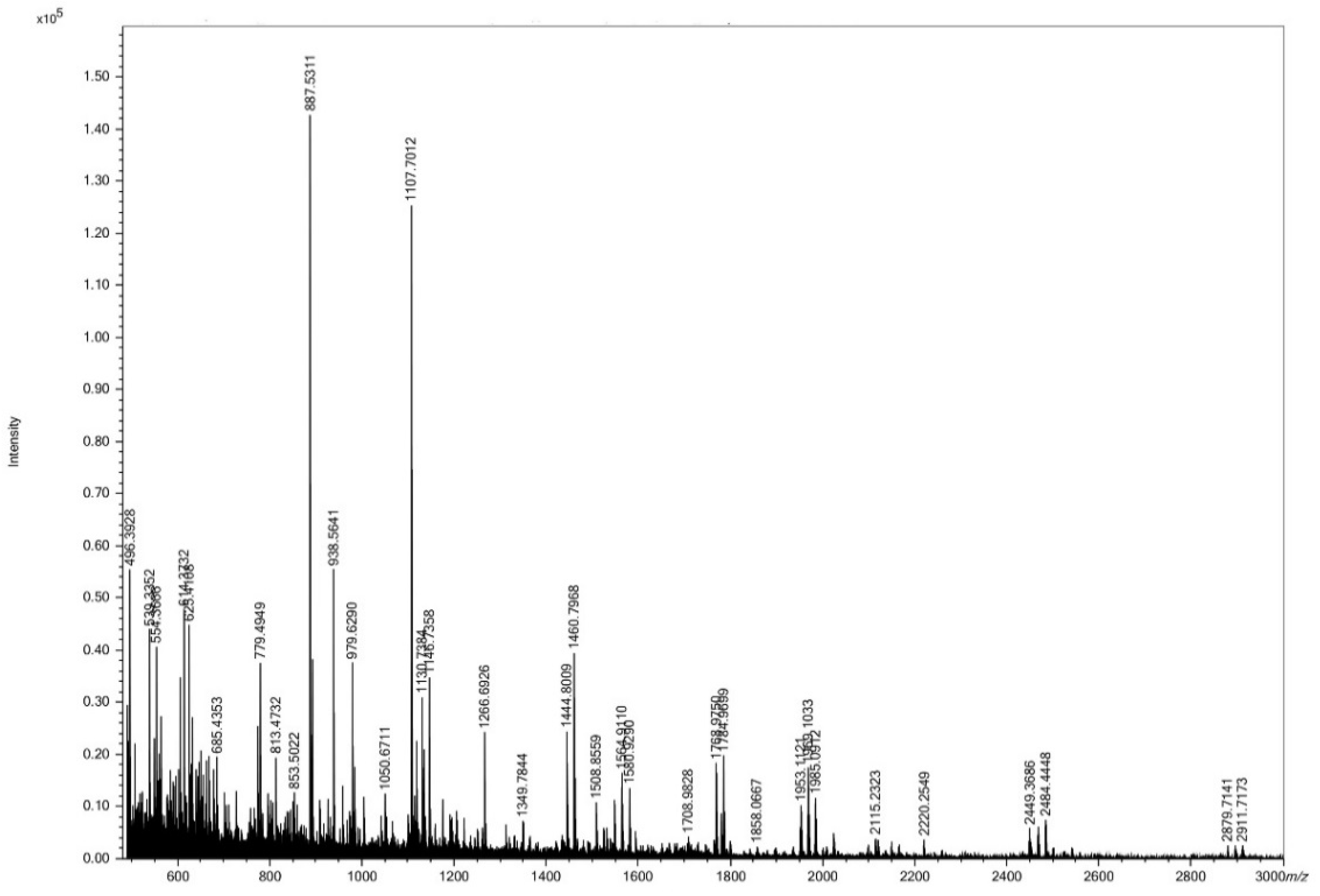
3.1. Size Distribution of HC
3.2. Impact of HC on Proliferation, Alkaline Phosphatase Activity and Mineralization of MC3T3-E1 Cells
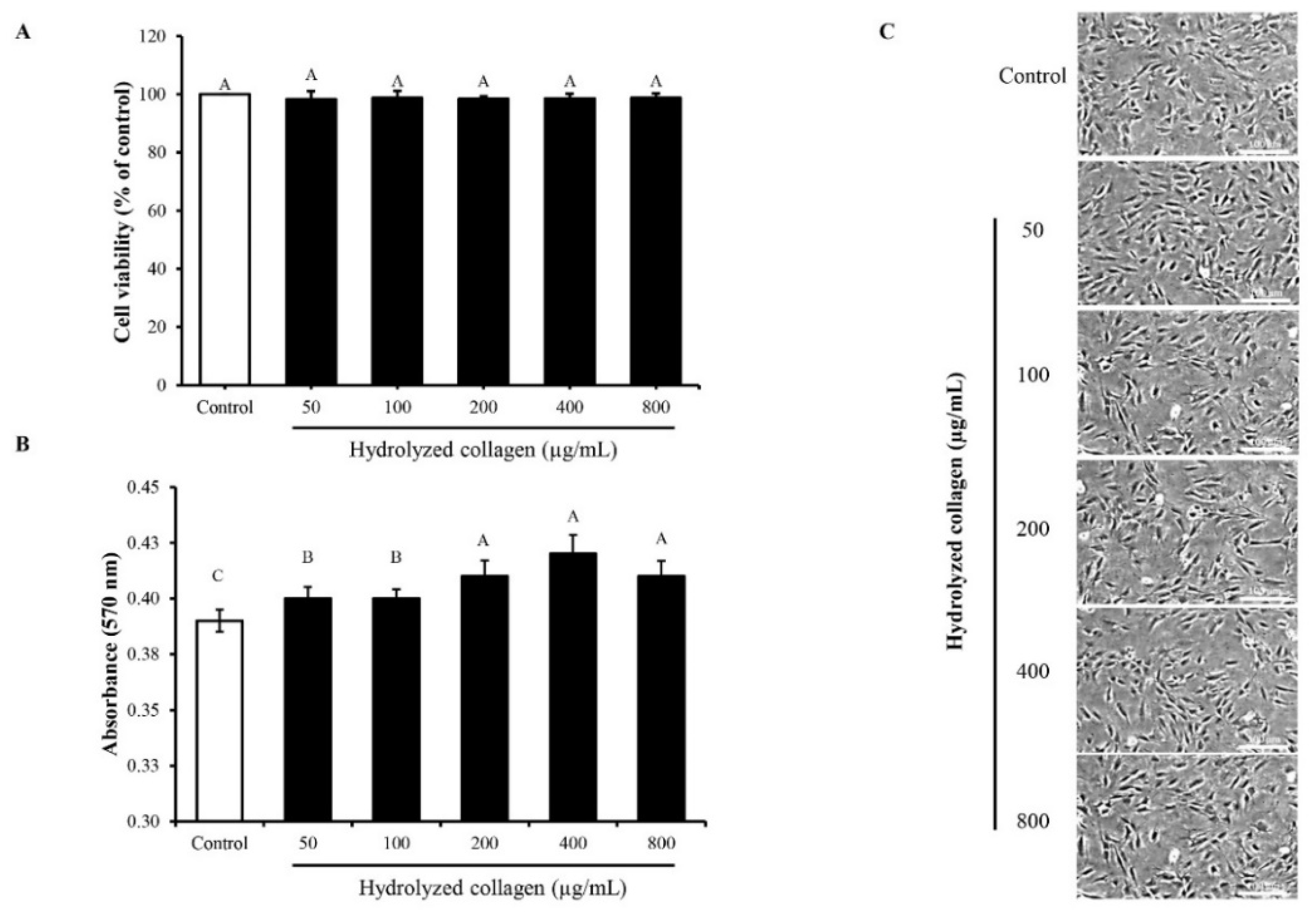
3.2.1. MC3T3-E1 Cell Proliferation
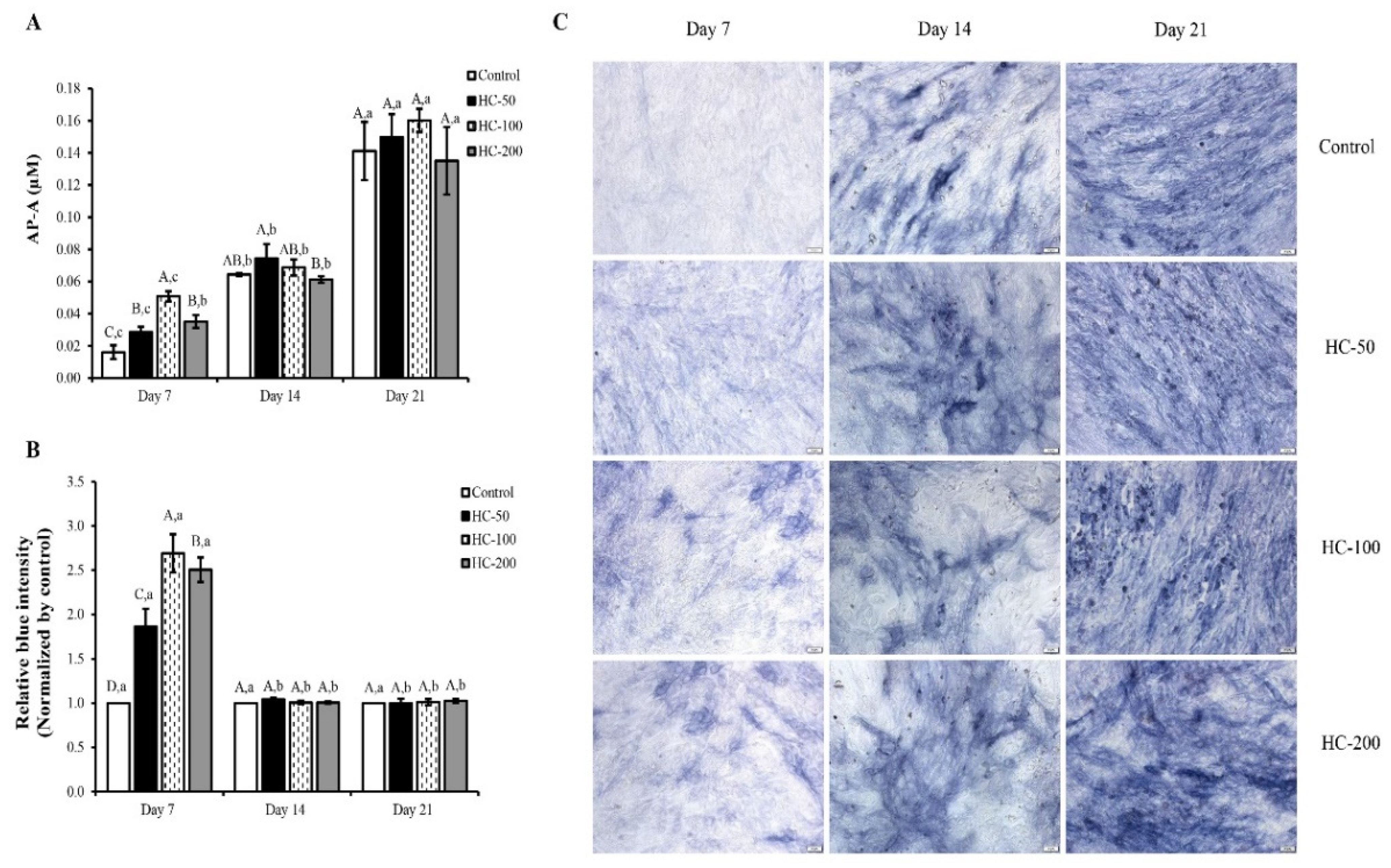
3.2.2. Alkaline Phosphatase Activity (AP-A)
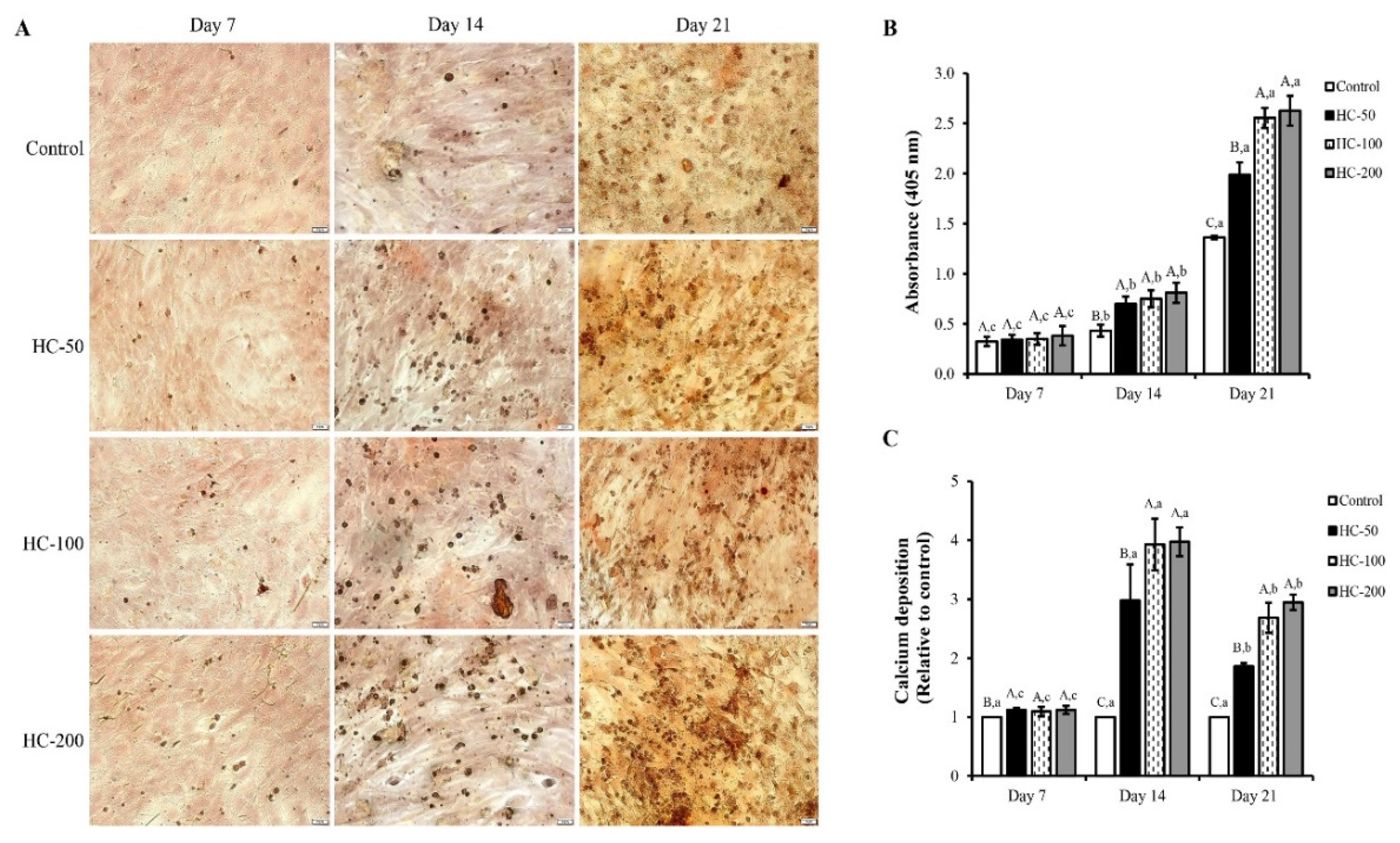
3.2.3. Mineralization of MC3T3-E1 Cells
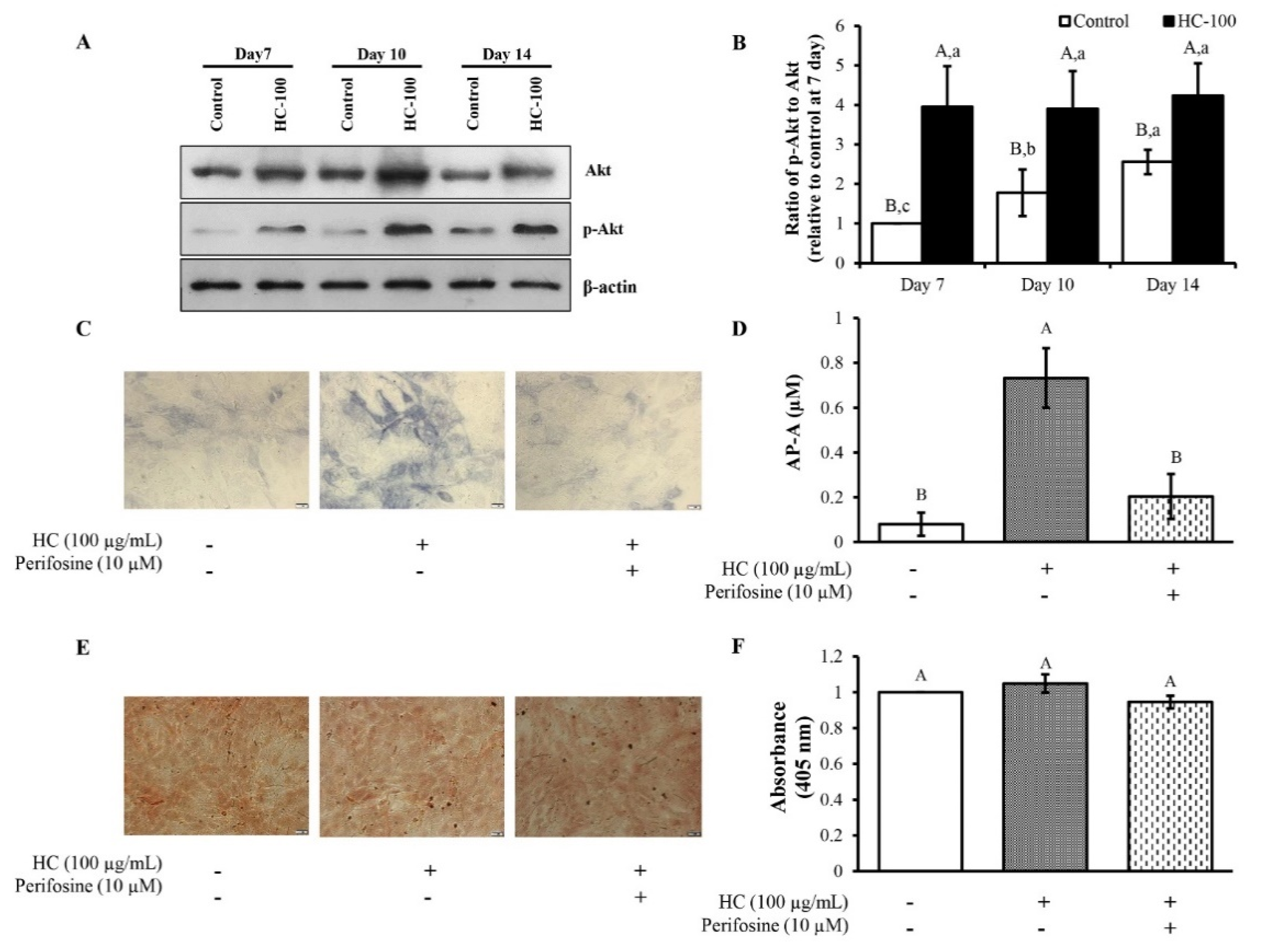
3.3. Effect of HC on RUNX2 Expression in MC3T3-E1 Cells
3.4. Effect of HC on Akt Signaling Pathway in MC3T3-E1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Hydrolyzed collagen from porcine lipase-defatted seabass skin: Antioxidant, fibroblast cell proliferation, and collagen production activities. J. Food Biochem. 2019, 43, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Effect of pulsed electric field-assisted process in combination with porcine lipase on defatting of seabass skin. J. Food Sci. 2019, 84, 1799–1805. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Aluko, R.E.; Benjakul, S. Enhanced Asian sea bass skin defatting using porcine lipase with the aid of pulsed electric field pretreatment and vacuum impregnation. Process Biochem. 2019, 86, 58–64. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. Technol. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Paisrisarn, P.; Tepaamorndech, S.; Khongkow, M.; Khemthong, P.; Kasamechonchung, P.; Klysubun, W.; Wutikhun, T.; Huang, L.; Chantarasakha, K.; Boonrungsiman, S. Alterations of mineralized matrix by lead exposure in osteoblast (MC3T3-E1) culture. Toxicol. Lett. 2018, 299, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J.H.; Choi, E.H.; Han, I. Promotion of osteogenic differentiation by non-thermal biocompatible plasma treated chitosan scaffold. Sci. Rep. 2019, 9, 3712. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Sukketsiri, W.; Aluko, R.E.; Sae-leaw, T.; Benjakul, S. Effect of hydrolyzed collagen from defatted Asian sea bass (Lates calcarifer) skin on fibroblast proliferation, migration and antioxidant activities. J. Food Sci. Technol. 2020, 58, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, B.; Lu, W.; Liu, J.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; Guo, Y. Cell proliferation stimulation ability and osteogenic activity of low molecular weight peptides derived from bovine gelatin hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef]
- Ye, M.; Jia, W.; Zhang, C.; Shen, Q.; Zhu, L.; Wang, L. Preparation, identification and molecular docking study of novel osteoblast proliferation-promoting peptides from yak (Bos grunniens) bones. RSC Adv. 2019, 9, 14627–14637. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Ogura, K.; Taniuchi, Y.; Kataoka, A.; Inoue, N.; Sugihara, F.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline promotes differentiation of MC3T3-E1 osteoblastic cells. Biochem. Biophys. Res. Commun. 2014, 453, 498–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimira, Y.; Odaira, H.; Nomura, K.; Taniuchi, Y.; Inoue, N.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline promotes osteogenic differentiation through Foxg1. Cell. Mol. Biol. Lett. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sae-leaw, T.; Karnjanapratum, S.; O′Callaghan, Y.C.; O′Keeffe, M.B.; FitzGerald, R.J.; O′Brien, N.M.; Benjakul, S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J. Food Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Guo, Y. Cell Growth Stimulation, Cell Cycle Alternation, and Anti-Apoptosis Effects of Bovine Bone Collagen Hydrolysates Derived Peptides on MC3T3-E1 Cells Ex Vivo. Molecules 2020, 25, 2305. [Google Scholar] [CrossRef]
- Wu, W.; He, L.; Li, C.; Zhao, S.; Liang, Y.; Yang, F.; Zhang, M.; Jin, G.; Ma, M. Phosphorylation of porcine bone collagen peptide to improve its calcium chelating capacity and its effect on promoting the proliferation, differentiation and mineralization of osteoblastic MC3T3-E1 cells. J. Funct. Foods 2020, 64, 103701. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.X.; Du, J.; Si, M.S.; Mo, J.J.; Qiao, S.C.; Lai, H.C. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J. Biomed. Mater. Res. Part A 2013, 101, 748–754. [Google Scholar] [CrossRef]
- Chu, N.; Salguero, A.L.; Liu, A.Z.; Chen, Z.; Dempsey, D.R.; Ficarro, S.B.; Alexander, W.M.; Marto, J.A.; Li, Y.; Amzel, L.M.; et al. Akt kinase activation mechanisms revealed using protein semisynthesis. Cell 2018, 174, 897–907.e14. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci. 2020, 245, 117389. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, K.A.; Boregowda, R.K.; Lasfar, A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol. Cancer 2015, 14, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-M.; Chen, P.-C.; Li, T.-M.; Fong, Y.-C.; Tang, C.-H. Si-Wu-tang extract stimulates bone formation through PI3K/Akt/NF-κB signaling pathways in osteoblasts. BMC Complement. Altern. Med. 2013, 13, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods 2021, 10, 1476. https://doi.org/10.3390/foods10071476
Chotphruethipong L, Binlateh T, Hutamekalin P, Aluko RE, Tepaamorndech S, Zhang B, Benjakul S. Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods. 2021; 10(7):1476. https://doi.org/10.3390/foods10071476
Chicago/Turabian StyleChotphruethipong, Lalita, Thunwa Binlateh, Pilaiwanwadee Hutamekalin, Rotimi E. Aluko, Surapun Tepaamorndech, Bin Zhang, and Soottawat Benjakul. 2021. "Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells" Foods 10, no. 7: 1476. https://doi.org/10.3390/foods10071476
APA StyleChotphruethipong, L., Binlateh, T., Hutamekalin, P., Aluko, R. E., Tepaamorndech, S., Zhang, B., & Benjakul, S. (2021). Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods, 10(7), 1476. https://doi.org/10.3390/foods10071476









