The Pro-Gly or Hyp-Gly Containing Peptides from Absorbates of Fish Skin Collagen Hydrolysates Inhibit Platelet Aggregation and Target P2Y12 Receptor by Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Preparation of Collagen Peptides (CPs)
2.3. Simulated Gastrointestinal Digestion
2.4. Uptake and Transport by Caco-2 Cells
2.5. Separation of the AH Absorbate (AHA) and PH Absorbate (PHA)
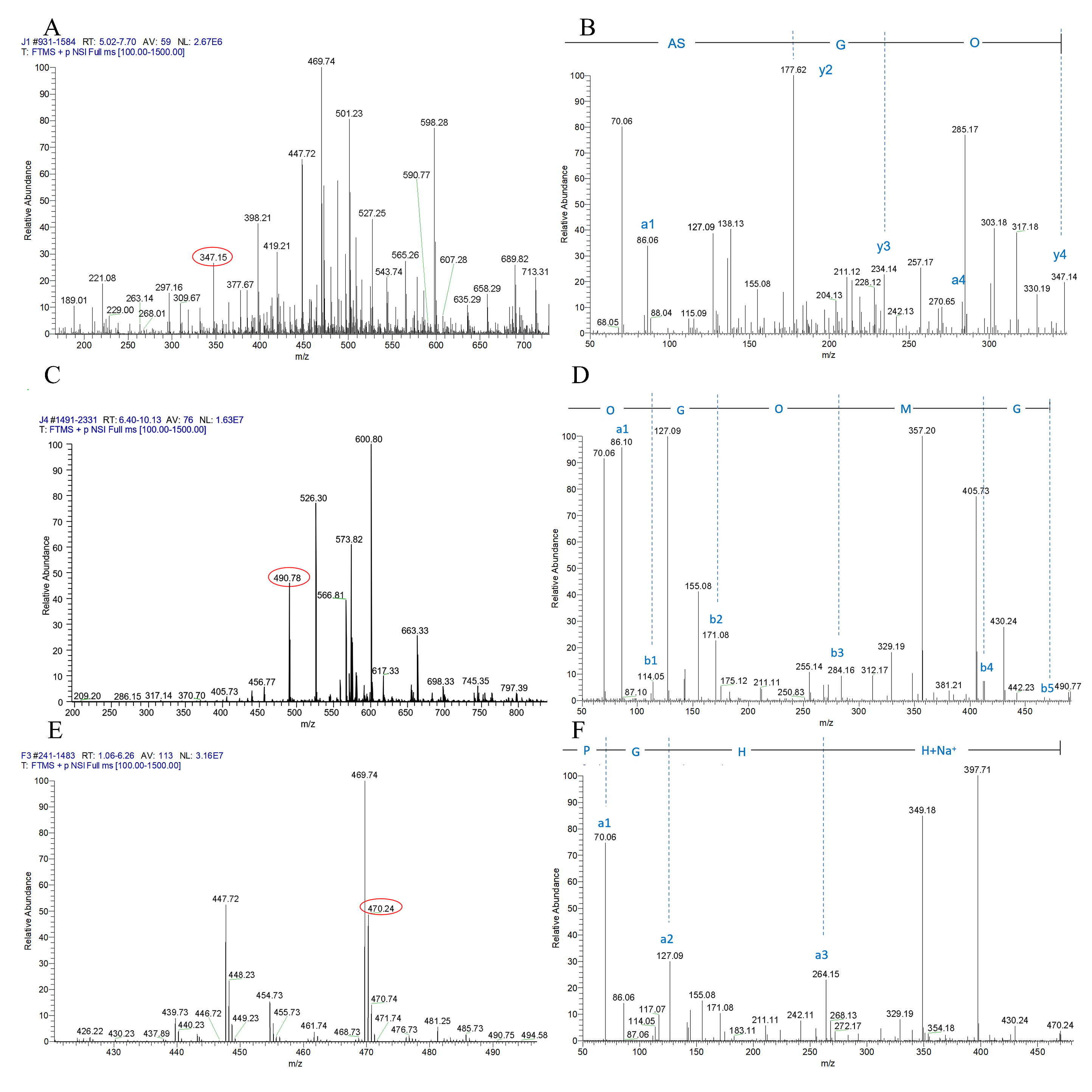
2.6. Peptide Sequencing Using LC-MS/MS
2.7. Antiplatelet Activity Assay In Vitro
2.8. Animals, Diets, and Treatments
2.8.1. Feeding Conditions and Animal
2.8.2. Ferric Chloride-Induced Arterial Thrombosis Model
2.8.3. Bleeding Assay and Determination of Thymus Index and Spleen Index
2.8.4. Determination of Thymus Index and Spleen Index
2.9. Molecular Docking
2.10. Statistics
3. Results
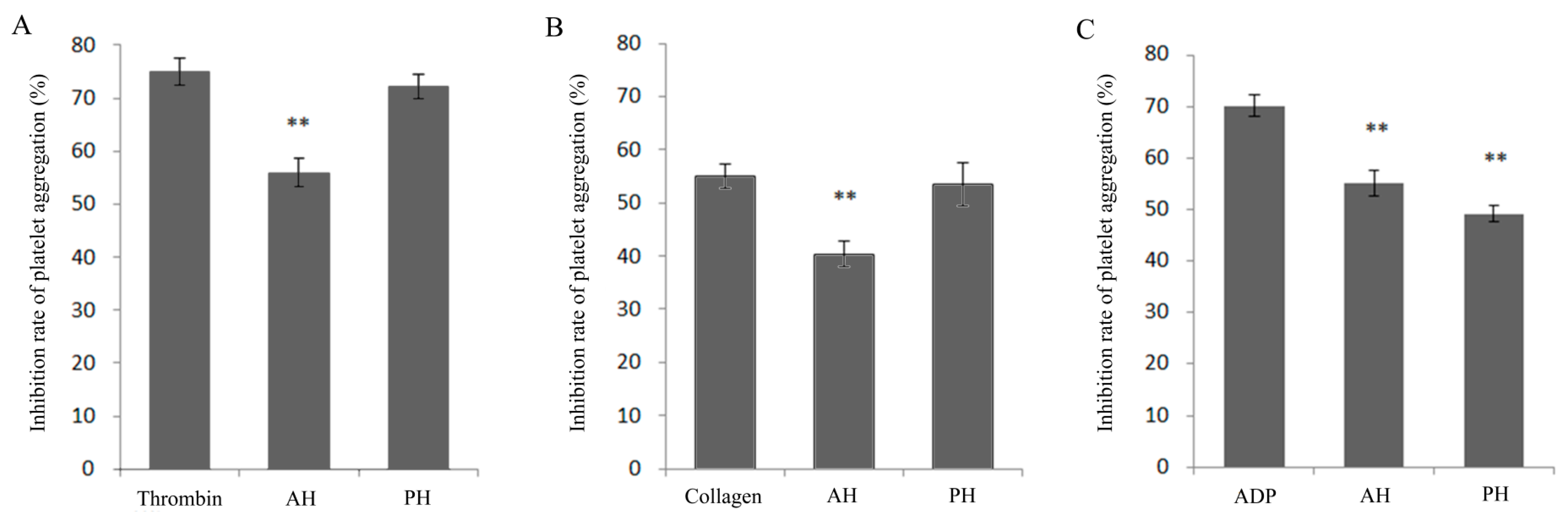
3.1. Effect of Alcalase-Hydrolysates (AH) and Protamex®-hydrolysates (PH) on Inhibiting Platelet Aggregation Induced by Collagen, Thrombin, and ADP In Vitro
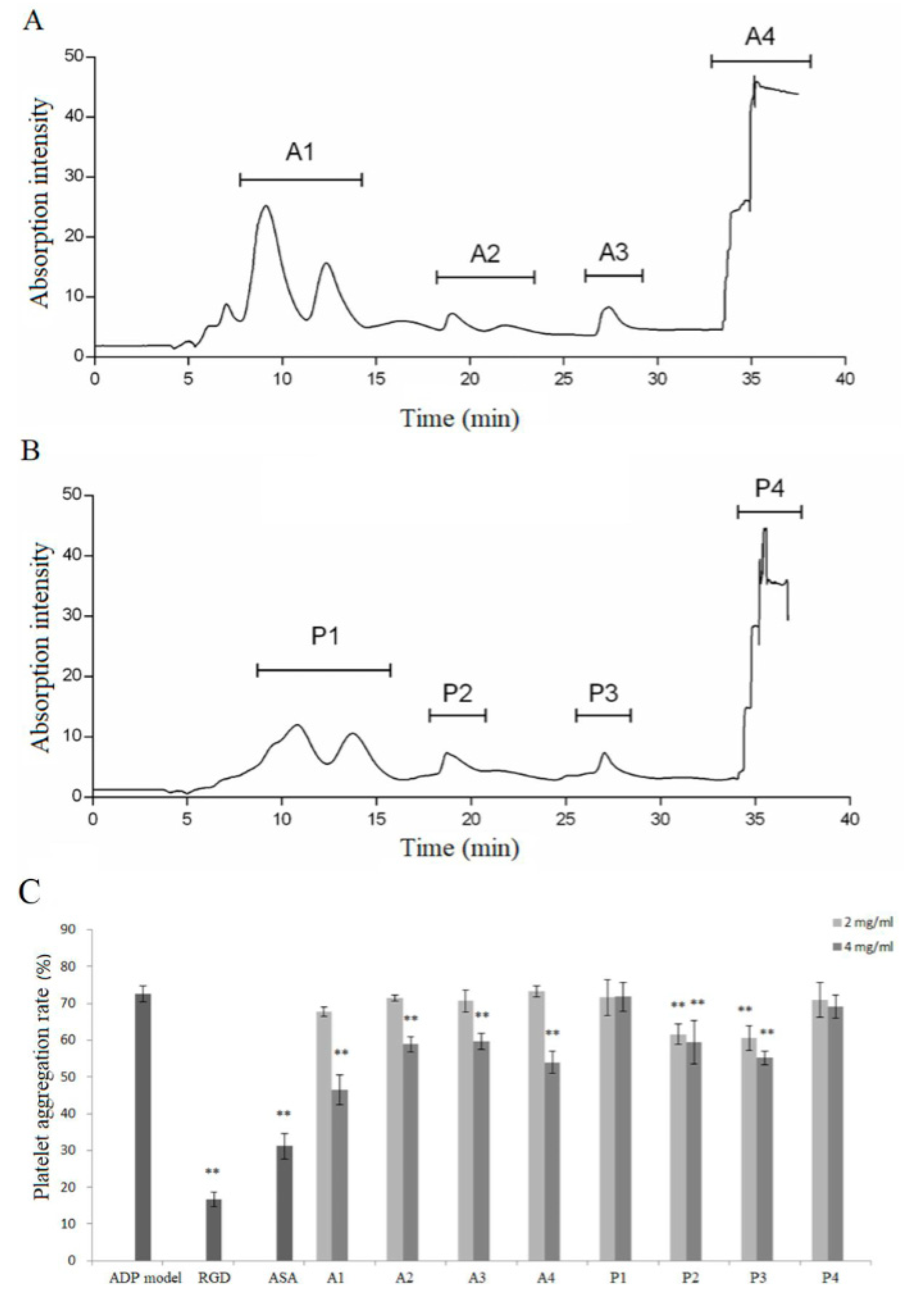
3.2. Separation and Identification of Antiplatelet Peptides from Collagen Hydrolysates after Simulated Gastrointestinal Digestion and Intestinal Absorption
3.3. Effect of Identified Peptides on Inhibiting Platelet Aggregation Induced by Collagen, Thrombin, and ADP In Vitro
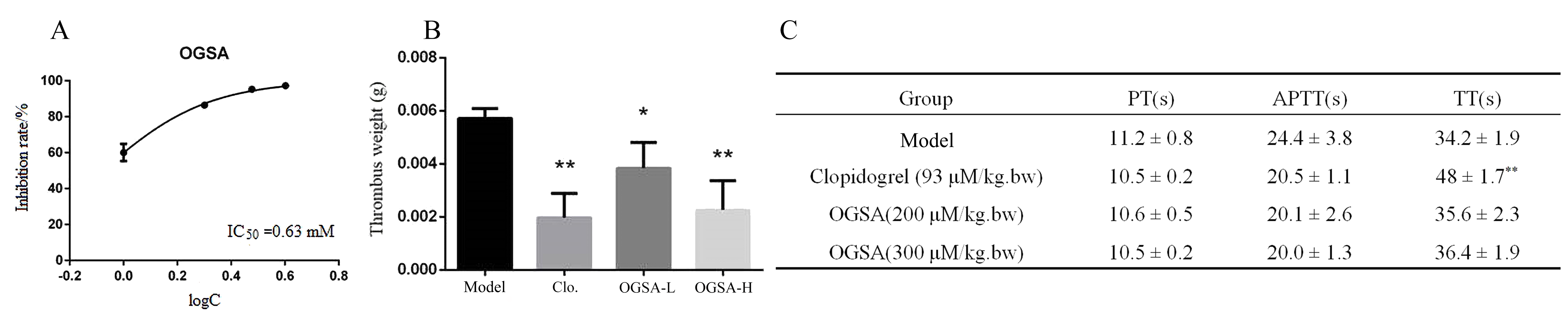
3.4. Tetrapeptide OGSA Inhibited Thrombus Formation In Vivo
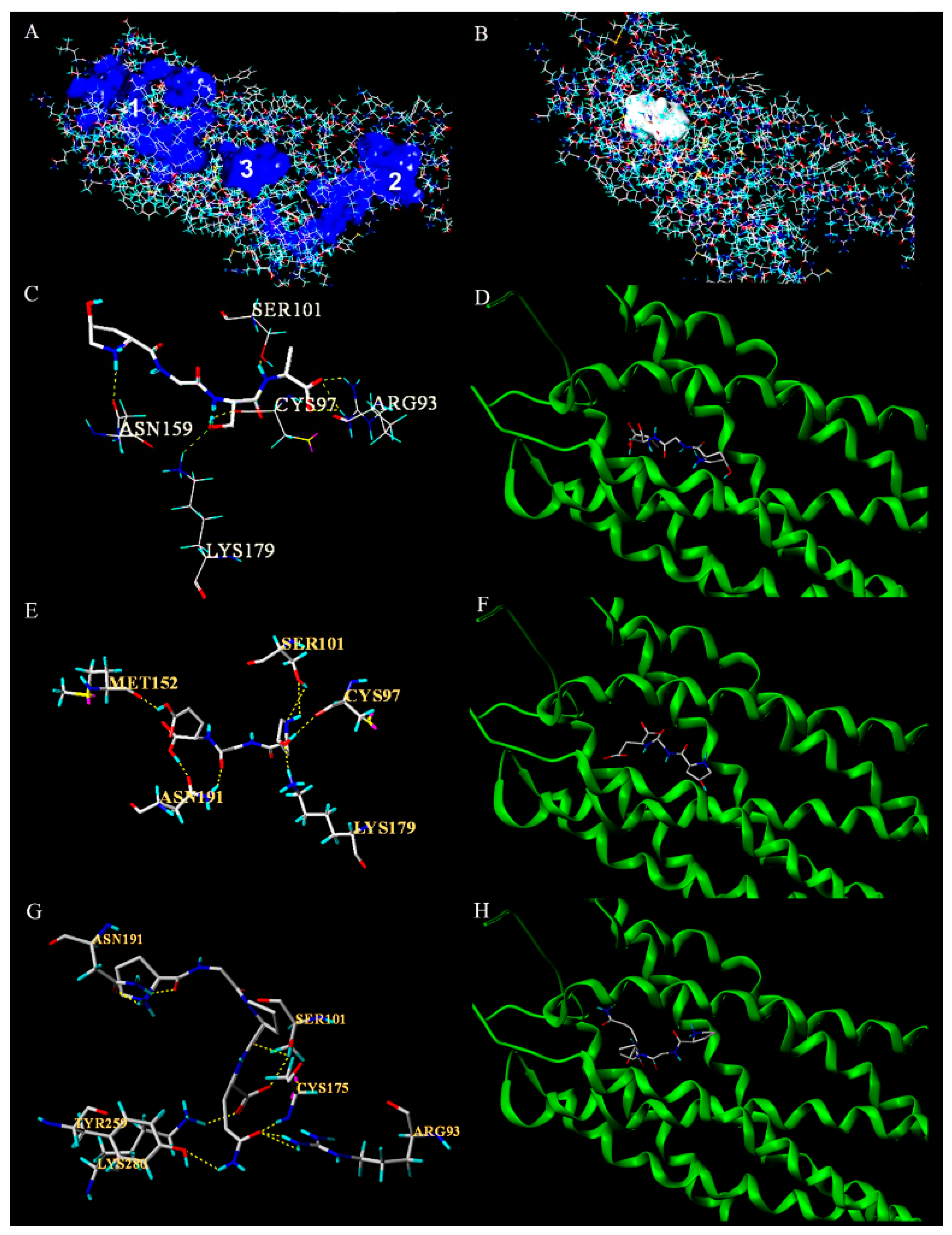
3.5. Molecular Docking of Antiplatelet Peptides with P2Y12 Receptor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | Absorbate of Alcalase hydrolysate |
| PHA | Absorbate of Protamex® hydrolysate |
| APTT | Activated partial thromboplastin time |
| ADP | Adenosine diphosphate |
| bw | Body weight |
| CCVd | Cardiocerebrovascular diseases |
| Clo | Clopidogrel |
| CPs | Collagen peptides |
| HP | High-dose peptides group |
| LP | Low-dose peptides group |
| N | Normal group |
| PRP | Platelet-rich plasma |
| PPP | Poor platelet plasma |
| PT | Prothrombin time |
| SI | Spleen index |
| TT | Thrombin time |
| TI | Thymus index |
References
- Ma, L.-Y.; Chen, W.-W.; Gao, R.-L.; Liu, L.-S.; Zhu, M.-L.; Wang, Y.-J.; Wu, Z.-S.; Li, H.-J.; Gu, D.-F.; Yang, Y.-J.; et al. China cardiovascular diseases report 2018: An updated summary. J. Geriatr. Cardiol. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Harrison, P. Platelet function analysis. Blood Rev. 2005, 19, 111–123. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kunapuli, S.P. P2Y12 receptor in platelet activation. Platelets 2011, 22, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Matsui, M.; Zheng, Y.W.; Sulciner, D.J.; Weiss, E.J.; Ludeman, M.J.; Coughlin, S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature 2000, 404, 609–613. [Google Scholar] [CrossRef]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M. Glycoprotein IIb/IIIa inhibitors. J. Invasive Cardiol. 2004, 16, 11S–16S. [Google Scholar] [PubMed]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef]
- Smith, J.B.; Mustard, J.F.; Jilma, B.; Fuchs, I.; Violi, F.; Pignatelli, P.; Pulcinelli, F.M.; De Gaetano, G.; Cerletti, C.; Rosenson, R.S.; et al. Aspirin. J. Thromb. Haemost. 2004, 2, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Angiolillo, D.J. Novel antiplatelet agents in acute coronary syndrome. Nat. Rev. Cardiol. 2015, 12, 30–47. [Google Scholar] [CrossRef] [PubMed]
- White, A.A.; Stevenson, D.D. Side effects from daily aspirin treatment in patients with AERD: Identification and management. Allergy Asthma Proc. 2011, 32, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Fukuta, H.; Niimura, T.; Zamami, Y.; Ishizawa, K.; Kimura, K.; Kamiya, T.; Ohte, N. Comparison of hemorrhagic risk between prasugrel and clopidogrel: A retrospective study using adverse drug event reporting databases. Int. J. Med. Sci. 2020, 17, 728–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, L.; Al-Majmaie, S.; Al-Groshi, A.; Rasul, A.; Sarker, S.D. Chalepin and chalepensin: Occurrence, biosynthesis and therapeutic potential. Molecules 2021, 26, 1609. [Google Scholar] [CrossRef]
- Elena Alanon, M.; Palomo, I.; Rodriguez, L.; Fuentes, E.; Arraez-Roman, D.; Segura-Carretero, A. Antiplatelet activity of natural bioactive extracts from mango (Mangifera Indica L.) and its by-products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow (A (R)): The first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Olas, B.; Wachowicz, B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets 2005, 16, 251–260. [Google Scholar] [CrossRef]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity, and interact with aspirin to enhance antiplatelet effects. TH Open Companion J. Thromb. Haemost. 2019, 3, e244–e258. [Google Scholar] [CrossRef]
- Karsdal, M. Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Academic Press: London, UK, 2016; pp. 10–15. [Google Scholar]
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.-M.; Chi, C.-F.; Luo, H.-Y.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef]
- Wang, J.; Luo, D.; Liang, M.; Zhang, T.; Yin, X.; Zhang, Y.; Yang, X.; Liu, W. Spectrum-effect relationships between high-performance liquid chromatography (HPLC) fingerprints and the antioxidant and anti-inflammatory activities of collagen peptides. Molecules 2018, 23, 3257. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.Y.; Xie, Y.Y.; Wen, B.T.; Ye, M.L.; Liu, Y.S.; Imam, K.; Cai, H.M.; Zhang, C.H.; Wang, F.Z.; Xin, F.J. Porcine bone collagen peptides promote osteoblast proliferation and differentiation by activating the PI3K/Akt signaling pathway. J. Funct. Foods 2020, 64, 11. [Google Scholar] [CrossRef]
- Khiari, Z.; Rico, D.; Belen Martin-Diana, A.; Barry-Ryan, C. Structure elucidation of ACE-inhibitory and antithrombotic peptides isolated from mackerel skin gelatine hydrolysates. J. Sci. Food Agric. 2014, 94, 1663–1671. [Google Scholar] [CrossRef]
- Maruyama, S.; Nonaka, I.; Tanaka, H. inhibitory effects of enzymatic hydrolysates of collagen and collagen-related synthetic peptides on fibrinogen thrombin clotting. Biochim. Biophys. Acta 1993, 1164, 215–218. [Google Scholar] [CrossRef]
- Nonaka, I.; Katsuda, S.I.; Ohmori, T.; Shigehisa, T.; Nakagami, T.; Maruyama, S. In vitro and in vivo anti-platelet effects of enzymatic hydrolysates of collagen and collagen-related peptides. Biosci. Biotechnol. Biochem. 1997, 61, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, L.; Luo, Y.; Zhang, S.; Li, B. Effects of collagen peptides intake on skin ageing and platelet release in chronologically aged mice revealed by cytokine array analysis. J. Cell. Mol. Med. 2018, 22, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Tian, Q.; Li, B. Novel Hyp-Gly-containing antiplatelet peptides from collagen hydrolysate after simulated gastrointestinal digestion and intestinal absorption. Food Funct. 2020, 11, 5553–5564. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, H.; Stassen, J.M.; Vermylen, J.; Deckmyn, H. inhibition of integrin function by a cyclic rgd-containing peptide prevents neointima formation. Circulation 1994, 90, 2203–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, B.; Li, B. Bioavailability of peptides from casein hydrolysate in vitro: Amino acid compositions of peptides affect the antioxidant efficacy and resistance to intestinal peptidases. Food Res. Int. 2016, 81, 188–196. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Ding, X.; Liu, X.; Li, Q.; Kong, Y. A novel selective inhibitor to thrombin-induced platelet aggregation purified from the leech Whitmania pigra. Biochem. Biophys. Res. Commun. 2016, 473, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, I.; Finkle, C.D.; Winocour, P.D. Effective use of BCH-2763, a new potent injectable direct thrombin inhibitor, in combination with tissue plasminogen activator (tPA) in a rat arterial thrombolysis model. Thromb. Haemost. 1998, 80, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, T.M.; Cho, J.S.; Gloviczki, P.; Mozes, G.; Rolle, R.; Miller, V.M. Thrombolysis for experimental deep venous thrombosis maintains valvular competence and vasoreactivity. J. Vasc. Surg. 2000, 31, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.R.; Kumar, Y.N.; Raghavendra, A.; Phanindra, V.; Madhava, G.; Ravi, N.; Bhaskar, M.; Raju, C.N. Design, synthesis, in silico and in vitro studies of substituted 1, 2, 3, 4-tetrahydro pyrimidine phosphorus derivatives. Comb. Chem. High Screen. 2015, 18, 862–871. [Google Scholar] [CrossRef]
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev. 1996, 96, 1027–1043. [Google Scholar] [CrossRef]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Geisler, T.; Gawaz, M.; Steinhubl, S.R.; Bhatt, D.L.; Storey, R.F.; Flather, M. Current strategies in antiplatelet therapy-Does identification of risk and adjustment of therapy contribute to more effective, personalized medicine in cardiovascular disease? Pharmacol. Ther. 2010, 127, 95–107. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, S.; Balius, T.E.; Singh, I.; Levit, A.; Moroz, Y.S.; O’Meara, M.J.; Che, T.; Algaa, E.; Tolmachova, K.; et al. Ultra-large library docking for discovering new chemotypes. Nature 2019, 566, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Hirono, S.; Kollman, P.A. calculation of the relative binding free-energy of 2′GMP AND 2′AMP to ribonuclease-T1 using molecular-dynamics free-energy perturbation approaches. J. Mol. Biol. 1990, 212, 197–209. [Google Scholar] [CrossRef]
- Canobbio, I.; Cipolla, L.; Guidetti, G.F.; Manganaro, D.; Visconte, C.; Kim, S.; Okigaki, M.; Falasca, M.; Kunapuli, S.P.; Torti, M. The focal adhesion kinase Pyk2 links Ca2+ signalling to Src family kinase activation and protein tyrosine phosphorylation in thrombin-stimulated platelets. Biochem. J. 2015, 469, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Gao, Z.-G.; Paoletta, S.; Zhang, D.; Han, G.W.; Li, T.; Ma, L.; Zhang, W.; Mueller, C.E.; et al. Agonist-bound structure of the human P2Y(12) receptor. Nature 2014, 509, 119–122. [Google Scholar] [CrossRef] [PubMed]
| Sample | Peptide a | Inhibition Rate of Platelet Aggregation % | ||
|---|---|---|---|---|
| Collagen Induced | Thrombin Induced | ADP b Induced | ||
| Control | RGD | 17.1 ± 3.1 | 22.7 ± 2.7 | 79.7 ± 2.8 |
| Aspirin d | 55.0 ± 7.2 | 6.1 ± 2.5 | 58.4 ± 5.5 | |
| A1 c | EGPAGPA | 16.4 ± 2.2 | 53.9 ± 2.1 | 90.7 ± 4.4 |
| GTOGT | 5.1 ± 27.7 | −2.7 ± 7.1 | 88.0 ± 4.4 | |
| PGPK | −18.7 ± 19.7 | −2.4 ± 5.9 | 69.3 ± 0.5 | |
| PGKP | −56.0 ± 10.7 | 0.1 ± 4.6 | 39.8 ± 1.9 | |
| PGPQ | −51.2 ± 26.5 | 0.9 ± 1.0 | 64.5 ± 16.2 | |
| PGQP | 65.6 ± 14.1 | −2.4 ± 2.9 | 56.2 ± 1.1 | |
| OGSA | −0.1 ± 24.2 | 15.5 ± 4.4 | 96.4 ± 2.0 | |
| OG | 6.5 ± 0.7 | −6.5 ± 2.2 | 56.6 ± 1.9 | |
| A4 c | VVGOKG | −9.0 ± 0.9 | 11.5 ± 2.0 | 96.0 ± 1.7 |
| OGOMG | 12.4 ± 19.4 | 0.3 ± 3.4 | 73.6 ± 7.4 | |
| P3 c | PGHH | 21.3 ± 8.7 | 19.6 ± 4.9 | 83.7 ± 3.5 |
| No. | Sequence a | IC50/mM b | PLC β2 c | PLC β3 c | P2Y12 c |
|---|---|---|---|---|---|
| 1 | OGE | 0.62 | −5.05 | −5.24 | −6.62 |
| 2 | OGSA | 0.63 | −5.92 | −3.88 | −5.73 |
| 3 | PGE | 0.93 | −5.22 | −4.72 | −6.09 |
| 4 | PGEOG | 0.97 | −4.57 | −4.44 | −5.71 |
| 5 | PGHH | 1.4 | −4.78 | −4.33 | −4.21 |
| 6 | OGOMG | 1.49 | −4.55 | −4.25 | −3.93 |
| 7 | PGPK | 2.79 | −6.24 | −3.83 | −3.27 |
| 8 | OG | 1.68 | −6.11 | −4.68 | −4.68 |
| 9 | PGKP | 2.57 | −4.6 | −3.68 | −2.92 |
| 10 | VGPOGPA | 2.73 | −4.84 | −3.11 | −1.40 |
| 11 | GTOGT | 3.71 | −2.00 | −2.04 | −1.46 |
| 12 | PGPQ | 3.56 | −6.47 | −5.66 | −4.53 |
| 13 | PGQP | 2.31 | −5.29 | −5.21 | −3.23 |
| Pearson correlation coefficient | 0.220 | 0.367 | 0.802 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Li, S.-M.; Li, B. The Pro-Gly or Hyp-Gly Containing Peptides from Absorbates of Fish Skin Collagen Hydrolysates Inhibit Platelet Aggregation and Target P2Y12 Receptor by Molecular Docking. Foods 2021, 10, 1553. https://doi.org/10.3390/foods10071553
Tian Q, Li S-M, Li B. The Pro-Gly or Hyp-Gly Containing Peptides from Absorbates of Fish Skin Collagen Hydrolysates Inhibit Platelet Aggregation and Target P2Y12 Receptor by Molecular Docking. Foods. 2021; 10(7):1553. https://doi.org/10.3390/foods10071553
Chicago/Turabian StyleTian, Qi, Shi-Ming Li, and Bo Li. 2021. "The Pro-Gly or Hyp-Gly Containing Peptides from Absorbates of Fish Skin Collagen Hydrolysates Inhibit Platelet Aggregation and Target P2Y12 Receptor by Molecular Docking" Foods 10, no. 7: 1553. https://doi.org/10.3390/foods10071553







