Effect of Grape Over-Ripening and Its Skin Presence on White Wine Alcoholic Fermentation in a Warm Climate Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Methodology
2.3. Statistical Analysis
3. Results and Discussion
3.1. Over-Ripening Effects in Grape Must Physicochemical Composition
3.2. Effect of Over-Ripening and Grape Skin (GS) Presence during Alcoholic Fermentation
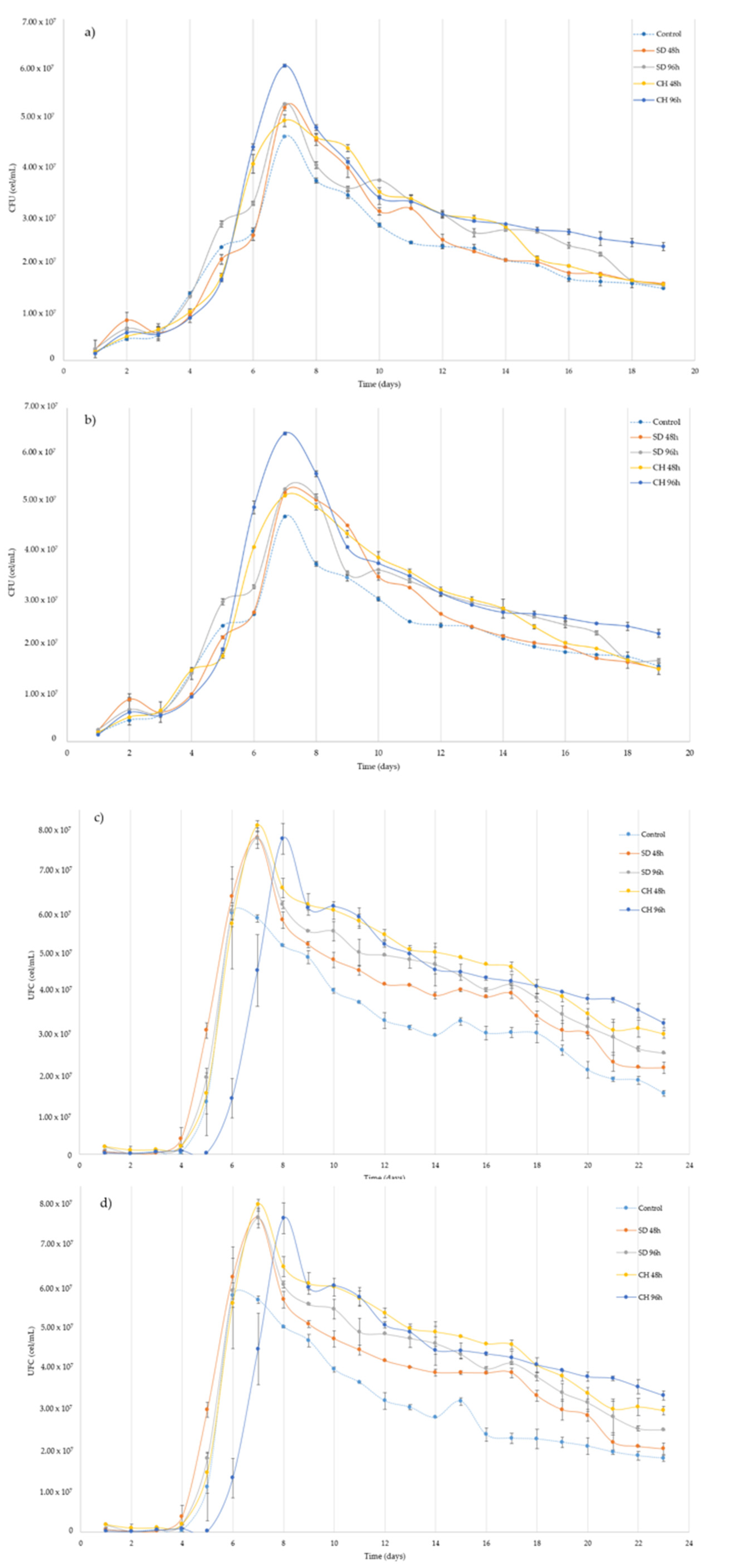
3.3. Over-Ripening and GS Presence Effect on the Alcoholic Fermentation kinetics
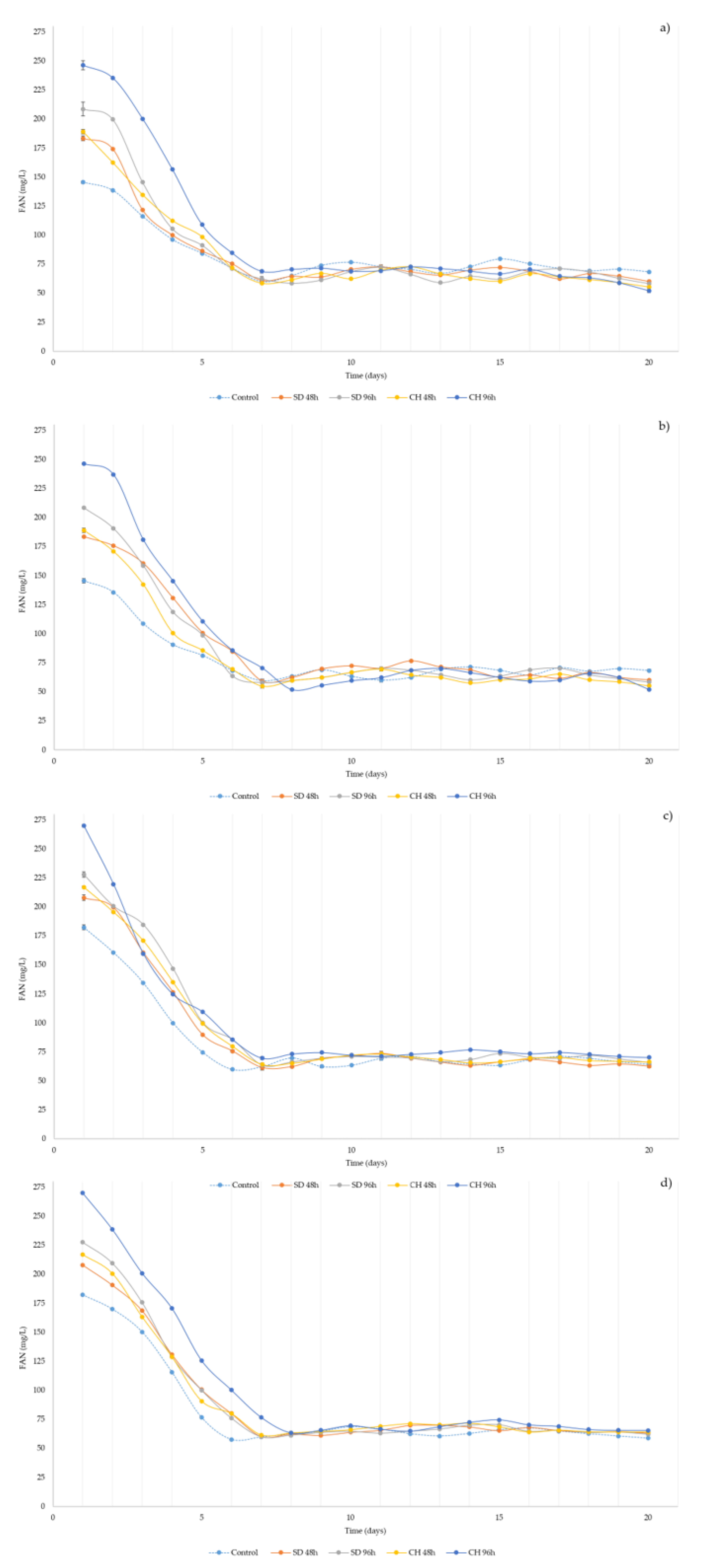
3.4. Over-Ripening and GS Presence Effect in Free Amino Nitrogen (FAN) during Alcoholic Fermentation
3.5. Effect of Over-Ripening and the Presence of GS on the Physicochemical Composition of Final Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Organization of Vine and Wine (OIV). State of the Vitiviniculture World Market: State of the Sector in 2019. Available online: https://www.oiv.int/public/medias/6679/en-oiv-state-of-the-vitiviniculture-world-market-2019.pdf (accessed on 3 April 2021).
- Food and Agriculture Organization of the United Nations (FAO). Food and Agriculture Data 2018. Available online: http://www.fao.org/faostat/ (accessed on 1 April 2021).
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Amerine, M.A. The Technology of Winemaking, 4th ed.; AVI Publishing Company: Westport, CT, USA, 1980. [Google Scholar]
- Schultz, H.R.; Jones, G.V. Climate induced historic and future changes in viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar] [CrossRef]
- Nemani, R.R.; White, M.A.; Cayan, D.R.; Jones, G.V.; Running, S.W.; Coughlan, J.C.; Peterson, D.L. Asymmetric warming over coastal California and its impact on the premium wine industry. Clim. Res. 2001, 19, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Mira de Orduña, R. Climate change associate effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Loira, I. Optimización de Parámetros Fermentativos de Calidad en Vinos Tintos de Zonas Cálidas. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2014. [Google Scholar]
- Henessy, K.J.; Whetton, P.H.; Webb, L.; McInnes, K.L. Climate change projections for Australian viticultural regions. Aust. N. Z. Grapegrow. Winemak. 2003, 469, 40. [Google Scholar]
- Godde, P.; Lattey, K.; Francis, L.; Gishen, M.; Cowey, G.; Holdstock, M.; Robinson, E.; Waters, E.; Skouroumounis, G.; Sefton, M.; et al. Towards offering wine to the consumer in optimal condition—The wine, the closures and other packaging variables. In A Review of AWRI Research Examining the Changes That Occur in Wine after Bottling. Part 1, Proceedings of the Enoforum 2005 Conference, Piacenza, Italy, 21–23 March 2005; Infowine: Ponte dell’Olio, Italy, 2005. [Google Scholar]
- Duchêne, E.; Schneider, C. Grapevine and climatic changes: A glance at the situation in Alsace. Agron. Sustain. Dev. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Jones, G.V. How Hot Is Too Hot; Wine Business Monthly: Sonoma, CA, USA, 2005; Volume 12. [Google Scholar]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Impact on Australian viticulture from greenhouse induced temperature change. In Proceedings of the MODSIM 2005 International Congress on Modelling and Simulation, Canberra, Australia, 12–15 December 2005; Modelling and Simulation Society of Australia and New Zealand: Canberra, Australia, 2005. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.; Tavares, R.M.; Soussa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout Grape Berry development and Fruit and Wine Quality. Food 2007, 13, 1–22. [Google Scholar]
- Jones, G.V.; Goodrich, G.B. Influence of climate variability on wine regions in the western USA and on wine quality in the Napa Valley. Clim. Res. 2008, 35, 241–254. [Google Scholar] [CrossRef]
- Fraga, H. Climate Change: A New Challenge for the Winemaking Sector. Agronomy 2020, 10, 1465. [Google Scholar] [CrossRef]
- Fraga, H.; Atauri, I.G.D.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Viticulture in Portugal: A review of recent trends and climate change projections. Oeno One 2017, 51, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Fraga, H.; Santos, J.A. Vineyard mulching as a climate change adaptation measure: Future simulations for Alentejo, Portugal. Agric. Syst. 2018, 164, 107–115. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. Oeno One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Ugaglia, A.A.; Peres, S. Knowledge dynamics and climate change issues in the wine industry: A literature review. J. Innov. Econ. Manag. 2017, 3, 105–125. [Google Scholar]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomaro in the world’s future wine-growing regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Galluzzi, G.; Seyoum, A.; Halewood, M.; López-Noriega, I.; Welch, E.W. The Role of Genetic Resources in Breeding for Climate change: The Case of Public Breeding Programmes in Eighteen Developing Countries. Plants 2020, 9, 1129. [Google Scholar] [CrossRef]
- Galletto, L.; Barisan, L.; Boatto, V.; ACCostantini, E.; Lorenzetti, R.; Pomarici, E.; Vecchio, R. More crop for drop–climate change and wine: An economic evaluation of a new drought-resistant rootstock. Recent Pat. Food Nutr. Agric. 2014, 6, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boatto, V.; Barisan, L.; Teo, G. Valutazione della risorsa irrigua di soccorso nella produzione del Conegliano Valdobbiadene Prosecco DOCG 1. Aestimum 2017, 70, 31. [Google Scholar]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate changes and food quality: The potential of microbial activities as mitigating strategies in the wine sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 654. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Genetical, Morphological and Pysicochemical Characterization of the Autochthonous Cultivar ‘Uva Rey’ (Vitis vinifera L.). Agronomy 2019, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions. Agronomy 2020, 10, 634. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process. Molecules 2018, 23, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Influence of the presence of Grape Skins during White Wine Alcoholic Fermentation. Agronomy 2021, 11, 452. [Google Scholar] [CrossRef]
- Esmaiili, M.; Sotudeh-Gharebagh, R.; Cronin, K.; Mousavi, M.A.E.; Rezazadeh, G. Grape drying: A review. Food Rev. Int. 2012, 23, 257–280. [Google Scholar] [CrossRef]
- López, I.; Morales, J.; Ramirez, P.; Instituto de Formación Agraria y Pesquera, IFAPA, Córdoba, Spain; Consejería de Agricultura y Pesca, Junta de Andalucía, Sevilla, Spain; Palencia, L.; Romero, C.; Sociedad Cooperativa AECOVI, Jerez de la Frontera, Cádiz, Spain. Personal communication, 2007.
- Palacios, V.; Roldán, A.; Jiménez-Cantizano, A.; Amores-Arrocha, A. Physicochemical and microbiological characterization of the sensory deviation responsable for the rigin of the special sherry wines “Palo Cortado” type. PLoS ONE 2018, 13, e0208330. [Google Scholar]
- OIV Office International de la vigne et du Vin. Recuéil des Methods Internationals D’analyse des Vins et des Moûts; Edition Oficielle: Paris, France, 2014. [Google Scholar]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of Protein and Total Usable Nitrogen in Beer and Wine Using a Microwell Ninhydrin Assay. J. Inst. Brew 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalycilic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- Almela, L.; Javaloy, S.; Fernández-López, J.A.; López-Roca, J.M. Varietal classification of Young red wines in terms of chemical and color parameters. J. Sci. Food Agric. 1996, 70, 173–180. [Google Scholar] [CrossRef]
- Heredia, F.J.; Troncoso, A.M.; Guzmán-Chozas, M. Multivariate characterization of aging status in red wines based on chromatic parameters. Food Chem. 1997, 60, 103–108. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Ruiz-Bejarano, M.J. Elaboración de Vinos Dulces de Andalucía a Partir de uvas Secadas Artificialmente. Ph.D Thesis, Universidad de Cádiz, Puerto Real, Spain, 2016. [Google Scholar]
- Chivite, J.; Raventós, M.; Castro, E. Gestión de pH en el vino de calidad, 1st ed.; Fundación para la Cultura del vino: Madrid, Spain, 2005; pp. 9–15. [Google Scholar]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozès, N.; Mas, A.; Guillamón, J.M. Effect of organic acids and nitrogen source on alcoholic fermentation: Study of their buffering capacity. J. Agric. Food Chem. 2003, 51, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Henshke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Bisson, L.F.; Butzke, C.E. Diagnosis and Rectification of Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Bisson, L.F. Stuck and Sluggish Fermentations. InfoWine 2005, 9, 107–119. [Google Scholar]
- Miño-Valdés, J.E. Fundamentos para Elaborar vino Blanco Común en un Desarrollo Tecnológico, 1st ed.; Editorial Universitaria, Universidad Nacional de Misiones: Posadas, Argentina, 2012; pp. 47–50. [Google Scholar]
- D’Amato, D.; Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. Effects of temperatura, ammonium and glucose concentracions on yeast growth in a model system wine. Int. J. Food Sci. Technol. 2006, 41, 1152–1157. [Google Scholar] [CrossRef]
- Hidalgo Togores, J. Transformaciones microbianas. Levaduras, bacterias y virus. In Tratado de Enología, Tomo I, 2nd ed.; Hidalgo-Togores, J., Ed.; Editorial Mundi-Prensa: Madrid, Spain, 2010; p. 118. [Google Scholar]
- Carbonell-Bejarano, P.; Martínez-Zapater, J.M. Estructura y Composición de la Uva y Su Contribución al Vino. SEBBM, Bioquímica del Vino. Available online: https://www.sebbm.es/revista/articulo.php?id=212&url=estructura-y-composicion-de-la-uva-y-su-contribucion-al-vino (accessed on 9 November 2020).
- Flamini, R.; De Rosso, M. Mass spectrometry in the analysis of grape and wine proteins. Expert Rev. Proteomic 2006, 3, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Dubordieu, D.; Doneche, B.; Lonvaud, A.; Glories, Y.; Maugean, A. Tratado de Enología, Tomo II, 3rd ed.; Hemisferio Sur-Mundi Prensa: Buenos Aires, Argentina, 2003. [Google Scholar]
- Barre, P.; Blondin, P.; Dequin, S.; Feuillat, M.; Sablayrolles, J.M.; Salmon, J.M. La Levure de Fermentation Alcoolique; Oenologie Technique et Documentation; Lovisier: Paris, France, 1998; pp. 414–495. [Google Scholar]
- Dizy, M.; Polo, M.C. Changes in concentration of nitrogenous compounds during fermentation of white grape musts at pilot plant scale. Food Sci. Technol. Int. 1996, 2, 87–93. [Google Scholar] [CrossRef]
- Fornairon-Bonneford, C.; Camarasa, C.; Moutounet, M.; Salmon, J.M. New Trends on yeast autolysis and wine ageing on lees: A bibliographical review. Int. J. Vine Wine Sci. 2001, 36, 49–69. [Google Scholar]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Ough, C.S. Substances extracted during skin contact with white musts. I. General wine composition and quality changes with contact time. Am. J. Enol. Vitic. 1969, 40, 208–213. [Google Scholar]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Antioxidant activity and phenolic profiles of Sauvignon Blanc wines made by various maceration techniques. Aust. J. Grape Wine Res. 2015, 21, 57–68. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vinw grape drying effect on volatile compounds and aromatic series in must from Pedro Ximenez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Gomez, E.; Laencina, J.; Martínez, A. Vinification effects on changes in volatile compounds of wine. J. Food Sci. 1994, 59, 406–409. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Corte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, M.; Pretorius, I.S. Yeast and its importance to wine aroma. South African J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, Y.; Watanabe, M. Effects of yeast strains and environmental conditions on formation of organic acid in must during fermentation. J. Ferment. Technol. 1981, 59, 27–32. [Google Scholar]
- Erasmus, D.J.; Van der Merwe, G.K.; Van Vuuren, H.J. Genome-vide expression analyses: Metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 2003, 3, 375–399. [Google Scholar] [CrossRef] [Green Version]
- Villar-Moreno, A.L. Sensibilidad al etanol en levaduras: Bases fisiológicas y análisis de métodos empleados en su determinación. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1992. [Google Scholar]
- López de Lerma, N.; Peinado, J.; Moreno, J.; Peinado, R.A. Antioxidant activity, browning and volatile Maillard compounds in Pedro Ximénez sweet wines under accelerated oxidative aging. LWT Food Sci. Technol. 2010, 43, 1557–1563. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, J.; Peinado, R.A. Antioxidant activity of musts from Pedro Ximénez grapes subjected to off-vine drying process. Food Chem. 2007, 107, 224–228. [Google Scholar] [CrossRef]
- Di Stefano, R.; Maggiortto, G. Antociani, acidi idrossicinnamici e flavone del frutto, delle foglie e ei tralci della vite. Riv. Vitic. Enol. 1995, 48, 51–65. [Google Scholar]
- Di Stefano, R.; Borsa, D.; Gentilizi, N.; Corino, L.; Tronfi, S. Evoluzione defli zuccheri, degli acidi fissi e dei composti fenolici dell’uva durante l’appassimento in frutaio. Riv. Vitic. Enol. 1997, 1, 33. [Google Scholar]
- Di Stefano, R.; Maggiortto, G.; Melia, V.; Di Bernardi, D.; Sparacio, A.; Fina, B.; Sparla, S. Evoluzione dei composti terpenici durante il proceso di appasimento dell’uva Zibibbo di Pantelleria. Enotecnico 1995, 10, 73. [Google Scholar]
- Nguela, J.M.; Vernhet, A.; Julien-Ortiz, A.; Sieczkowski, N.; Mouret, J.-R. Effect of grape must polyphenols on yeast metabolism during alcoholic fermentation. Food Res. Int. 2019, 121, 161–175. [Google Scholar] [CrossRef] [PubMed]
- CIE Comission Internationale de l’Éclairage. Technical Report. Colorimetry, 2nd ed.; CIE: Viena, Austria, 1986. [Google Scholar]

| Control | SD48h | SD96h | CH48h | CH96h | |
|---|---|---|---|---|---|
| 2018 | |||||
| pH | 3.470 ± 0.014 a | 3.440 ± 0.014 b | 3.420 ± 0.028 c | 3.300 ± 0.014 d | 3.200 ± 0.014 e |
| TA (g/L) | 3.630 ± 0.117 a | 3.640 ± 0.175 a | 3.920 ± 0.058 b | 4.106 ± 0.058 c | 4.996 ± 0.058 d |
| FAN (mg/L) | 145.600 ± 0.000 a | 183.400 ± 1.980 b | 208.600 ± 5.940 c | 189.000 ± 1.980 b | 246.400 ± 3.960 d |
| °Bé | 11.300 ± 0.140 a | 12.800 ± 0.140 b | 13.500 ± 0.140 c | 12.800 ± 0.000 b | 15.000 ± 0.140 d |
| 2019 | |||||
| pH | 3.360 ± 0.021 a | 3.290 ± 0.042 b | 3.230 ± 0.035 c | 3.280 ± 0.078 b | 3.230 ± 0.070 c |
| TA | 3.620 ± 0.080 a | 4.310 ± 0.053 b | 5.525 ± 0.053 c | 5.063 ± 0.043 d | 5.780 ± 0.070 e |
| FAN (mg/L) | 162.500 ± 2.256 a | 200.230 ± 1.978 b | 224.600 ± 1.450 c | 207.650 ± 2.465 b | 265.130 ± 3.472 d |
| °Bé | 12.180 ± 0.020 a | 12.770 ± 0.040 b | 13.910 ± 0.090 c | 14.210 ± 0.060d c | 15.680 ± 0.030 e |
| 2018 | |||||
| Control | SD48h | SD96h | CH48h | CH96h | |
| Without GS | |||||
| TA (g/L) | 4.629 ± 0.027 a | 4.763 ± 0.067 a | 4.905 ± 0.080 a | 5.102 ± 0.241 b | 5.554 ± 0.013 b |
| VA (g/L) | 0.162 ± 0.012 a | 0.184 ± 0.031 a | 0.400 ± 0.024 b | 0.231 ± 0.012 a | 0.366 ± 0.021 b |
| % Alc. | 11.854 ± 0.182 a | 13.430 ± 0.060 a | 14.633 ± 0.159 a,b | 13.656 ± 0.398 a | 16.584 ± 0.016 c |
| RS (g/L) | 1.418 ± 0.285 a | 1.922 ± 0.330 b | 2.220 ± 0.509 b | 1.733 ± 0.107 a,b | 2.998 ± 0.264 c |
| TPI | 7.990 ± 0.141 a | 6.540 ± 0.170 b | 6.090 ± 0.269 b | 6.450 ± 0.891 b | 8.160 ± 0.085 a |
| Abs 420 | 0.074 ± 0.015 a | 0.093 ± 0.008 a | 0.093 ± 0.001 a | 0.109 ± 0.008 b | 0.110 ± 0.002 b |
| L* | 96.834 ± 0.923 a | 98.279 ± 0.057 a | 98.664 ± 0.173 a | 98.372 ± 0.071 a | 98.247 ± 0.127 a |
| a* | 0.160 ± 0.119 a | 0.420 ± 0.020 b | 0.470 ± 0.033 b | 0.600 ± 0.064 c | 0.430 ± 0.023 b |
| b* | 10.484 ± 2.874 a | 5.120 ± 0.572 b | 4.860 ± 0.246 b | 5.683 ± 0.492 b | 6.168 ± 0.009 b |
| H* | 91.019 ± 0.931 a | 94.671 ± 0.301 a | 95.526 ± 0.664 a | 96.021 ± 0.115 a | 93.970 ± 0.217 a |
| With 20% GS | |||||
| TA (g/L) | 4.413 ± 0.102 a | 4.569 ± 0.140 a,b | 4.769 ± 0.097 b | 4.958 ± 0.305 b,c | 5.068 ± 0.198 c |
| VA (g/L) | 0.361 ± 0.068 a | 0.412 ± 0.006 a,c | 0.580 ± 0.100 b | 0.428 ± 0.168 c,d | 0.551 ± 0.136 b,d |
| % Alc. | 11.970 ± 0.256 a | 13.569 ± 0.147 a,b | 14.896 ± 0.253 a,b | 13.852 ± 0.539 a,b | 16.489 ± 0.187 c |
| RS (g/L) | 1.257 ± 0.149 a | 1.567 ± 0.698 a | 2.541 ± 0.410 b | 1.710 ± 0.205 a | 3.056 ± 0.423 b |
| TPI | 8.690 ± 0.157 a | 7.214 ± 0.099 a | 7.724 ± 0.301 a | 7.158 ± 0.249 a | 8.879 ± 3.265 a |
| Abs 420 | 0.158 ± 0.008 a | 0.087 ± 0.005 b | 0.048 ± 0.001 c | 0.087 ± 0.003 b | 0.099 ± 0.001 b |
| L* | 98.698 ± 0.587 a | 100.025 ± 0.147 a | 101.259 ± 0.257 a | 99.995 ± 0.009 a | 102.025 ± 0.298 a |
| a* | 0.153 ± 0.111 a | 0.411 ± 0.015 b | 0.468 ± 0.019 b,c | 0.530 ± 0.054 c | 0.384 ± 0.069 b |
| b* | 5.699 ± 0.547 a | 3.568 ± 0.413 b | 2.567 ± 0.154 b | 2.541 ± 0.056 b | 2.354 ± 0.016 b |
| H* | 93.545 ± 0.931 a | 97.541 ± 0.149 a | 98.035 ± 0.761 a | 98.221 ± 0.431 a | 96.028 ± 0.199 a |
| 2019 | |||||
| Control | SD 48 h | SD 96 h | CH 48 h | CH 96 h | |
| Without GS | |||||
| TA (g/L) | 5.570 ± 0.098 a | 5.810 ± 0.104 a | 6.480 ± 0.057 b | 6.320 ± 0.421 b | 6.460 ± 0.268 b |
| VA (g/L) | 0.189 ± 0.030 a | 0.214 ± 0.012 a | 0.256 ± 0.036 b | 0.296 ± 0.016 b | 0.489 ± 0.080 c |
| % Alc. | 10.756 ± 0.430 a | 12.380 ± 0.320 a,d | 14.299 ± 0.190 b | 13.420 ± 0.598 b,d | 16.240 ± 0.480 c |
| RS (g/L) | 1.356 ± 0.018 a | 1.976 ± 0.143 b | 1.447 ± 0.169 a,b | 1.238 ± 0.188 a | 4.813 ± 0.268 c |
| TPI | 6.513 ± 0.091 a | 4.976 ± 0.100 b | 3.790 ± 0.082 c | 5.713 ± 0.712 b | 10.268 ± 0.55 d |
| Abs 420 | 0.040 ± 0.010 a | 0.051 ± 0.010 a,d | 0.062 ± 0.001 b,d | 0.073 ± 0.010 b | 0.110 ± 0.01 c |
| L* | 97.563 ± 1.235 a | 98.593 ± 0.147 a | 97.305 ± 0.846 a | 95.168 ± 0.992 a | 96.436 ± 0.589 a |
| a* | 0.5 ± 0.006 a | 0.769 ± 0.110 b | 0.782 ± 0.015 b | 0.988 ± 0.036 c | 0.846 ± 0.087 b |
| b* | 10.312 ± 0.653 a | 11.241 ± 0.216 a | 11.983 ± 0.549 a | 10.673 ± 0.630 a | 14.297 ± 0.55 b |
| H* | 97.536 ± 2.541 a | 98.631 ± 0.964 a | 97.995 ± 0.966 a | 104.531 ± 2.174 a | 93.631 ± 0.501 a |
| With 20% GS | |||||
| TA (g/L) | 5.170 ± 0.070 a | 5.460 ± 0.070 b | 6.118 ± 0.050 c | 6.025 ± 0.560 c | 6.165 ± 0.150 c |
| VA (g/L) | 0.230 ± 0.030 a | 0.240 ± 0.010 a,b | 0.280 ± 0.010 b | 0.340 ± 0.010 c | 0.620 ± 0.030 d |
| % Alc. | 10.860 ± 0.910 a | 12.430 ± 0.440 b | 14.390 ± 0.260 c | 13.390 ± 1.470 b,c | 16.480 ± 0.360 d |
| RS (g/L) | 1.200 ± 0.050 a | 1.450 ± 0.090 a,b | 1.640 ± 0.001 a,b | 1.770 ± 0.140 b | 4.510 ± 0.080 c |
| TPI | 4.320 ± 0.160 a | 5.620 ± 0.100 b | 7.260 ± 0.070 c | 6.350 ± 0.880 b,c | 10.974 ± 0.550 d |
| Abs 420 | 0.140 ± 0.010 a | 0.058 ± 0.010 b | 0.063 ± 0.001 b | 0.071 ± 0.010 b | 0.780 ± 0.010 b |
| L* | 100.220 ± 0.430 a | 100.110 ± 0.050 a | 100.190 ± 0.000 a | 97.990 ± 2.020 a | 98.900 ± 0.140 a |
| a* | 0.511 ± 0.130 a | 0.862 ± 0.090 b | 0.852 ± 0.010 b | 1.053 ± 0.250 c | 0.911 ± 0.030 b,c |
| b* | 3.330 ± 0.300 a | 4.280 ± 0.640 a,b | 4.780 ± 0.010 a,b | 3.660 ± 0.490 a | 9.900 ± 0.550 b |
| H* | 100.290 ± 1.810 a | 101.480 ± 1.220 a | 100.100 ± 0.130 a | 106.480 ± 5.660 a | 95.270 ± 0.490 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Effect of Grape Over-Ripening and Its Skin Presence on White Wine Alcoholic Fermentation in a Warm Climate Zone. Foods 2021, 10, 1583. https://doi.org/10.3390/foods10071583
Sancho-Galán P, Amores-Arrocha A, Palacios V, Jiménez-Cantizano A. Effect of Grape Over-Ripening and Its Skin Presence on White Wine Alcoholic Fermentation in a Warm Climate Zone. Foods. 2021; 10(7):1583. https://doi.org/10.3390/foods10071583
Chicago/Turabian StyleSancho-Galán, Pau, Antonio Amores-Arrocha, Víctor Palacios, and Ana Jiménez-Cantizano. 2021. "Effect of Grape Over-Ripening and Its Skin Presence on White Wine Alcoholic Fermentation in a Warm Climate Zone" Foods 10, no. 7: 1583. https://doi.org/10.3390/foods10071583
APA StyleSancho-Galán, P., Amores-Arrocha, A., Palacios, V., & Jiménez-Cantizano, A. (2021). Effect of Grape Over-Ripening and Its Skin Presence on White Wine Alcoholic Fermentation in a Warm Climate Zone. Foods, 10(7), 1583. https://doi.org/10.3390/foods10071583









