Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of RG-I-Rich Apple Pectin-Derived Samples
2.2.1. Generation of RG-I-Rich Apple Pectin-Derived Samples
Enzymatic Degradation of Residual Starch
Generation of RG-I-Rich Pectin Samples by Means of a β-Eliminative Reaction
2.2.2. Generation of RG-I-Rich Apple Pectin-Derived Sample with Modified RG-I-Related Sugars by Means of Acid Hydrolysis
2.3. Structural Characterization of the Pectin Samples
2.3.1. Galacturonic Acid Content
2.3.2. Degree of Methyl Esterification
2.3.3. Molecular Weight Distribution
2.3.4. Neutral Sugar Content
2.3.5. Protein Content
2.4. Physicochemical Properties of the Pectin Samples
2.4.1. ζ-Potential Measurements
2.4.2. Dynamic Interfacial Tension
2.4.3. Flow Behavior
2.5. Preparation of Pectin-Containing Emulsions
2.6. Physical Stability Evaluation of the Pectin-Containing Emulsions
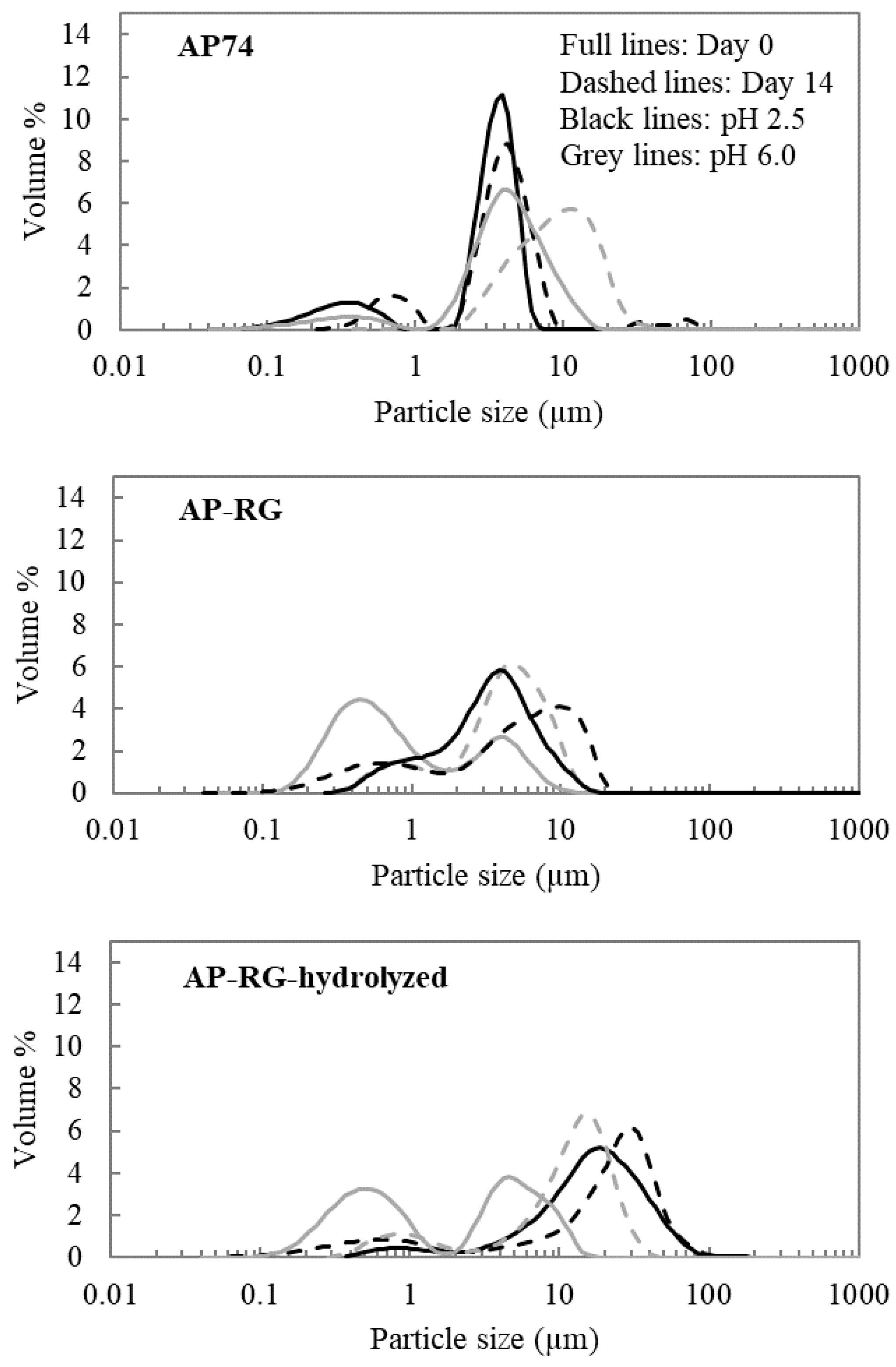
2.6.1. Volume-Weighted Particle Size Distribution
2.6.2. Individual Oil Droplet Size
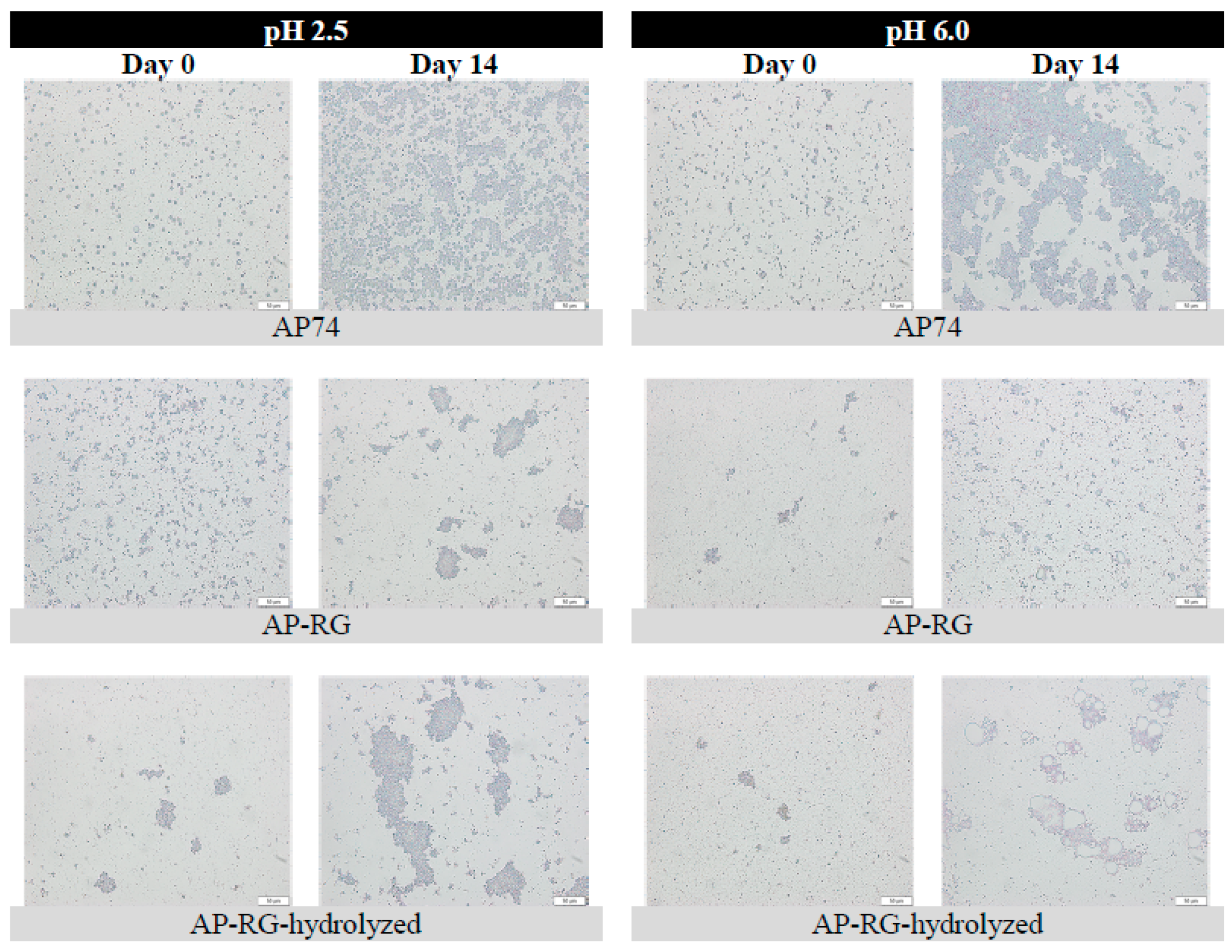
2.6.3. Microstructure
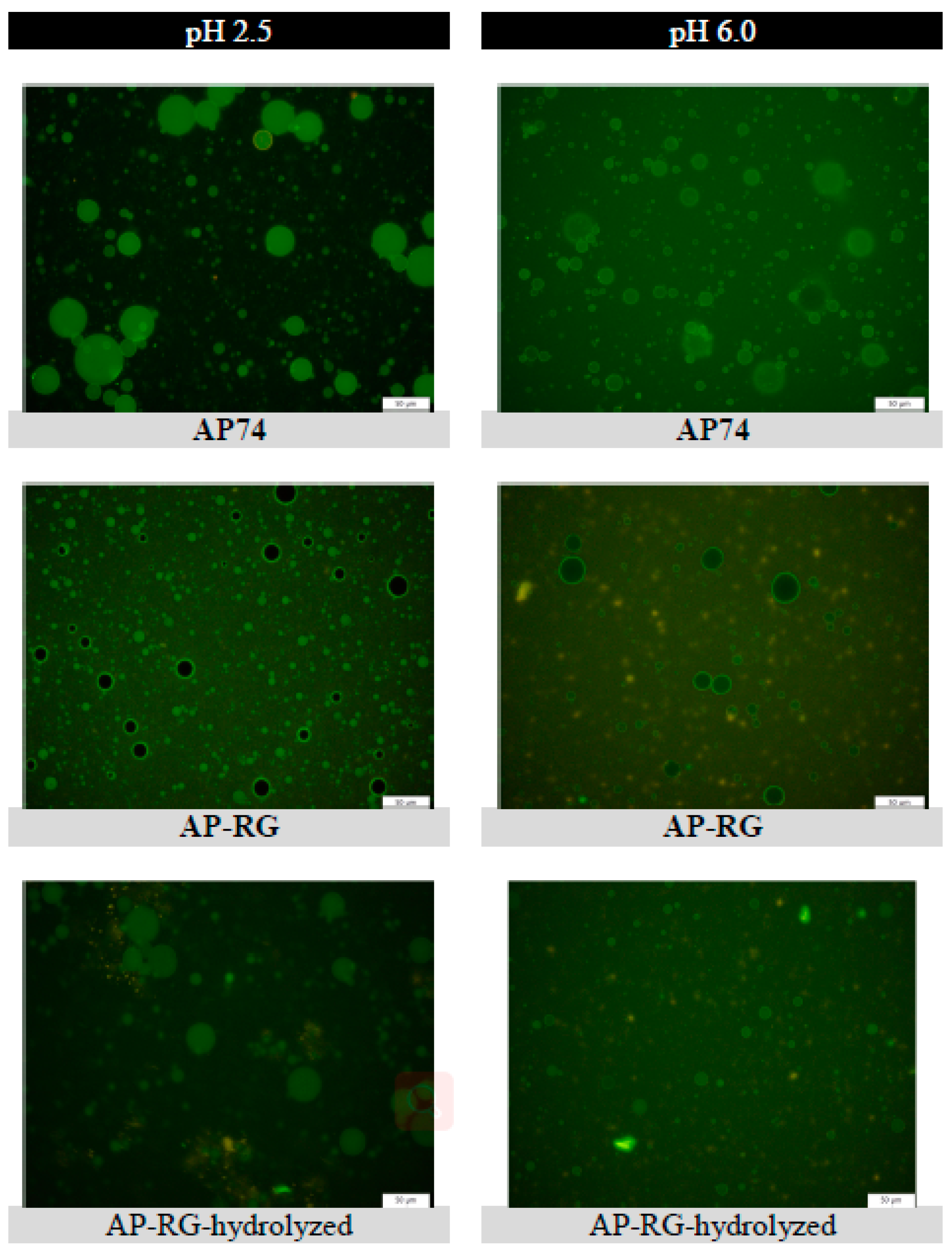
2.6.4. Fluorescent Microscopy
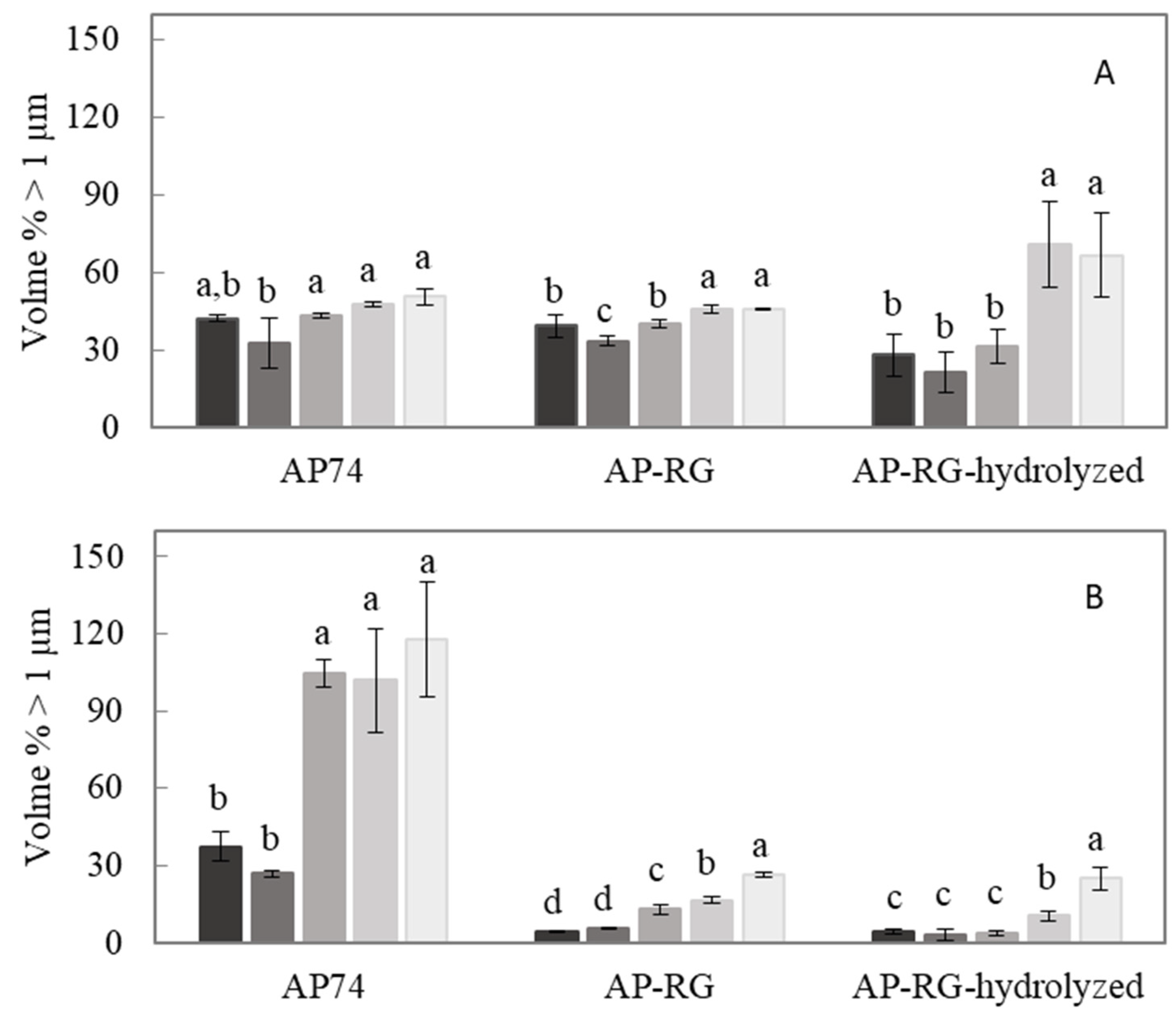
2.6.5. Accelerated Physical Stability Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization of Apple Pectin and RG-I-Rich Apple Pectin Samples
3.1.1. Galacturonic Acid and Neutral Sugar Content
3.1.2. Degree of Methylesterification
3.1.3. Protein Content
3.1.4. Molecular Weight Distribution
3.2. Physicochemical Properties of the Pectin Samples
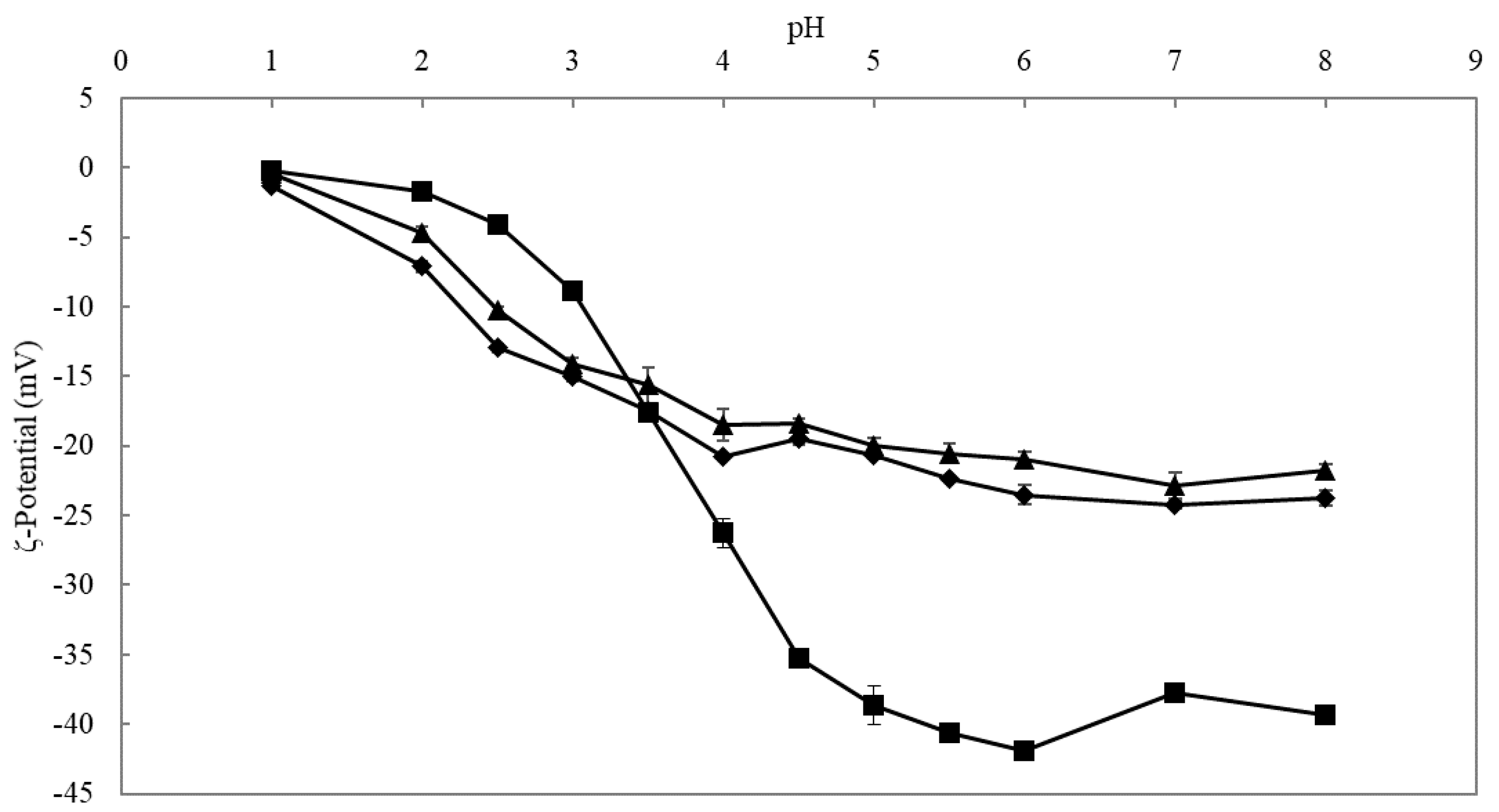
3.2.1. ζ-Potential
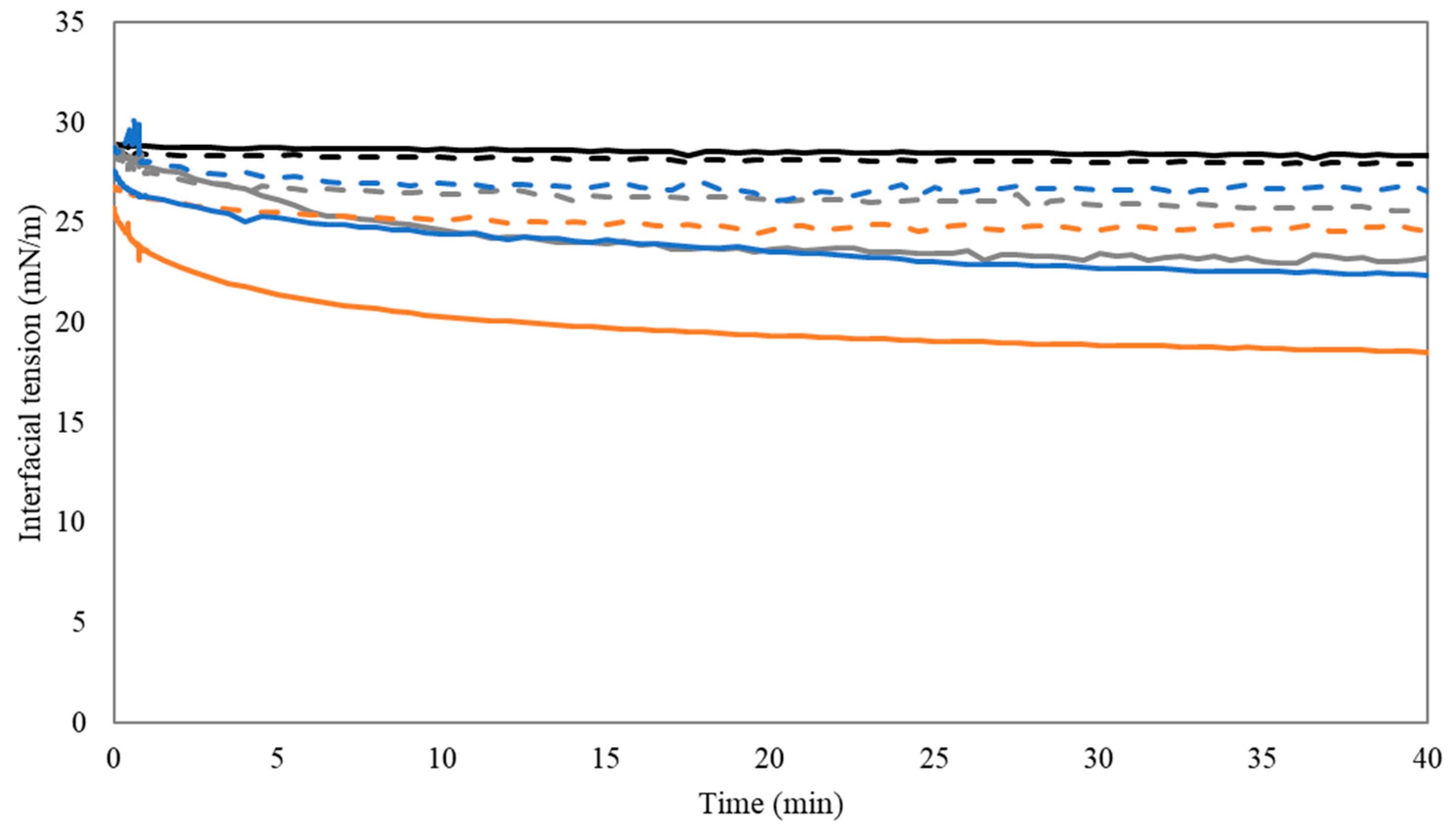
3.2.2. Dynamic Interfacial Tension
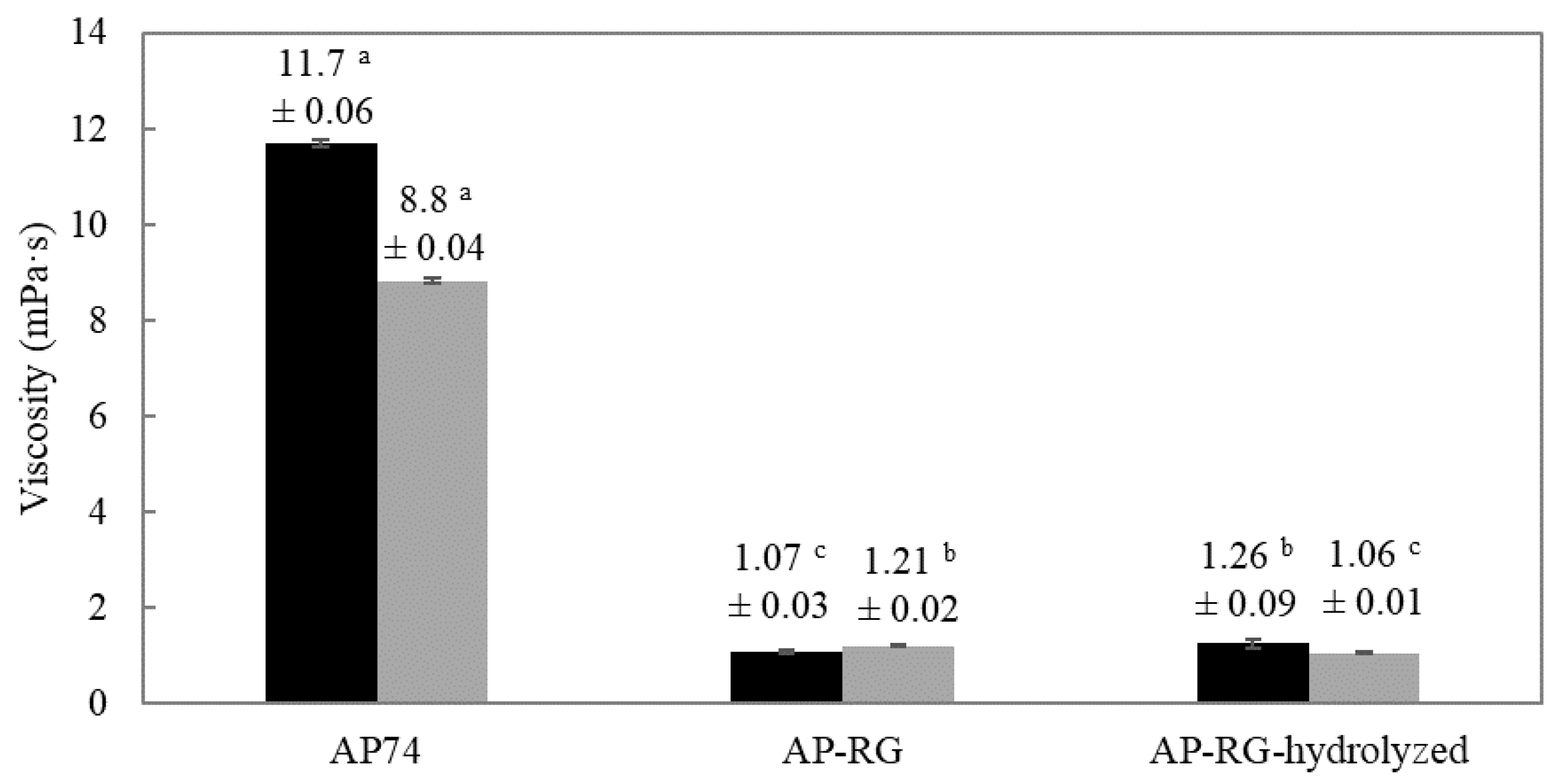
3.2.3. Determination of Flow Properties
3.3. Physical Stability Evaluation of the Pectin-Containing Emulsions
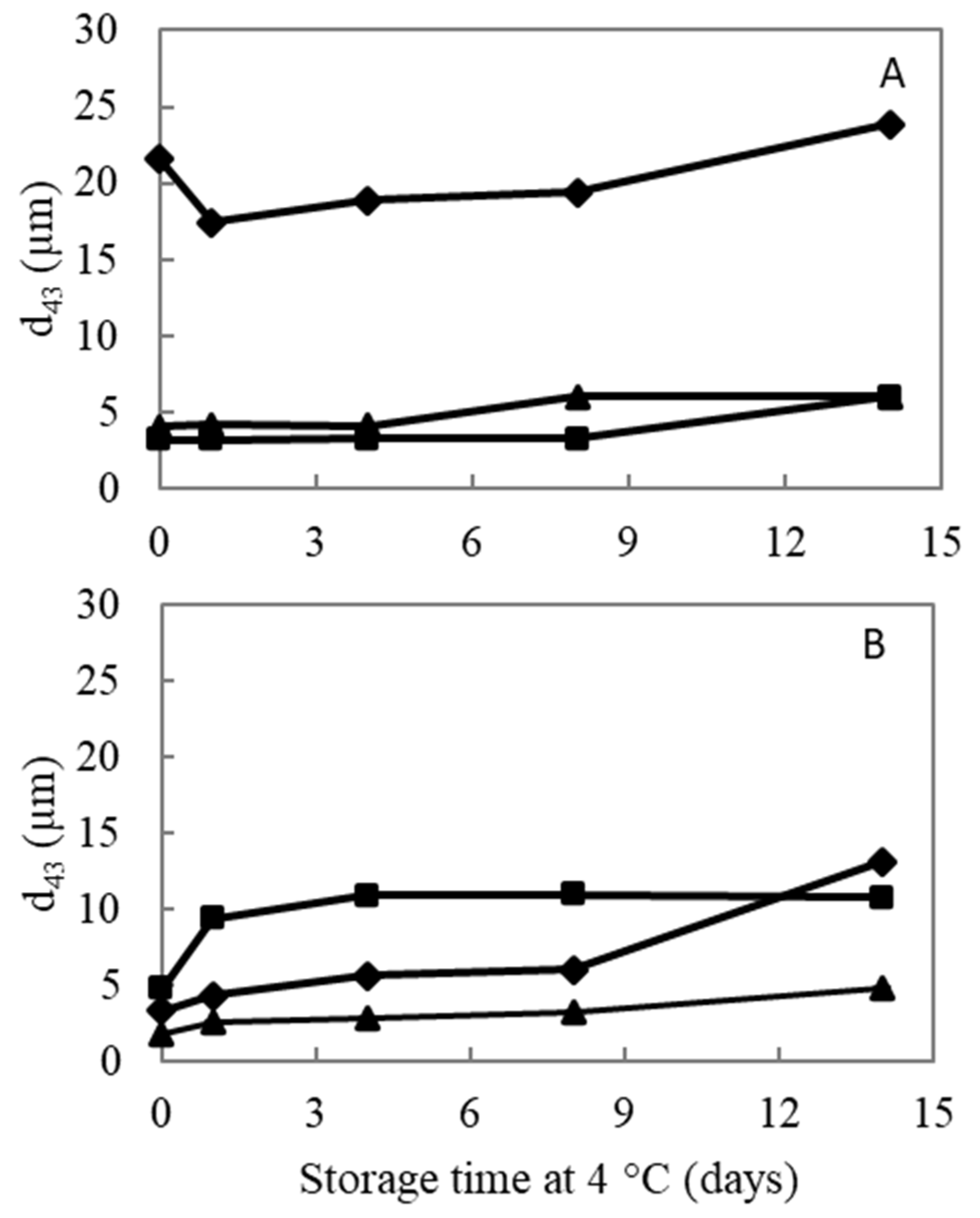
3.3.1. Particle Size and Microscopic Assessment
3.3.2. Microscopic Visualization of Fluorescently Labelled Pectin Molecules in Coarse Emulsions
3.3.3. Accelerated Physical Stability Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Van Buren, J.P. Function of Pectin in Plant Tissue Structure and Firmness. In The Chemistry and Technology of Pectin; Elsevier: Amsterdam, The Netherland, 1991. [Google Scholar]
- Njoroge, D.M.; Kinyanjui, P.K.; Makokha, A.O.; Christiaens, S.; Shpigelman, A.; Sila, D.N.; Hendrickx, M.E. Extraction and characterization of pectic polysaccharides from easy- and hard-to-cook common beans (Phaseolus vulgaris). Food Res. Int. 2014, 64, 314–322. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Brazilian Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Jafari, F.; Khodaiyan, F.; Kiani, H.; Hosseini, S.S. Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohydr. Polym. 2017, 157, 1315–1522. [Google Scholar] [CrossRef] [PubMed]
- Girma, E.; Worku, M.T. Extraction and Characterization of Pectin from Selected Fruit Peel Waste. Int. J. Sci. Res. Publ. 2016, 6, 447–454. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
- Neckebroeck, B.; Verkempinck, S.H.E.; Van Audenhove, J.; Bernaerts, T.; de Wilde d’Estmael, H.; Hendrickx, M.E.; Van Loey, A.M. Structural and emulsion stabilizing properties of pectin rich extracts obtained from different botanical sources. Food Res. Int. 2021, 141, 110087. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G.J. Structural Characterization of Pectic Hairy Regions Isolated from Apple Cell Walls; Elsevier: Amsterdam, The Netherland, 1995; ISBN 9054854057. [Google Scholar]
- Calvete-Torre, I.; Munoz-Almagro, N.; Pacheco, T.; Anton, M.J.; Dapena, E.; Ruiz, L.; MArgolles, A.; Villamiel, M.; Moreno, J. Apple pomace derived from mono-varietal ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Wu, X.; Mort, A. Structure of a Rhamnogalacturonan Fragment from Apple Pectin: Implications for Pectin Architecture. Int. J. Carbohydr. Chem. 2014, 2014, 347381. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; MacDougall, A.J.; Morris, V.J. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr. Res. 2010, 345, 487–497. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Jamsazzadeh Kermani, Z.; Moelants, K.R.N.; Ngouémazong, E.D.; Van Loey, A.; Hendrickx, M.E.G. Process–Structure–Function Relations of Pectin in Food. Crit. Rev. Food Sci. Nutr. 2016, 56, 1021–1042. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Koch, L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Effect of Molecular Weight Reduction, Acetylation and Esterification on the Emulsification Properties of Citrus Pectin. Food Biophys. 2015, 10, 217–227. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Al-Assaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, X.; Li, X.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z. gang Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll. 2002, 16, 249–256. [Google Scholar] [CrossRef]
- Neckebroeck, B.; Verkempinck, S.H.E.; Vaes, G.; Wouters, K.; Magnée, J.; Hendrickx, M.E.; Van Loey, A.M. Advanced insight into the emulsifying and emulsion stabilizing capacity of carrot pectin subdomains. Food Hydrocoll. 2020, 102. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Structure-Function Relationships in Pectin Emulsification. Food Biophys. 2018, 13, 71–79. [Google Scholar] [CrossRef]
- Nakamura, A.; Maeda, H.; Corredig, M. Emulsifying properties of enzyme-digested soybean soluble polysaccharide. Food Hydrocoll. 2006, 20, 1029–1038. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, W.; Gao, W.; Tian, G.; Zhao, C.; Bao, Y.; Cui, J.; Lian, Y.; Zheng, J. Effect of mesoscopic structure of citrus pectin on its emulsifying properties: Compactness is more important than size. J. Colloid Interface Sci. 2020, 570, 80–88. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, W.Y.; Chen, T.T.; Yan, J.K. Effects of structural and conformational characteristics of citrus pectin on its functional properties. Food Chem. 2021, 339, 128064. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, Y.; Chen, W.; Adie, K.; Liu, D.; Yin, Y. Citrus pectin modified by microfluidization and ultrasonication: Improved emulsifying and encapsulation properties. Ultrason. Sonochem. 2021, 70, 105322. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, S.J.; Zheng, X.L.; Wang, X.X.; Bao, Q.D.; Guo, X.M. Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohydr. Polym. 2013, 98, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Yu, Y.; Huang, X. Effect of ultrasonic treatment on rheological and emulsifying properties of sugar beet pectin. Food Sci. Nutr. 2020, 8, 4266–4275. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, H.; Zhang, T.; Guo, X. Investigation into the polymerization and changes of physicochemical properties of sugar beet pectin through controlled dry-heating. Food Hydrocoll. 2021, 110, 106212. [Google Scholar] [CrossRef]
- Lin, J.; Yu, S.; Ai, C.; Zhang, T.; Guo, X. Emulsion stability of sugar beet pectin increased by genipin crosslinking. Food Hydrocoll. 2020, 101, 105459. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Pectins of different origin and their performance in forming andstabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Saavedra Isusi, G.I.; Bindereif, B.; Karbstein, H.P.; van der Schaaf, U.S. Polymer or microgel particle: Differences in emulsifying properties of pectin as microgel or as individual polymer chains. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124793. [Google Scholar] [CrossRef]
- Li, M.; Karboune, S. Laccase-catalyzed conjugation of potato protein (PPT) with selected pectic polysaccharides (PPS): Conjugation efficiency and emulsification properties. Food Chem. 2021, 342, 128212. [Google Scholar] [CrossRef]
- Santiago, J.S.J.; Kyomugasho, C.; Maheshwari, S.; Jamsazzadeh Kermani, Z.; Van de Walle, D.; Van Loey, A.M.; Dewettinck, K.; Hendrickx, M.E. Unravelling the structure of serum pectin originating from thermally and mechanically processed carrot-based suspensions. Food Hydrocoll. 2018, 77, 482–493. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. Thermal and high pressure high temperature processes result in distinctly different pectin non-enzymatic conversions. Food Hydrocoll. 2014, 39, 251–263. [Google Scholar] [CrossRef]
- De Roeck, A.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Effect of high pressure/high temperature processing on cell wall pectic substances in relation to firmness of carrot tissue. Food Chem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Gwala, S.; Christiaens, S.; Jamsazzadeh Kermani, Z.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Pectin nanostructure influences pectin-cation interactions and in vitro-bioaccessibility of Ca2+, Zn2+, Fe2+ and Mg2+-ions in model systems. Food Hydrocoll. 2017, 62, 299–310. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Willemsen, K.L.D.D.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. Microscopic evidence for Ca2+ mediated pectin-pectin interactions in carrot-based suspensions. Food Chem. 2015. [Google Scholar] [CrossRef][Green Version]
- Detloff, T.; Sobisch, T.; Lerche, D. Instability Index. Dispers. Lett. Tech. 2014, 4, 1–4. [Google Scholar]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.C.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of low molecular weight pectins via electron beam irradiation and their potential prebiotic functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S.; Orsat, V. Microwave-assisted alkaline extraction of galactan-rich rhamnogalacturonan I from potato cell wall by-product. Food Chem. 2016, 190, 495–505. [Google Scholar] [CrossRef]
- Hu, R.; Lin, L.; Liu, T.; Liu, S. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure. Bioresour. Technol. 2010, 101, 3586–3594. [Google Scholar] [CrossRef]
- Schols, H.A.; Posthumus, M.A.; Voragen, A.G.J. Structural features of hairy regions of pectins isolated from apple juice produced by the liquefaction process. Carbohydr. Res. 1990, 206, 117–129. [Google Scholar] [CrossRef]
- Rodríguez-Chong, A.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Hydrolysis of sugar cane bagasse using nitric acid: A kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS PharmSciTech 2008, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Schiffman, J.D. Characterization of self-Assembled polyelectrolyte complex nanoparticles formed from chitosan and pectin. Langmuir 2014, 30, 3441–3447. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U. Pectin-water interactions in foods—From powder to gel. Food Hydrocoll. 2018, 78, 109–119. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Hua, X.; Yang, H.; Din, P.; Chi, K.; Yang, R. Rheological properties of deesterified pectin with different methoxylation degree. Food Biosci. 2018, 23, 91–99. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 9781498726696. [Google Scholar]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s−1. J. Food Process. 2014, 2014, 234583. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Pectin: Technological and Physiological Properties; Springer: Cham, Switzerland, 2020; ISBN 9783030534202. [Google Scholar]
- Reddy, S.R.; Fogler, H.S. Emulsion stability: Delineation of different particle loss mechanisms. J. Colloid Interface Sci. 1981, 79, 105–113. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids and Emulsion Stability; Woodhead Publishing Limited: Sawston, UK, 2009; ISBN 9781845695873. [Google Scholar]



| Property | Samples | ||
|---|---|---|---|
| Pectin-Related Sugars (% w/w) | AP74 | AP-RG | AP-RG-Hydrolyzed |
| GalA | 64.74 a ± 0.59 | 19.71 c ± 0.93 | 26.57 b ± 0.90 |
| Fucose | 0.16 a ± 0.03 | ND | ND |
| Rhamnose | 1.06 c ± 0.06 | 4.14 a ± 0.11 | 2.29 b ± 0.13 |
| Arabinose | 3.89 b ± 0.23 | 18.67 a ± 0.05 | 1.79 c ± 0.07 |
| Galactose | 4.42 c ± 0.71 | 22.64 b ± 0.01 | 29.27 a ± 0.14 |
| Xylose | 0.30 c ± 0.12 | 1.04 b ± 0.01 | 1.53 a ± 0.03 |
| Other sugars | |||
| Glucose | 14.41 a ± 0.26 | 0.66 b ± 0.01 | 1.00 b ± 0.05 |
| Mannose | 0.05 b ± 0.03 | 0.48 a ± 0.02 | 0.53 a ± 0.06 |
| Pectin domains (% w/w) | |||
| HG (GalA-Rha) | 63.68 a ± 0.06 | 15.57 c ± 0.11 | 24.28 b ± 0.13 |
| RG (2Rha+Ara+Gal) | 10.42 c ± 0.81 | 49.59 a ± 0.17 | 35.63 b ± 0.18 |
| Sugar ratios | |||
| Linearity (GalA/Fuc+Rha+Ara+Gal+Xyl) (Ratio 1) | 5.59 a ± 0.53 | 0.35 b ± 0.01 | 0.69 b ± 0.01 |
| RG-I contribution (Rha/GalA) (Ratio 2) | 0.02 c ± 0.01 | 0.25 a ± 0.01 | 0.10 b ± 0.01 |
| Branching of RG-I (Ara+Gal/Rha) (Ratio 3) | 7.88 b ± 1.30 | 9.91 a, b ± 0.26 | 12.53 a ± 0.72 |
| HG/RG (Ratio 4) | 6.12 a ± 0.47 | 0.31 b ± 0.01 | 0.68 b ± 0.01 |
| Mw (kDa) | 356.00 a ± 28.28 | 49.55 b ± 0.35 | 21.55 b ± 0.07 |
| Protein content (% w/w) | 1.52 a ± 0.01 | 1.41 a ± 0.44 | 1.13 a ± 0.15 |
| DM (%) | 73.66 a ± 2.24 | <3% | <3% |
| Sample Code | Average Particle Size of Freshly Prepared Emulsions (µm) | ηsunflowewr oil/η1% w/v pectin solution | ||
|---|---|---|---|---|
| pH 2.5 | pH 6.0 | pH 2.5 | pH 6.0 | |
| AP74 | 3.22 b ± 0.02 | 4.78 a ± 0.05 | 3.80 b ± 0.0001 | 5.05 c ± 0.0001 |
| AP-RG | 4.06 b ± 0.32 | 1.76 c ± 0.20 | 41.69 a ± 0.0001 | 36.87 b ± 0.0001 |
| AP-RG-hydrolyzed | 21.57 a ± 1.43 | 3.34 b ± 0.35 | 35.87 a ± 0.0001 | 42.03 a ± 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humerez-Flores, J.N.; Verkempinck, S.H.E.; Kyomugasho, C.; Moldenaers, P.; Van Loey, A.M.; Hendrickx, M.E. Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties. Foods 2021, 10, 1586. https://doi.org/10.3390/foods10071586
Humerez-Flores JN, Verkempinck SHE, Kyomugasho C, Moldenaers P, Van Loey AM, Hendrickx ME. Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties. Foods. 2021; 10(7):1586. https://doi.org/10.3390/foods10071586
Chicago/Turabian StyleHumerez-Flores, Jessika N., Sarah H. E. Verkempinck, Clare Kyomugasho, Paula Moldenaers, Ann M. Van Loey, and Marc E. Hendrickx. 2021. "Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties" Foods 10, no. 7: 1586. https://doi.org/10.3390/foods10071586
APA StyleHumerez-Flores, J. N., Verkempinck, S. H. E., Kyomugasho, C., Moldenaers, P., Van Loey, A. M., & Hendrickx, M. E. (2021). Modified Rhamnogalacturonan-Rich Apple Pectin-Derived Structures: The Relation between Their Structural Characteristics and Emulsifying and Emulsion-Stabilizing Properties. Foods, 10(7), 1586. https://doi.org/10.3390/foods10071586







