Wine Pomace Product Inhibit Listeria monocytogenes Invasion of Intestinal Cell Lines Caco-2 and SW-480
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Wine Pomace Products (WPP)
2.3. WPP Microbiological Quality
2.4. Characterization of Phenolic Compounds
2.5. Quencher Antioxidant Capacity
2.6. Bacteria Strains, Culture Conditions, and Treatment with WPP
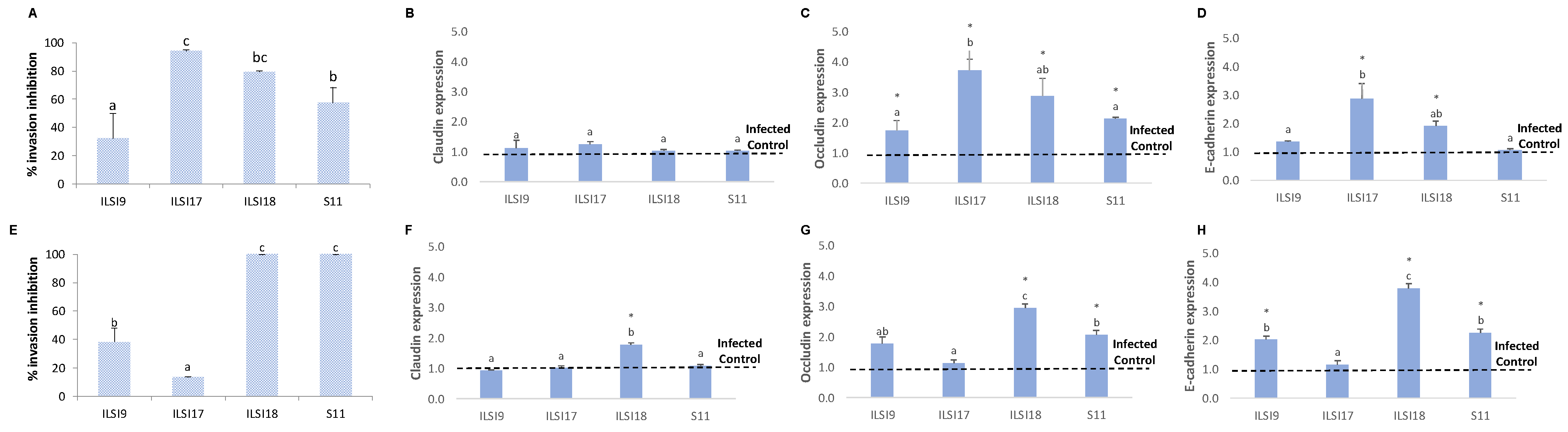
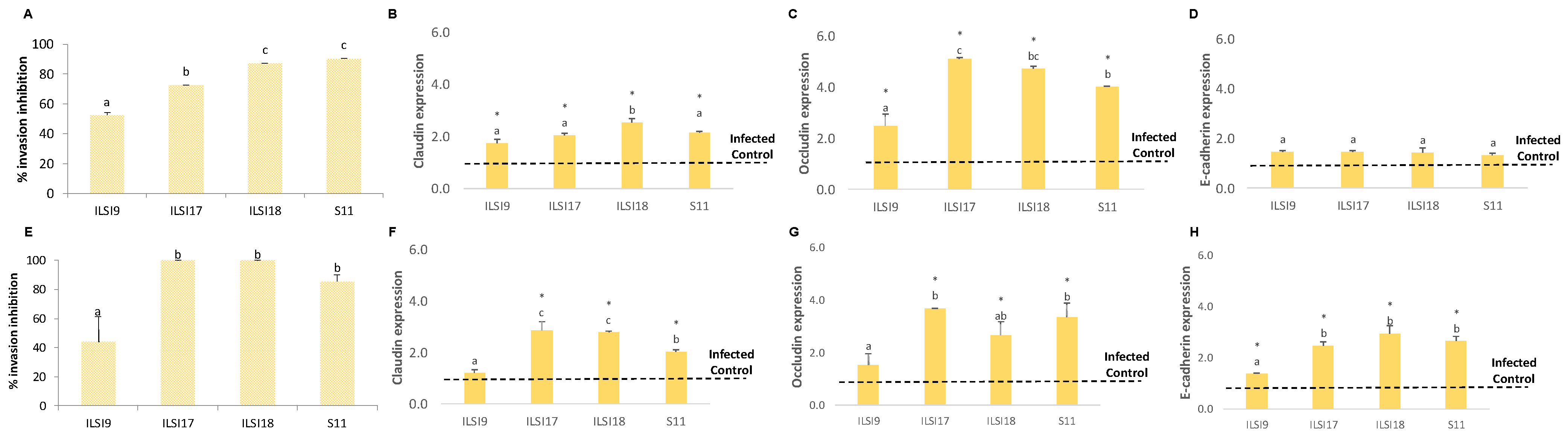
2.7. In Vitro Virulence Assays
2.8. Analysis of Adherent and Tight Junctions by Quantitative Real-Time PCR (q-PCR)
2.9. Statistical Assays
3. Results and Discussion
3.1. Effect of WPP on the Invasive Capacity of Listeria monocytogenes in Caco-2 and SW480 Cell Lines
3.2. Antivirulence Effect of L. monocytogenes Treated with WPP on Caco-2 Cells and SW480 Cell Lines
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jordan, K.; McAuliffe, O. Listeria monocytogenes in Foods. Adv. Food Nutr. Res. 2018, 86, 181–213. [Google Scholar]
- Allerberger, F.; Wagner, M. Listeriosis: A Resurgent Foodborne Infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, J.; Tan, W.; Zhang, Y.; Wang, H.; Zhou, X.; Liu, S.; Feng, H.; Li, W.; Niu, X.; et al. Fisetin Inhibits Listeria Monocytogenes Virulence by Interfering with the Oligomerization of Listeriolysin O. J. Infect. Dis. 2014, 211, 1376–1387. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- Rebagliati, V.; Philippi, R.; Rossi, M.; Troncoso, T. Prevention of Foodborne Listeriosis. Indian J. Pathol. Microbiol. 2009, 52, 145. [Google Scholar]
- Wang, J.; Liu, B.; Teng, Z.; Zhou, X.; Wang, X.; Zhang, B.; Lu, G.; Niu, X.; Yang, Y.; Deng, X. Phloretin Attenuates Listeria Monocytogenes Virulence Both In Vitro and In Vivo by Simultaneously Targeting Listeriolysin O and Sortase, A. Front. Cell. Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef]
- Bierne, H.; Mazmanian, S.K.; Trost, M.; Pucciarelli, M.G.; Liu, G.; Dehoux, P.; Genome, L.; Jänsch, L.; Portillo, F.G.; Schneewind, O.; et al. Inactivation of the SrtA Gene in Listeria Monocytogenes Inhibits Anchoring of Surface Proteins and Affects Vir-Ulence. Mol. Microbiol. 2002, 43, 869–881. [Google Scholar] [CrossRef]
- Drolia, R.; Bhunia, A.K. Crossing the Intestinal Barrier via Listeria Adhesion Protein and Internalin, A. Trends Microbiol. 2019, 27, 408–425. [Google Scholar] [CrossRef]
- Vera, A.; González, G.; Domínguez, M. Principales Factores De Virulencia De Listeria Monocytogenes Y Su regulación. Rev. Chilena Infectol. 2013, 30, 407–416. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L.; del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz, P. Antioxidant and Antimicrobial Properties of Wine Byproducts and Their Potential Uses in the Food Industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [PubMed]
- Magariños, H.L.E.; Sahr, C.; Selaive, S.D.C.; Costa, M.E.; Figuerola, F.E.; Pizarro, O.A. In Vitro Inhibitory Effect of Cranberry (Vaccinium Macrocarpum Ait.) Juice on Pathogenic Microorganisms. Appl. Biochem. Microbiol. 2008, 44, 300–304. [Google Scholar] [CrossRef]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäe, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Anti-Bacterial and Antioxidative Properties of Different Parts of Garden Rhubarb, Blackcurrant, Chokeberry and Blue Honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef]
- Nazer, A.; Kobilinsky, A.; Tholozan, J.-L.; Dubois-Brissonnet, F. Combinations of Food Antimicrobials at Low Levels to Inhibit the Growth of Salmonella Sv. Typhimurium: A Synergistic Effect? Food Microbiol. 2005, 22, 391–398. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.-J.; Haroutounian, S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.A.L.; Del Pino-García, R.; Rivero-Pérez, M.A.D.; Muñiz-Rodríguez, P. Alternative Natural Seasoning to Improve the Microbial Stability of Low-Salt Beef Patties. Food Chem. 2017, 227, 122–128. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic Compounds, Antioxidant, and Antibacterial Properties of Pomace Extracts from Four Virginia—Grown Grape Varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Antonić, B.; Jančková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Me-Ta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.; Cabral, L.M. Grape by-Product Extracts Against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Modulation of Akt-p38-MAPK/Nrf2/SIRT1 and NF-κB Pathways by Wine Pomace Product in Hyperglycemic Endothelial Cell Line. J. Funct. Foods 2019, 58, 255–265. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The Protective Effects of Wine Pomace Products on the Vascular Endothelial Barrier Function. Food Funct. 2020, 11, 7878–7891. [Google Scholar] [CrossRef] [PubMed]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation. Cell Host Microbe 2018, 23, 470–484.e7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E. Optimization of a Solid-Phase Extraction Method Using Copolymer Sorbents for Isolation of Phenolic Compounds in Red Wines and Quantification by HPLC. J. Agric. Food Chem. 2008, 56, 11560–11570. [Google Scholar] [CrossRef]
- Rivero-Pérez, M.; Muñiz, P.; González-SanJosé, M.L. Contribution of Anthocyanin Fraction to the Antioxidant Properties of Wine. Food Chem. Toxicol. 2008, 46, 2815–2822. [Google Scholar] [CrossRef]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-Sanjosé, M.L.; Muñiz, P. Adaptation and Validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) Antioxidant Capacity Assays in Model Products Obtained from Residual Wine Pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef]
- Fugett, M.; Fortes, E.; Nnoka, E.; Wiedmann, C. International Life Sciences Institute North America Listeria Monocytogenes Strain Collection: Development of Standard Listeria Monocytogenes Strain Sets for Research and Validation studies. J. Food Prot. 2006, 69, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the Major Listeria Monocytogenes Serovars by Multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed]
- Melero, B.; Stessl, B.; Manso, B.; Wagner, M.; Esteban-Carbonero, Ó.J.; Hernández, M.; Rovira, J.; Rodriguez-Lázaro, D. Listeria Monocytogenes Colonization in a Newly Established Dairy Processing Facility. Int. J. Food Microbiol. 2019, 289, 64–71. [Google Scholar] [CrossRef]
- Pricope, L.; Nicolau, A.; Wagner, M.; Rychli, K. The Effect of Sublethal Concentrations of Benzalkonium Chloride on Inva-Siveness and Intracellular Proliferation of Listeria Monocytogenes. Food Control 2013, 31, 230–235. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic Compounds and Antioxidant Activity from Red Grape Marc Extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Rivero-Pérez, M.L.; Muñiz, M.D.; González-Sanjosé, P. Antioxidant Profile of Red Wines Evaluated by Total Antioxidant Capacity, Scavenger Activity, and Biomarkers of Oxidative Stress methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef]
- Orsi, R.H.; Bakker, H.D.; Wiedmann, M. Listeria Monocytogenes Lineages: Genomics, Evolution, Ecology, and Phenotypic Characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; El-Gebali, S.; Natoli, M.; Sengstag, T.; Delorenzi, M.; Bentz, S.; Bouzourene, H.; Rumbo, M.; Felsani, A.; Siissalo, S.; et al. Defining New Criteria for Selection of Cell-Based Intestinal Models Using Publicly Available Databases. BMC Genomics 2012, 13, 274. [Google Scholar] [CrossRef]
- Yoon, W.-H.; Lee, S.-K.; Song, K.-S.; Kim, J.-S.; Kim, T.-D.; Li, G.; Yun, E.-J.; Heo, J.Y.; Jung, Y.-J.; Park, J.-I.; et al. The Tumorigenic, Invasive and Metastatic Potential of Epithelial and Round Subpopulations of the SW480 Human Colon Cancer Cell Line. Mol. Med. Rep. 2008, 1, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Dynamic Contacts: Rearranging Adherens Junctions to Drive Epithelial Remodelling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhang, B.; Niu, X.; Song, M.; Luo, Z.; Lu, G.; Liu, B.; Zhao, X.; Wang, J.; et al. Inhibition of Sortase A by Chalcone Prevents Listeria Monocytogenes Infection. Biochem. Pharmacol. 2016, 106, 19–29. [Google Scholar] [CrossRef]
- Goebel, W.; Gonza, B.; Domi, G.; de Patoge, G. Listeria Pathogenesis and Molecular Virulence Determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar]
- Moroni, O.; Kheadr, E.; Boutin, Y.; Lacroix, C.; Fliss, I. Inactivation of Adhesion and Invasion of Food-Borne Listeria Mono-Cytogenes by Bacteriocin-Producing Bifidobacterium Strains of Human Origin. Appl. Environ. Microbiol. 2006, 72, 6894–6901. [Google Scholar] [CrossRef]
- Selma, M.V.; Larrosa, V.; Beltrán, D.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.; Espín, J.C. Resveratrol and Some Glucosyl, Glucosylacyl, and Glucuronide Derivatives Reduce Escherichia Coli O157:H7, Salmonella Typhimurium, and Listeria Mono-Cytogenes Scott A Adhesion to Colonic Epithelial Cell Lines. J. Agric. Food Chem. 2012, 60, 7367–7374. [Google Scholar] [CrossRef]
- Manso, B.; Melero, B.; Stessl, B.; Fernández-Natal, I.; Jaime, I.; Hernández, M.; Wagner, M.; Rovira, J.; Rodríguez-Lázaro, D. Characterization of Virulence and Persistence Abilities of Listeria Monocytogenes Strains Isolated from Food Processing Premises. J. Food Prot. 2019, 82, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Melero, B.; Manso, B.; Stessl, B.; Hernández, M.; Wagner, M.; Rovira, J.; Rodríguez-Lázaro, D. Distribution and Persistence of Listeria Monocytogenes in a Heavily Contaminated Poultry Processing Facility. J. Food Prot. 2019, 82, 1524–1531. [Google Scholar] [CrossRef]
- Ciolacu, L.; Nicolau, A.I.; Wagner, M.; Rychli, K. Listeria Monocytogenes Isolated from Food Samples from a Romanian Black Market Show Distinct Virulence Profiles. Int. J. Food Microbiol. 2015, 209, 44–51. [Google Scholar] [CrossRef]
- Biazik, J.M.; Jahn, K.A.; Su, Y.; Wu, Y.N.; Braet, F. Unlocking the Ultrastructure of Colorectal Cancer Cells in Vitro Using Selective Staining. World J. Gastroenterol. 2010, 16, 2743–2753. [Google Scholar] [CrossRef]
- Swaminathan, P.; Cabanes, B.; Zhang, D.; Cossart, W. Listeria Monocytogenes, Food Microbiology: Fundamentals and Frontiers, 3rd ed.; Doyle, M., Beuchat, L., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 457–491. [Google Scholar]
- Cajnko, M.M.; Marušić, M.; Kisovec, M.; Rojko, N.; Benčina, M.; Caserman, S.; Anderluh, G. Listeriolysin O Affects the Per-Meability of Caco-2 Monolayer in a Pore-Dependent and Ca2+-Independent Manner. PLoS ONE 2015, 10, e0130471. [Google Scholar] [CrossRef]
- Latorre, E.; Pradilla, A.; Chueca, B.; Pagán, R.; Layunta, E.; Alcalde, A.I.; Mesonero, J.E. Listeria Monocytogenes Inhibits Serotonin Transporter in Human Intestinal Caco-2 Cells. Microb. Ecol. 2016, 72, 730–739. [Google Scholar] [CrossRef]
- Mohan, V.; Wibisono, W.R.; de Hoop, L.; Summers, G.; Fletcher, G.C. Identifying Suitable Listeria Innocua Strains as Surro-Gates for Listeria Monocytogenes for Horticultural Products. Front. Microbiol. 2019, 10, 2281. [Google Scholar] [CrossRef]
- Rodríguez-Vaquero, M.J.; Fernández Aredes, P.A.; de Nadra, M.C.M. Effect of Phenolic Compound Mixtures on the Viability of Listeria Monocytogenes in Meat Model. Food Technol. Biotechnol. 2011, 49, 83–88. [Google Scholar]
- Opoku-Temeng, C.; Sintim, H.O. Inhibition of Cyclic Diadenylate Cyclase, DisA, by Polyphenols. Sci. Rep. 2016, 6, 25445. [Google Scholar] [CrossRef] [PubMed]
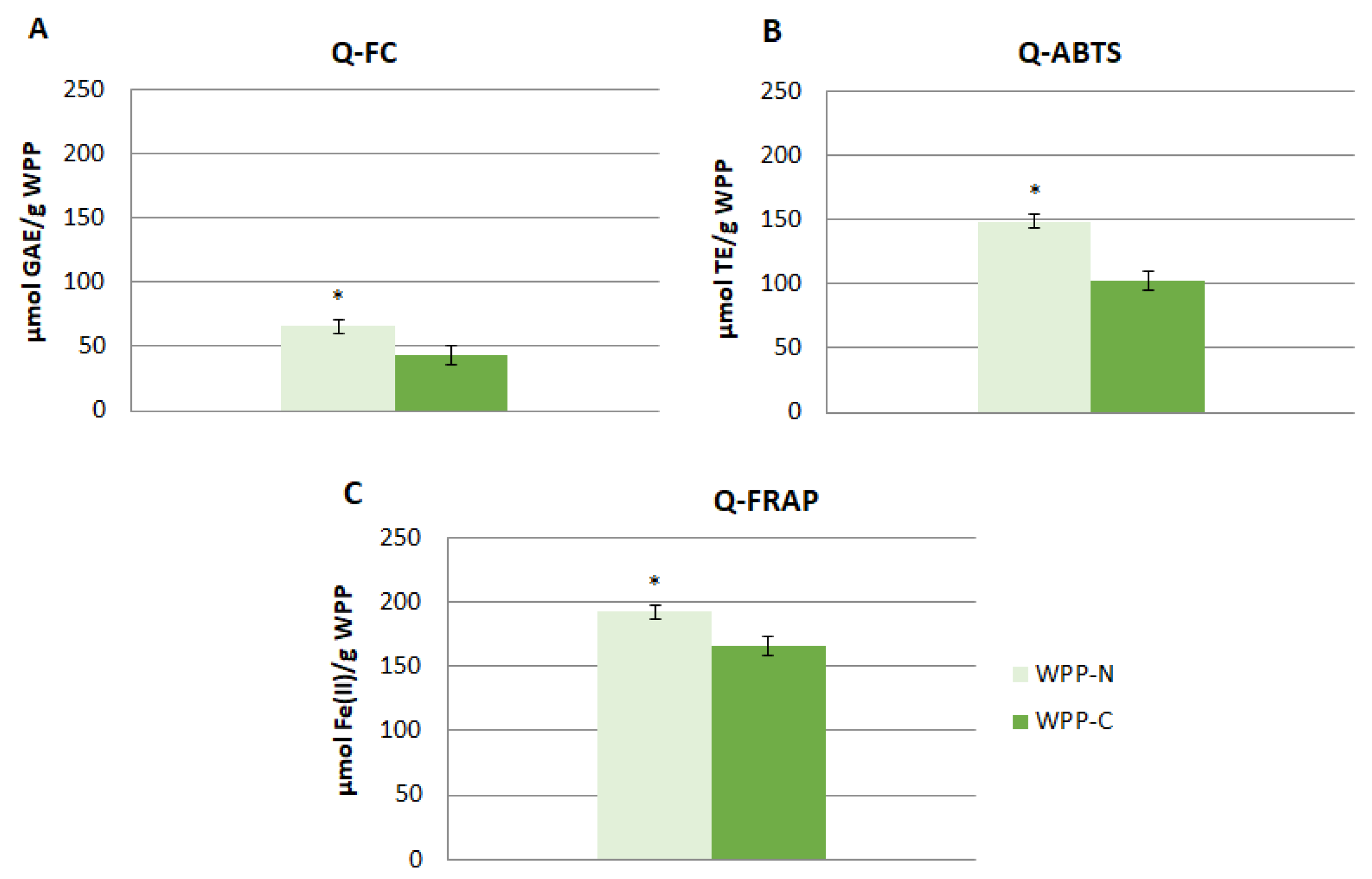

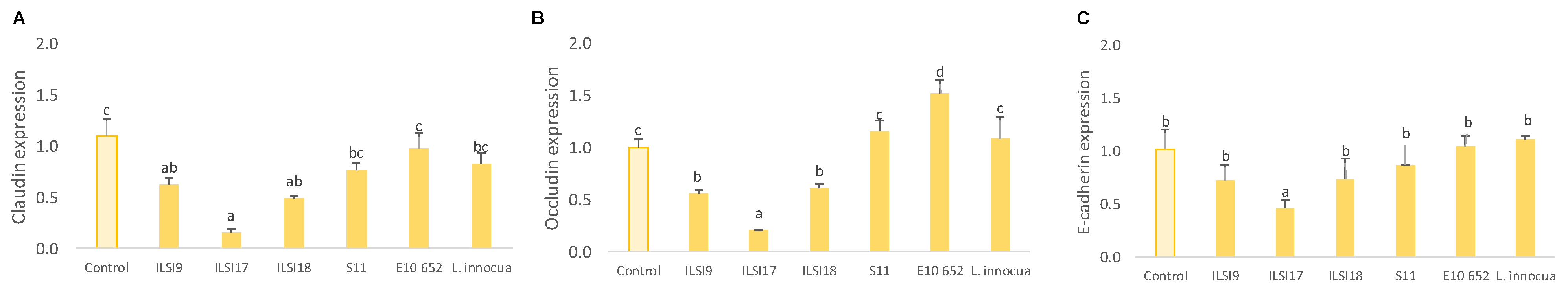
| Phenolic Compounds | WPP-N | WPP-C |
|---|---|---|
| Gallic acid | 266 ± 4 | 343 ± 1 * |
| Protocatechuic acid | 12.1 ± 0.3 | 78.2 ± 0.7 * |
| Gentisic acid | 13.4 ± 0.2 | 41.3 ± 0.4 * |
| Vanillic acid | 8.58 ± 0.20 | 12.6 ± 0.5 * |
| Syringic acid | 13.4 ± 0.2 | 1.18 ± 0.06 * |
| Ethyl gallate | 42.3 ± 0.1 | 23.6 ± 0.3 * |
| Total Hydroxybenzoic acids | 357± 3 | 500 ± 1 * |
| p-Coumaric acid | 9.93 ± 0.02 | 10.7 ± 0.1 * |
| Caffeic acid | 32.2 ± 0.5 | 65.1 ± 0.7 * |
| Total Hydroxycinnamic acids | 42.2 ± 0.4 | 75.9 ± 0.4 * |
| Catechin | 118 ± 2 | 71.3 ± 0.4 * |
| Epicatechin | 249 ± 1 | 153 ± 1 * |
| Epicatechingallate | 543 ± 3 | 672 ± 8 * |
| Procyanidin B1 | 186 ± 1 | 380 ± 5 * |
| Procyanidin B2 | 42.7 ± 2.2 | 33.1 ± 0.2 * |
| Total Flavan-3-ols | 1139 ± 2 | 1311 ± 9 * |
| t-resveratrol | 3.75 ± 0.03 | 1.81 ± 0.08 * |
| t-piceid | 1.10 ± 0.03 | 0.68 ± 0.05 * |
| Total Stilbenes | 4.83 ± 0.01 | 2.49 ± 0.03 * |
| Delphinidin derivatives | 221 ± 13 | 41.2 ± 4.7 * |
| Cyanidin derivatives | 9.37 ± 0.03 | 3.74 ± 0.16 * |
| Petunidin derivatives | 169 ± 19 | 11.1 ± 0.1 * |
| Peonidin derivatives | 24.0 ± 1 | 4.38 ± 0.02 * |
| Malvidin derivatives | 789 ± 65 | 140 ± 8 * |
| Total Anthocyanins | 1225 ± 98 | 201 ± 13 * |
| TOTAL PHENOLIC COMPOUNDS | 2768 ± 93 | 2090 ± 4 * |
| (cfu/mL) | |||
|---|---|---|---|
| Serogroup | 24-h Growth | 48-h Growth | |
| L. innocua | - | NI | NI |
| ILSI9 | 1/2b, 3b, 7 | 1.1 · 103 ± 7.7 · 102 a | 8.3 · 102 ± 3.0 · 102 * α |
| ILSI17 | 1/2c, 3c | 8.8 · 104 ± 3.6 · 103 c | 5.9 · 102 ± 1.0 · 101 * α |
| ILSI18 | 1/2a, 3a | 6.7 · 103 ± 1.4 · 103 b | 1.5 · 102 ± 5.0 · 101 * α |
| S11 | 1/2a, 3a | 9.4 · 102 ± 4.5 · 102 a | 9.6 · 102 ± 3.3 · 102 β |
| E10.652 | 1/2a, 3a | NI | NI |
| (cfu/mL) | |||
|---|---|---|---|
| Serogroup | 24-h Growth | 48-h Growth | |
| L. innocua | - | NI | NI |
| ILSI9 | 1/2b, 3b, 7 | 2.7 · 103 ± 1.7 · 103 a | 9.8 · 102 ± 2.6 · 102 * α |
| ILSI17 | 1/2c, 3c | 3.9 · 107 ± 4.8 · 106 b | 5.2 · 107 ± 4.1 · 106 * β |
| ILSI18 | 1/2a, 3a | 1.3 · 103 ± 2.5 · 102 a | 4.3 · 101 ± 0.5 · 101 * α |
| S11 | 1/2a, 3a | 5.1 · 102 ± 8.2 · 101 a | 9.3 · 102 ± 1.3 · 102 * α |
| E10.652 | 1/2a, 3a | NI | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerardi, G.; Rivero-Pérez, M.D.; Cavia-Saiz, M.; Melero, B.; Salinero-Zorita, A.; González-SanJosé, M.L.; Muñiz, P. Wine Pomace Product Inhibit Listeria monocytogenes Invasion of Intestinal Cell Lines Caco-2 and SW-480. Foods 2021, 10, 1485. https://doi.org/10.3390/foods10071485
Gerardi G, Rivero-Pérez MD, Cavia-Saiz M, Melero B, Salinero-Zorita A, González-SanJosé ML, Muñiz P. Wine Pomace Product Inhibit Listeria monocytogenes Invasion of Intestinal Cell Lines Caco-2 and SW-480. Foods. 2021; 10(7):1485. https://doi.org/10.3390/foods10071485
Chicago/Turabian StyleGerardi, Gisela, María D. Rivero-Pérez, Mónica Cavia-Saiz, Beatriz Melero, Alicia Salinero-Zorita, María L. González-SanJosé, and Pilar Muñiz. 2021. "Wine Pomace Product Inhibit Listeria monocytogenes Invasion of Intestinal Cell Lines Caco-2 and SW-480" Foods 10, no. 7: 1485. https://doi.org/10.3390/foods10071485
APA StyleGerardi, G., Rivero-Pérez, M. D., Cavia-Saiz, M., Melero, B., Salinero-Zorita, A., González-SanJosé, M. L., & Muñiz, P. (2021). Wine Pomace Product Inhibit Listeria monocytogenes Invasion of Intestinal Cell Lines Caco-2 and SW-480. Foods, 10(7), 1485. https://doi.org/10.3390/foods10071485







