1H NMR Study of the In Vitro Digestion of Highly Oxidized Soybean Oil and the Effect of the Presence of Ovalbumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Subject of Study
2.2. In Vitro Gastrointestinal Digestion
2.3. Lipid Extraction of the Digestates
2.4. Analysis by 1H NMR
2.4.1. Operating Conditions
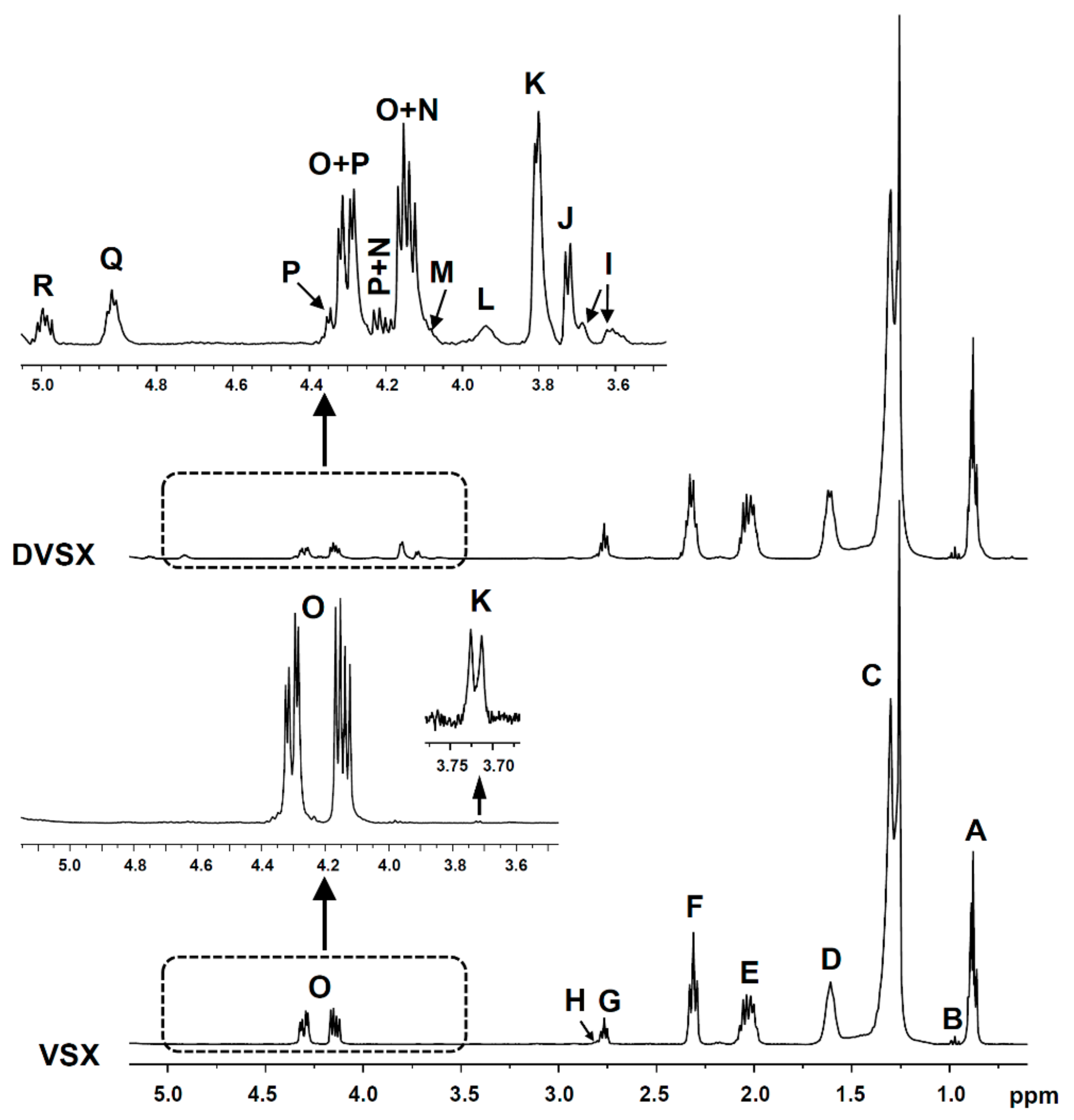
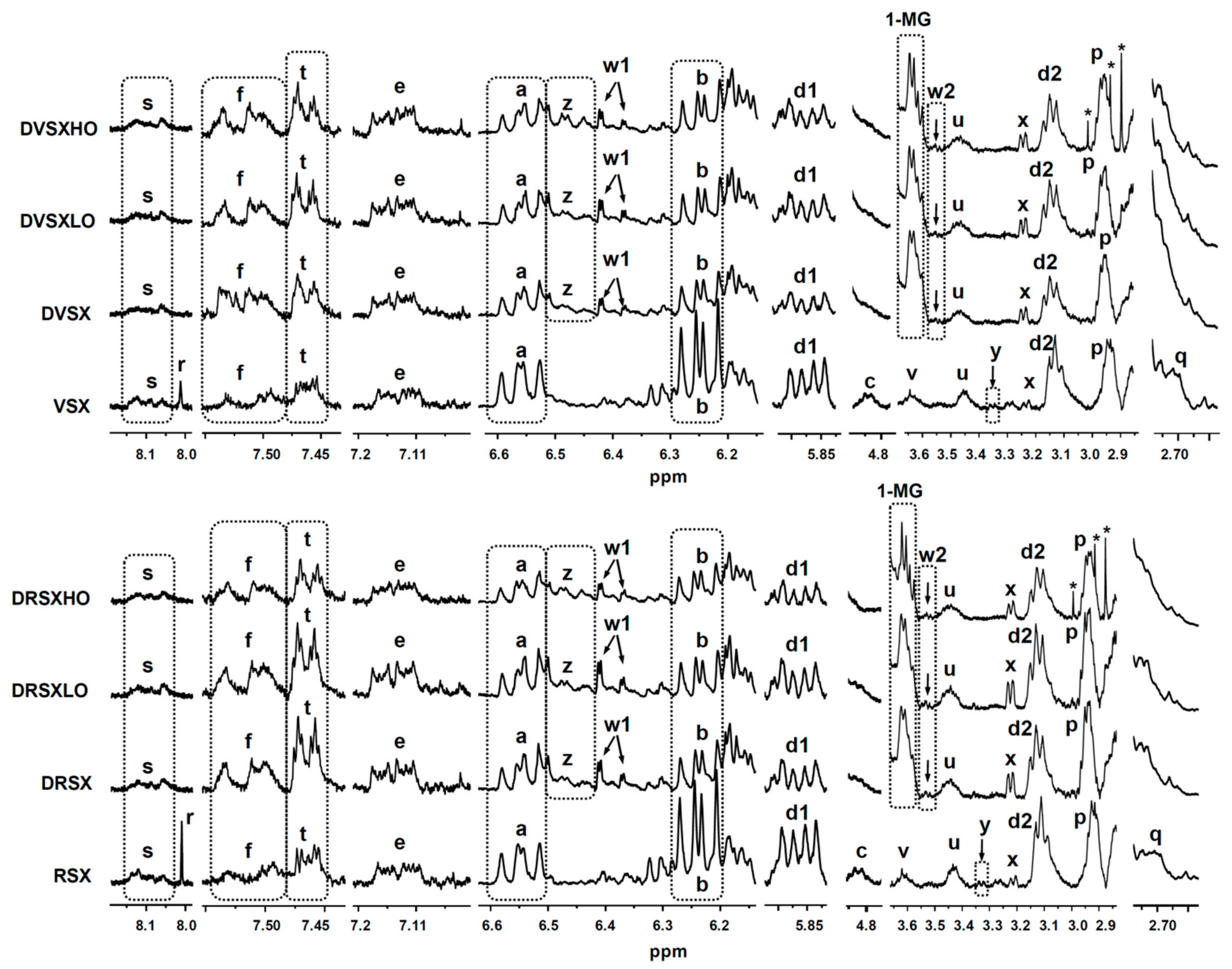
2.4.2. Identification of Some Compounds and Structures
2.4.3. Quantification from 1H NMR Spectral Data
2.5. Statistical Analysis
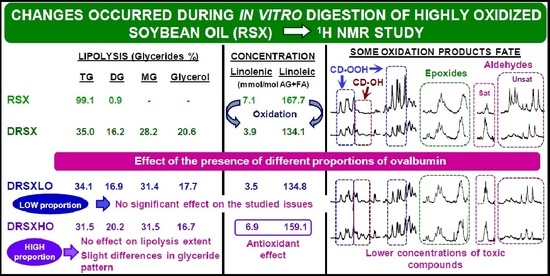
3. Results and Discussion
3.1. Composition of the Oil Samples Subject of Study
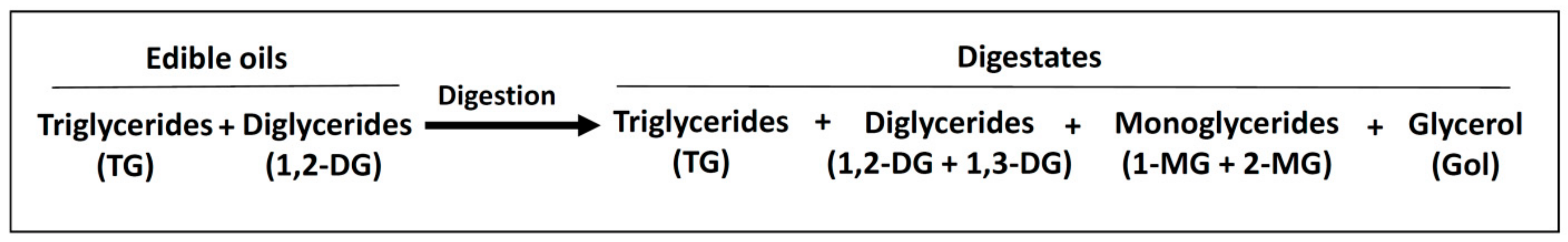
3.2. Lipolysis Extent Reached in the In Vitro Digestion of the Several Samples and Consequences
3.2.1. In the In Vitro Digestion of VSX and RSX Samples
3.2.2. In the In Vitro Digestion of VSX and RSX Samples with Ovalbumin Added
3.3. Changes in the Concentration of the Most Unsaturated Fatty Acyl Groups of the Oil Occurred during the In Vitro Digestion and Consequences
3.3.1. In the In Vitro Digestion of VSX and RSX Samples
3.3.2. In the In Vitro Digestion of VSX and RSX Samples with Ovalbumin Added
3.4. Concentration of oil Oxidation Derivatives in the Digestates versus That of the Samples Submitted to Digestion
3.4.1. Concentrations of Oxidation Derived Compounds in DVSX and DRSX Samples
3.4.2. Concentrations of Oxidation Derived Compounds in the Digestates of Samples Containing Ovalbumin Added, DVSXLO, DRSXLO, DVSXHO and DRSXHO
3.4.3. Bioaccessibility of Oxidation Compounds
3.5. Some Remarks about the Relevance of the Presence of Oxidation Products in the Gastrointestinal Tract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–785S. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. F. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Marmesat, S.; Velasco, J.; Dobarganes, M.C. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J. Chromatogr. A 2008, 1211, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L.; Weisshaar, R.; Matthäus, B. Epoxy fatty acids in used frying fats and oils, edible oils and chocolate and their formation in oils during heating. Eur. J. Lipid Sci. Technol. 2016, 118, 425–434. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Pérez-Camino, M.C.; Dobarganes, M.C. In vitro action of pancreatic lipase on complex glycerides from thermally oxidized oils. Lipid/Fett 1992, 94, 307–312. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Pérez-Camino, M.C.; Dobarganes, M.C. Evaluation of hydrolysis and absorption of thermally oxidized olive oil in non-absorbed lipids in the rat. Ann. Nutr. Metab. 1993, 37, 121–128. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; García-Martínez, M.C.; Holgado, F. Changes and effects of dietary oxidized lipids in the gastrointestinal tract. Lipid Insights 2008, 2, 11–19. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Influence of minor components on lipid bioaccessibility and oxidation during in vitro digestion of soybean oil. J. Sci. Food Agric. 2019, 99, 4793–4800. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. The key role of ovalbumin in lipid bioaccessibility and oxidation product profile during the in vitro digestion of slightly oxidized soybean oil. Food Funct. 2019, 10, 4440–4451. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Behaviour of non-oxidized and oxidized flaxseed oils, as models of omega-3 rich lipids, during in vitro digestion. Occurrence of epoxidation reactions. Food Res. Int. 2017, 97, 104–115. [Google Scholar] [CrossRef]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Polyunsaturated lipids and vitamin A oxidation during cod liver oil in vitro gastrointestinal digestion. Antioxidant effect of added BHT. Food Chem. 2017, 232, 733–743. [Google Scholar] [CrossRef]
- Larsson, K.; Harrysson, H.; Havenaar, R.; Alminger, M.; Undeland, I. Formation of malondialdehyde (MDA), 4-hydroxy-2-hexenal (HHE) and 4-hydroxy-2-nonenal (HNE) in fish and fish oil during dynamic gastrointestinal in vitro digestion. Food Funct. 2016, 7, 1176–1187. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. 1H NMR and SPME-GC/MS study of hydrolysis, oxidation and other reactions occurring during in vitro digestion of non-oxidized and oxidized sunflower oil. Formation of hydroxy-octadecadienoates. Food Res. Int. 2017, 91, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, C.; Larsson, K.; Carlsson, N.G.; Comi, I.; Scheers, N.; Vegarud, G.; Undeland, I. Formation of reactive aldehydes (MDA, HHE, HNE) during the digestion of cod liver oil: Comparison of human and porcine in vitro digestion models. Food Funct. 2016, 7, 1401–1412. [Google Scholar] [CrossRef]
- Van Hecke, T.; Vossen, E.; Hemeryck, L.Y.; Bussche, J.V.; Vanhaecke, L.; De Smet, S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem. 2015, 187, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated alpha, beta-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.F.; Williamson, K.C.; Newman, J.W.; Morisseau, C.; Hammock, B.D. Metabolism of monoepoxides of methyl linoleate: Bioactivation and detoxification. Arch. Biochem. Biophys. 2000, 376, 420–432. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Li, J.; Wang, Y.; Liu, Y. Epoxy stearic acid, an oxidative product derived from oleic acid, induces cytotoxicity, oxidative stress, and apoptosis in HepG2 cells. J. Agric. Food Chem. 2018, 66, 5237–5246. [Google Scholar] [CrossRef]
- Lundstrom, S.L.; Balgoma, D.; Wheelock, A.M.; Haeggstrom, J.Z.; Dahlen, S.E.; Wheelock, C.E. Lipid mediator profiling in pulmonary disease. Curr. Pharm. Biotechnol. 2011, 12, 1026–1052. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Jacklyn, R.G.; Majchrzak-Hong, S.; Zamora, D.; Horowitz, M.S.; et al. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Crowley, J.R.; Alejandro, M.A.; Shoulars, K.; Casajuna, N.; Mani, S.; Reyna, A.; Sharp, J. Leukotoxin diols from ground corncob bedding disrupt estrous cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environ. Health. Perspect. 2005, 113, 1698–1704. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Oxylipins associated to current diseases detected for the first time in the oxidation of corn oil as a model system of oils rich in omega-6 polyunsaturated groups. A global, broad and in-depth study by 1H NMR spectroscopy. Antioxidants 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ashida, H.; Minamoto, S.; Danno, G.I.; Natake, M. The effects of orally administered linoleic acid and its autoxidation products on intestinal mucosa in rat. J. Nutr. Sci. Vitaminol. 1988, 34, 363–373. [Google Scholar] [CrossRef]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.A.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Olivero-David, R.; Paduano, A.; Fogliano, V.; Vitaglione, P.; Bastida, S.; González-Muñoz, M.J.; Benedí, J.; Sacchi, R.; Sánchez-Muniz, F.J. Effect of thermally oxidized oil and fasting status on the short-term digestibility of ketolinoleic acids and total oxidized fatty acids in rats. J. Agric. Food Chem. 2011, 59, 4684–4691. [Google Scholar] [CrossRef] [PubMed]
- Penumetcha, M.; Khan, N.; Parthasarathy, S. Dietary oxidized fatty acids: An atherogenic risk? J. Lipid Res. 2000, 41, 1473–1480. [Google Scholar] [CrossRef]
- Wilson, R.; Fernie, C.E.; Scrimgeour, C.M.; Lyall, K.; Smyth, L.; Riemersma, R.A. Dietary epoxy fatty acids are absorbed in healthy women. Eur. J. Clin. Investig. 2002, 32, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Emami, S.; Hennebelle, M.; Morgan, R.K.; Lerno, L.A.; Slupsky, C.M.; Lein, P.J.; Taha, A.Y. Linoleic acid-derived 13-hydroxyoctadecadienoic acid is absorbed and incorporated into rat tissues. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ashida, H. Catabolic fate of dietary trilinoleoylglycerol hydroperoxides in rat gastrointestines. Biochim. Biophys. Acta Lipids Lipid Metab. 1998, 1393, 336–348. [Google Scholar] [CrossRef]
- Nakatsugawa, K.I.; Kaneda, T. Absorption and metabolism of methyl linoleate hydroperoxides in rats. J. Jpn. Oil Chem. Soc. 1983, 32, 361–366. [Google Scholar] [CrossRef]
- Goicoechea, E.; Van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, E.; Brandon, E.F.; Blokland, M.H.; Guillén, M.D. Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem Toxicol. 2011, 49, 115–124. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Zhao, M.T.; Xie, H.; Hu, X.; Ma, X.C.; Zhang, J.H.; Bai, Y.H.; Zhou, D.Y. Formation and disappearance of aldehydes in fried clam during simulated gastrointestinal digestion. Food Funct. 2020, 11, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Chalvardjian, A.; Morris, L.J.; Holman, R.T. Effect of dietary epoxyoleic acid upon rats. J. Nutr. 1962, 76, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Effect of the presence of protein on lipolysis and lipid oxidation occurring during in vitro digestion of highly unsaturated oils. Food Chem. 2017, 235, 21–33. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Effect of the enrichment of corn oil with alpha-or gamma-tocopherol on its in vitro digestion studied by 1H NMR and SPME-GC/MS; formation of hydroperoxy-, hydroxy-, keto-dienes and keto-E-epoxy-E-monoenes in the more alpha-tocopherol enriched samples. Antioxidants 2020, 9, 246. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Van de Kamp, E.; Rompelberg, C. Development and Applicability of An In Vitro Digestion Model in Assessing the Bioaccessibility of Contaminants from Food; Report No. 320102002; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2004; Available online: http://www.rivm.nl/bibliotheek/rapporten/320102002.pdf (accessed on 29 June 2021).
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A study by 1H NMR on the influence of some factors affecting lipid in vitro digestion. Food Chem. 2016, 211, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Ruiz, A. High resolution 1H nuclear magnetic resonance in the study of edible oils and fats. Trends Food Sci. Technol. 2001, 12, 328–338. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 2003, 105, 502–507. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: Study by 1H nuclear magnetic resonance. J. Agric. Food Chem. 2009, 57, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Monitoring by 1H nuclear magnetic resonance of the changes in the composition of virgin linseed oil heated at frying temperature. Comparison with the evolution of other edible oils. Food Control. 2012, 28, 59–68. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Nakashima, F.; Shibata, T.; Uchida, K.; Guillén, M.D. A dual perspective of the action of lysine on soybean oil oxidation process obtained by combining 1H NMR and LC–MS: Antioxidant effect and generation of lysine–aldehyde adducts. Antioxidants 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Yusta, A.; Guillén, M.D. Deep-frying food in extra virgin olive oil: A study by 1H nuclear magnetic resonance of the influence of food nature on the evolving composition of the frying medium. Food Chem. 2014, 150, 429–437. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Molina, M.; Yahuaca-Júarez, B.; Ibargoitia, M.L.; Guillén, M.D. Changes provoked by nixtamalization and tortilla making in the lipids of two corn varieties. A study by 1H NMR. Food Chem. 2020, 313. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Assessment of soybean oil oxidative stability from rapid analysis of its minor component profile. Molecules 2020, 25, 4860. [Google Scholar] [CrossRef] [PubMed]
- Gamage, P.T.; Matsushita, S. Interactions of the autoxidized products of linoleic acid with enzyme proteins. Agric. Biol. Chem. 1973, 37, 1–8. [Google Scholar] [CrossRef]
- Matsushita, S. Specific interactions of linoleic acid hydroperoxides and their secondary degraded products with enzyme proteins. J. Agric. Food Chem. 1975, 23, 150–154. [Google Scholar] [CrossRef]
- Matsushita, S.; Kobayashi, M. Effect of linoleic acid hydroperoxides on pepsin activity. Agric. Biol. Chem. 1970, 34, 825–829. [Google Scholar] [CrossRef]
- Matsushita, S.; Kobayashi, M.; Nitta, Y. Inactivation of enzymes by linoleic acid hydroperoxides and linoleic acid. Agric. Biol. Chem. 1970, 34, 817–824. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Benedí, J.; Bastida, S.; Olivero-David, R.; González-Muñoz, M.J. Enzymes and thermally oxidized oils and fats. In Frying of Foods. Oxidation, Nutrient and Non-Nutrient Antioxidants, Biologically Active Compounds and High Temperatures; Boskou, D., Elmadfa, I., Eds.; Taylor and Francis Group: Boca Raton, FL, USA, 2011; pp. 105–155. [Google Scholar]
- Arroyo, R.; Sánchez-Muniz, F.J.; Cuesta, C.; Sinisterra, J.V.; Sánchez-Montero, J.M. Thermoxidation of substrate models and their behavior during hydrolysis by porcine pancreatic lipase. J. Am. Oil Chem. Soc. 1997, 74, 1509–1516. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Dobarganes, M.C. Assessments on the digestibility of oxidized compounds from [1-14C] linoleic acid using a combination of chromatographic techniques. J. Chromatogr. B Biomed. Sci. Appl. 1995, 675, 1–8. [Google Scholar] [CrossRef]
- Jira, W.; Spiteller, G.; Richter, A. Increased levels of lipid oxidation products in rheumatically destructed bones of patients suffering from rheumatoid arthritis. Z. Naturforsch. C 1998, 53, 1061–1071. [Google Scholar] [CrossRef]
- Udilova, N.; Jurek, D.; Marian, B.; Gille, L.; Schulte-Hermann, R.; Nohl, H. Induction of lipid peroxidation in biomembranes by dietary oil components. Food Chem Toxicol. 2003, 41, 1481–1489. [Google Scholar] [CrossRef]
- Zhao, B.; Sakurai, Y.; Shibata, K.; Kikkawa, F.; Tomoda, Y.; Mizukami, H. Cytotoxic fatty acid ketodienes from egg plants. Jpn. J. Food Chem. Saf. 2014, 21, 42–47. [Google Scholar]
- Kenmogne-Domguia, H.B.; Moisan, S.; Viau, M.; Genot, C.; Meynier, A. The initial characteristics of marine oil emulsions and the composition of the media inflect lipid oxidation during in vitro gastrointestinal digestion. Food Chem. 2014, 152, 146–154. [Google Scholar] [CrossRef]
- Larsson, K.; Cavonius, L.; Alminger, M.; Undeland, I. Oxidation of cod liver oil during gastrointestinal in vitro digestion. J. Agric. Food Chem. 2012, 60, 7556–7564. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Nam, K.C.; Ahn, D.U. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poultry Sci. 2014, 93, 2678–2686. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Lipid hydroperoxide reactivity with proteins and amino acids: A review. J. Agric. Food Chem. 1979, 27, 220–229. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef]
- Schneider, C.; Tallman, K.A.; Porter, N.A.; Brash, A.R. Two distinct pathways of formation of 4-hydroxynonenal mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001, 276, 20831–20838. [Google Scholar] [CrossRef]
- Schneider, C.; Boeglin, W.E.; Yin, H.; Stec, D.F.; Hachey, D.L.; Porter, N.A.; Brash, A.R. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids 2005, 40, 1155–1162. [Google Scholar] [CrossRef]
- Gardner, H.W.; Kleiman, R. Degradation of linoleic acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions: II. Specificity in formation of fatty acid epoxides. Biochim. Biophys. Acta Lipids Lipid Metab. 1981, 665, 113–125. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Christie, W.W.; Durand, T.; Rossi, J.C.; Vidal, J.P.; Kasyanov, S.P.; Akulina, V.N.; Bezuglov, V.V. Synthesis of keto-and hydroxydienoic compounds from linoleic acid. Chem. Phys. Lipids 1997, 85, 125–134. [Google Scholar] [CrossRef]
- Gardner, H.W. Decomposition of linoleic acid hydroperoxides. Enzymic reactions compared with nonenzymic. J. Agric. Food Chem. 1975, 23, 129–136. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, J.; Sayre, L.M. Synthesis of six epoxyketooctadecenoic acid (EKODE) isomers, their generation from nonenzymatic oxidation of linoleic acid, and their reactivity with imidazole nucleophiles. J. Org. Chem. 2007, 72, 9471–9480. [Google Scholar] [CrossRef]
- Onyango, A.N. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem. Phys. Lipids 2012, 165, 777–786. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Modification of bovine serum albumin structure following reaction with 4,5-(E)-epoxy-2-(E)-heptenal. Chem Res. Toxicol. 2000, 13, 501–508. [Google Scholar] [CrossRef]
- Schaich, K.M. Co-oxidation of proteins by oxidizing lipids. In Lipid Oxidation Pathways; Kamal-Eldin, A., Min, D.B., Eds.; AOCS Press: Urbana, IL, USA, 2008; Volume 2, pp. 183–274. [Google Scholar]
- Van Hecke, T.; Vossen, E.; Bussche, J.V.; Raes, K.; Vanhaecke, L.; De Smet, S. Fat content and nitrite-curing influence the formation of oxidation products and NOC-specific DNA adducts during in vitro digestion of meat. PLoS ONE 2014, 9, e101122. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. 2020, 141. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Destaillats, F.; Robert, F.; Skibsted, L.H.; Dionisi, F. Formation and hydrolysis of triacylglycerol and sterols epoxides: Role of unsaturated triacylglycerol peroxyl radicals. Free Radical Biol. Med. 2004, 37, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Catalioto, R.M.; Maggi, A.C.; Giuliani, S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr. Med. Chem. 2011, 18, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Hayakawa, M.; Sugiyama, S.; Ajioka, M.; Ito, T.; Satake, T.; Ozawa, T. Cardiovascular effects of leukotoxin (9, 10-epoxy-12-octadecenoate) and free fatty acids in dogs. Cardiovasc. Res. 1988, 22, 213–218. [Google Scholar] [CrossRef]
- Mubiru, E.; Shrestha, K.; Papastergiadis, A.; De Meulenaer, B. Improved gas chromatography-flame ionization detector analytical method for the analysis of epoxy fatty acids. J. Chrom. A 2013, 1318, 217–225. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Bordeaux, O.; Márquez-Ruiz, G.; Dobarganes, C. Formation and evolution of monoepoxy fatty acids in thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried-food outlets. J. Agric. Food Chem. 2004, 5, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Plopper, C.G.; Lakritz, J.; Storms, D.H.; Hammock, B.D. Leukotoxin-diol: A putative toxic mediator involved in acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2001, 25, 434–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamal-Eldin, A.; Márquez-Ruiz, G.; Dobarganes, C.; Appelqvist, L.Å. Characterisation of aldehydic acids in used and unused frying oils. J. Chromatogr. A 1997, 776, 245–254. [Google Scholar] [CrossRef]


| TG% | 1,2-DG% | 1,3-DG% | 2-MG% | 1-MG% | Gol% | |
|---|---|---|---|---|---|---|
| VSX | 99.01 ± 0.18 b | 0.50 ± 0.00 a | - | - | - | nd |
| DVSX | 35.62 ± 0.60 a | 13.23 ± 1.76 b | 3.46 ± 1.09 a | 19.02 ± 1.35 a | 8.25 ± 0.85 b | 20.43 ± 2.61 a |
| DVSXLO | 34.93 ± 0.88 a | 14.74 ± 0.76 bc | 2.78 ± 0.92 a | 21.90 ± 2.60 a | 7.26 ± 0.20 b | 18.39 ± 3.83 a |
| DVSXHO | 30.21 ± 3.60 a | 16.58 ± 0.22 c | 2.96 ± 0.94 a | 28.90 ± 2.59 b | 5.59 ± 0.65 a | 15.77 ± 0.37 a |
| RSX | 99.08 ± 0.08 d | 0.90 ± 0.06 a | - | - | - | nd |
| DRSX | 35.02 ± 0.47 b | 13.23 ± 1.18 b | 2.98 ± 0.29 a | 20.92 ± 1.04 a | 7.27 ± 1.47 b | 20.59 ± 1.50 a |
| DRSXLO | 34.09 ± 0.11 b | 14.34 ± 0.55 b | 2.51 ± 0.02 a | 23.95 ± 1.87 b | 7.44 ± 0.22 b | 17.68 ± 1.40 a |
| DRSXHO | 31.51 ± 0.49 a | 17.01 ± 1.59 c | 3.22 ± 1.35 a | 27.16 ± 0.75 c | 4.35 ± 1.16 a | 16.74 ± 1.86 a |
| Linolenic | Linoleic | |
|---|---|---|
| VSX | 10.2 ± 0.4 b | 174.4 ± 2.3 c |
| DVSX | 7.0 ± 1.0 a | 148.4 ± 9.5 a |
| DVSXLO | 7.1 ± 0.6 a | 147.8 ± 2.9 a |
| DVSXHO | 7.6 ± 0.6 a | 161.7 ± 3.6 b |
| RSX | 7.1 ± 0.5 b | 167.7 ± 5.9 b |
| DRSX | 3.9 ± 1.1 a | 134.1 ± 9.3 a |
| DRSXLO | 3.5 ± 0.4 a | 134.8 ± 3.6 a |
| DRSXHO | 6.9 ± 0.7 b | 159.1 ± 7.5 b |
| Samples Derived from Virgin Soybean Oil | Samples Derived from Refined Soybean Oil | |||||||
|---|---|---|---|---|---|---|---|---|
| VSX | DVSX | DVSXLO | DVSXHO | RSX | DRSX | DRSXLO | DRSXHO | |
| Monohydroperoxy-conjugated dienes | ||||||||
| Hydroperoxy-(Z,E)-dienes | 15.24 ± 0.46 c | 8.83 ± 1.04 b | 8.32 ± 0.67 b | 6.90 ± 0.78 a | 11.98 ± 1.99 b | 9.07 ± 0.95 a | 8.88 ± 1.76 a | 7.46 ± 0.71 a |
| Hydroperoxy-(E,E)-dienes | 23.84 ± 0.64 b | 12.53 ± 1.32 a | 12.15 ± 2.66 a | 9.74 ± 0.87 a | 24.44 ± 1.61 b | 12.09 ± 0.93 a | 11.29 ± 1.12 a | 11.03 ± 1.31 a |
| Total hydroperoxy-dienes | 39.08 ± 1.10 c | 21.36 ± 2.36 b | 20.47 ± 3.33 b | 16.64 ± 1.66 a | 36.42 ± 3.59 b | 21.15 ± 1.89 a | 20.18 ± 2.87 a | 18.49 ± 2.02 a |
| Dihydroperoxy-non-conjugated dienes | 5.12 ± 0.23 | - | - | - | 5.25 ± 0.51 | - | - | - |
| Hydroperoxy-epoxy-(E)-monoenes | 9.19 ± 0.34 b | 5.79 ± 1.08 a | 5.58 ± 0.13 a | 4.48 ± 0.94 a | 14.20 ± 2.04 b | 6.82 ± 1.01 a | 6.79 ± 0.61 a | 5.13 ± 0.43 a |
| Monohydroxy-conjugated dienes | ||||||||
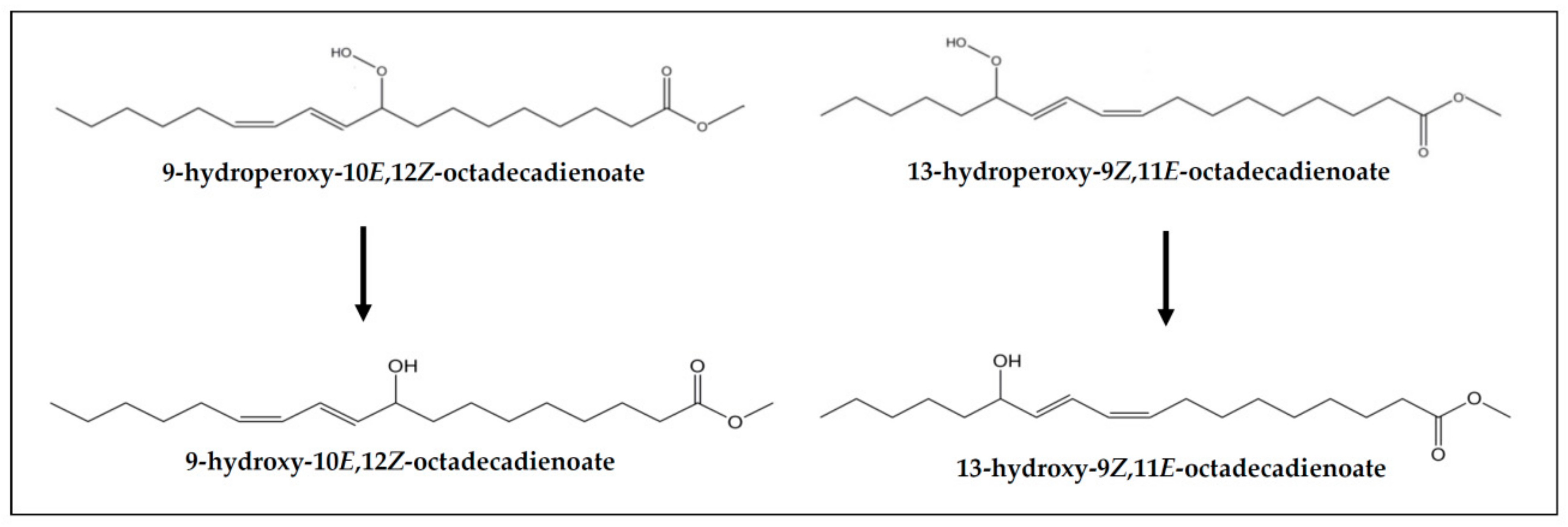
| Hydroxy-(Z,E)-dienes | - | 2.35 ± 0.38 a | 2.42 ± 0.34 a | 5.03 ± 1.09 b | - | 2.04 ± 0.40 a | 2.47 ± 0.31 a | 4.55 ± 0.66 b |
| Hydroxy-(E,E)-dienes | - | nd | nd | nd | - | nd | nd | nd |
| Keto-conjugated dienes | ||||||||
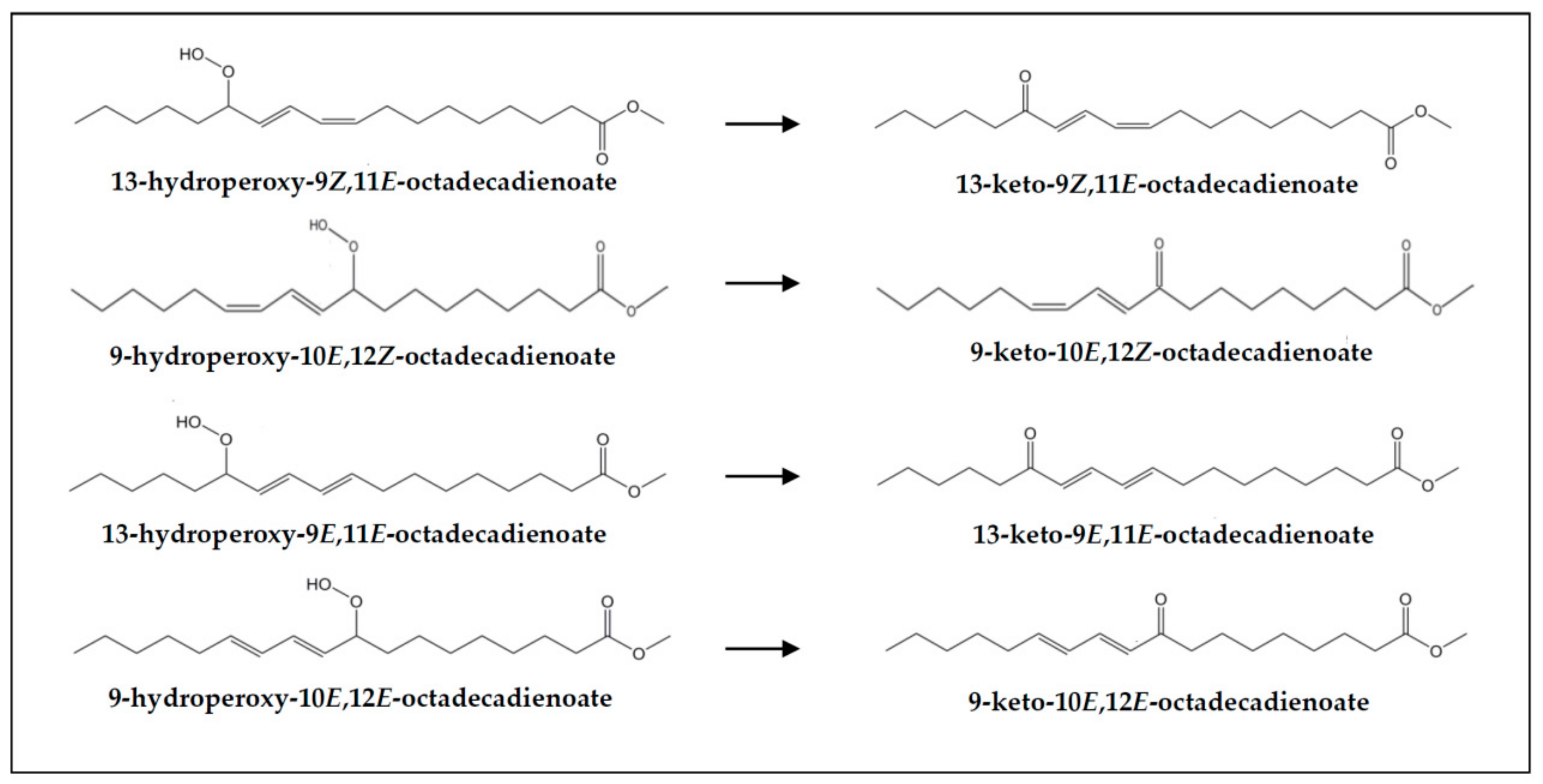
| Keto-(Z,E)-dienes | 2.02 ± 0.19 a | 3.79 ± 0.57 b | 3.55 ± 0.46 b | 2.98 ± 0.55 b | 1.63 ± 0.10 a | 3.31 ± 0.40 b | 3.20 ± 0.25 b | 2.80 ± 0.54 b |
| Keto-(E,E)-dienes | 3.97 ± 0.13 a | 5.42 ± 0.72 b | 5.15 ± 0.45 b | 4.81 ± 0.24 b | 4.31 ± 0.28 a | 5.68 ± 1.02 a | 5.41 ± 0.86 a | 4.95 ± 0.66 a |
| Aldehydes | ||||||||
| n-Alkanals | 1.59 ± 0.16 a | 4.27 ± 0.53 c | 3.75 ± 0.72 bc | 3.15 ± 0.29 b | 2.25 ± 0.15 a | 4.71 ± 0.87 b | 4.50 ± 1.10 b | 3.55 ± 0.51 ab |
| 4-OOH-+4-OH-(E)-2-alkenals | 7.48 ± 0.30 c | 2.24 ± 0.34 b | 1.78 ± 0.16 b | 1.24 ± 0.18 a | 9.01 ± 0.58 b | 2.25 ± 0.14 a | 2.16 ± 0.64 a | 1.76 ± 0.30 a |
| 4,5-Epoxy-(E)-2-alkenals | 0.62 ± 0.06 b | 0.42 ± 0.10 a | 0.34 ± 0.10 a | - | 0.87 ± 0.23 b | 0.38 ± 0.17 a | 0.34 ± 0.16 a | - |
| (E,E)-2,4-Alkadienals | 0.91 ± 0.11 b | 0.90 ± 0.10 b | 0.92 ± 0.02 b | 0.64 ± 0.11 a | 0.99 ± 0.18 a | 0.91 ± 0.19 a | 0.89 ± 0.14 a | 0.70 ± 0.15 a |
| (E)-2-Alkenals | 3.23 ± 0.21 d | 2.63 ± 0.53 c | 1.79 ± 0.20 b | 0.80 ± 0.16 a | 3.95 ± 0.19 d | 2.36 ± 0.30 c | 1.77 ± 0.24 b | 1.11 ± 0.18 a |
| (Z)-2-Alkenals | 0.64 ± 0.28 | - | - | - | 0.47 ± 0.11 | - | - | - |
| 4-Oxo-(E)-2-alkenals | 0.16 ± 0.06 | - | - | - | 0.22 ± 0.05 | - | - | - |
| 2,3-Epoxy-alkanals | - | 0.35± 0.03 a | 0.31 ± 0.03 a | 0.29 ± 0.04 a | 0.22 ± 0.07 a | 0.39 ± 0.10 a | 0.39 ± 0.07 a | 0.31 ± 0.04 a |
| Monoepoxides | ||||||||
| (Z)-Monoepoxides | 11.52 ± 1.21 a | 11.22 ± 0.61 a | 10.74 ± 0.57 a | 11.79 ± 0.59 a | 14.22 ± 0.42 a | 13.40 ± 0.74 a | 13.16 ± 1.28 a | 12.67 ± 0.83 a |
| (E)-Monoepoxides | 2.14 ± 0.02 | nd | nd | nd | 2.89 ± 0.16 | nd | nd | nd |
| Formic acid | 0.67 ± 0.07 | APS | APS | APS | 0.68 ± 0.02 | APS | APS | APS |
| Formate groups | 3.73 ± 0.48 a | 3.61 ± 0.35 a | 3.63 ± 0.44 a | 4.00 ± 0.38 a | 4.00 ± 0.83 a | 4.01 ± 0.34 a | 3.97 ± 0.27 a | 3.82 ± 0.67 a |
| 5-Alkyl-(5H)-furan-2-one derivatives | 1.36 ± 0.01 a | 1.90 ± 0.43 b | 1.68 ± 0.37 ab | 1.50 ± 0.14 a | 1.49 ± 0.15 a | 2.67 ± 0.06 c | 2.24 ± 0.24 b | 1.71 ± 0.13 a |
| Other potential hydroxy- or ether-derivatives | ||||||||
| Giving signal “u” (3.43 ppm) | 2.65 ± 0.17 b | 2.36± 0.32 ab | 2.27 ± 0.27 ab | 1.89 ± 0.27 a | 3.25 ± 0.27 b | 3.16 ± 0.59 b | 3.10 ± 0.14 b | 1.99 ± 0.39 a |
| Giving signal “v” (3.62 ppm) | 1.85 ± 0.12 | nd | nd | nd | 2.13 ± 0.19 | nd | nd | nd |
| Keto-epoxy-(E)-monoenes | ||||||||
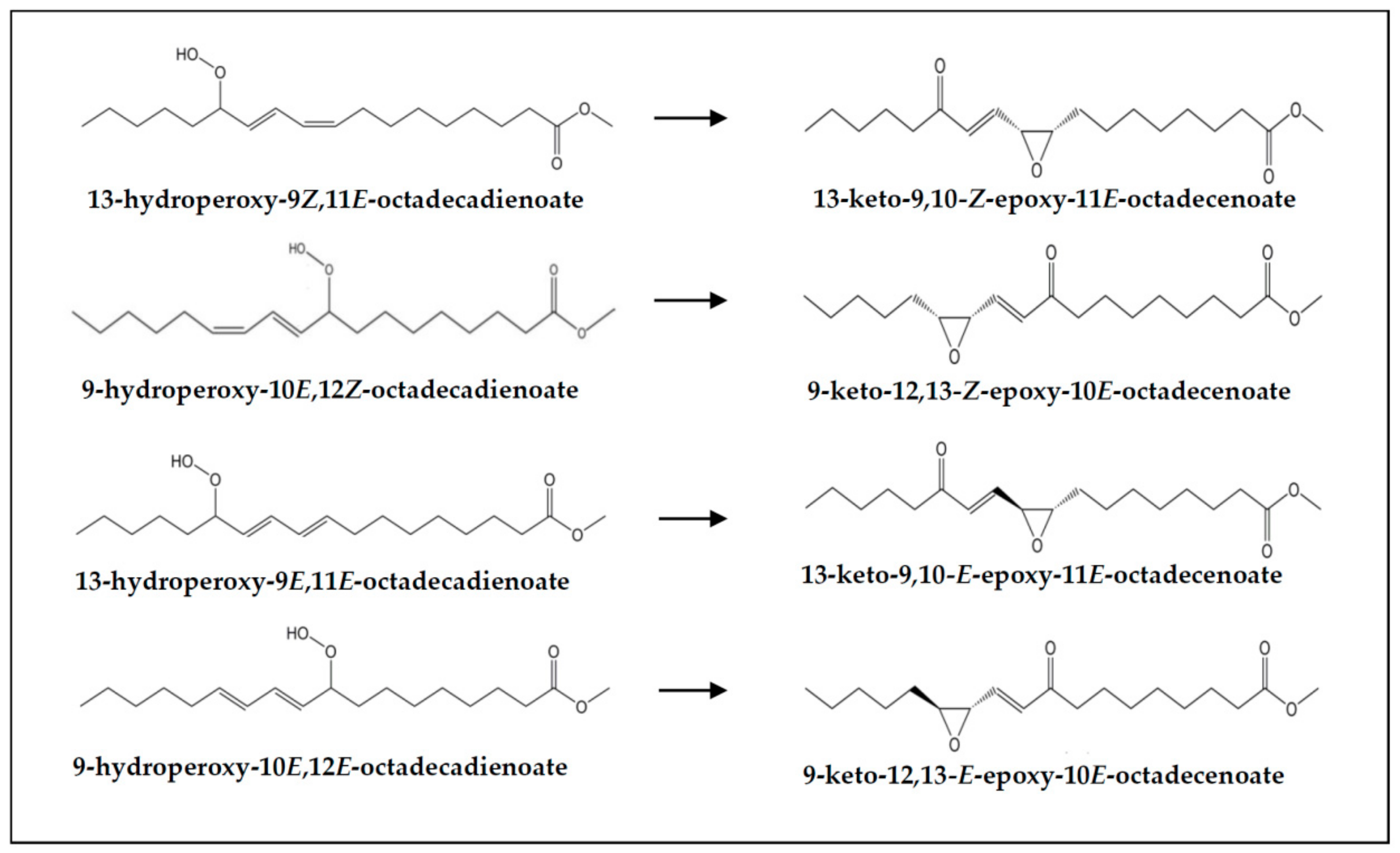
| Non-vicinal keto-epoxy-(E)-monoenes | 1.34 ± 0.04 a | 2.68 ± 0.47 b | 2.81 ± 0.30 b | 2.54 ± 0.15 b | 1.66 ± 0.09 a | 3.56 ± 0.43 c | 3.50 ± 0.21 c | 2.85 ± 0.21 b |
| Non-vicinal keto-(E)-epoxy-(E)-monoenes nd | 1.98 ± 0.49 b | 2.03 ± 0.22 b | 1.58 ± 0.19 ab | nd | 2.64 ± 0.43 b | 2.51 ± 0.19 b | 1.89 ± 0.18 a | |
| Non-vicinal keto-(Z)-epoxy-(E)-monoenes nd | 0.70 ± 0.04 a | 0.78 ± 0.13 a | 0.96 ± 0.09 b | nd | 0.92 ± 0.17 a | 0.99 ± 0.06 a | 0.96 ± 0.11 a | |
| Vicinal keto-(E)-epoxy-(E)-monoenes | 0.29 ± 0.04 | - | - | - | 0.28 ± 0.05 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. 1H NMR Study of the In Vitro Digestion of Highly Oxidized Soybean Oil and the Effect of the Presence of Ovalbumin. Foods 2021, 10, 1573. https://doi.org/10.3390/foods10071573
Martin-Rubio AS, Sopelana P, Ibargoitia ML, Guillén MD. 1H NMR Study of the In Vitro Digestion of Highly Oxidized Soybean Oil and the Effect of the Presence of Ovalbumin. Foods. 2021; 10(7):1573. https://doi.org/10.3390/foods10071573
Chicago/Turabian StyleMartin-Rubio, Ana S., Patricia Sopelana, María L. Ibargoitia, and María D. Guillén. 2021. "1H NMR Study of the In Vitro Digestion of Highly Oxidized Soybean Oil and the Effect of the Presence of Ovalbumin" Foods 10, no. 7: 1573. https://doi.org/10.3390/foods10071573
APA StyleMartin-Rubio, A. S., Sopelana, P., Ibargoitia, M. L., & Guillén, M. D. (2021). 1H NMR Study of the In Vitro Digestion of Highly Oxidized Soybean Oil and the Effect of the Presence of Ovalbumin. Foods, 10(7), 1573. https://doi.org/10.3390/foods10071573