Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dough Ingredients and Sourdough Preparation
2.2. Enumeration of Lactic Acid Bacteria and Yeasts
2.3. Bread-Making
2.4. Crumb Grain Characteristics of Breads
2.5. Chemical Characteristics of Breads
2.6. Profile of Volatile Organic Compounds
2.7. Prediction of Glycaemic Index
2.8. Prediction of Antioxidant Activity
2.9. Sensory Analysis
2.10. Statistical Analyses
3. Results
3.1. Main Features of Sourdoughs
3.2. Crumb Grain and Chemical Characterization of Breads
3.3. Profile of Volatile Organic Compounds in Breads
3.4. Prediction of Glycaemic Index and Antioxidant Activity of Breads
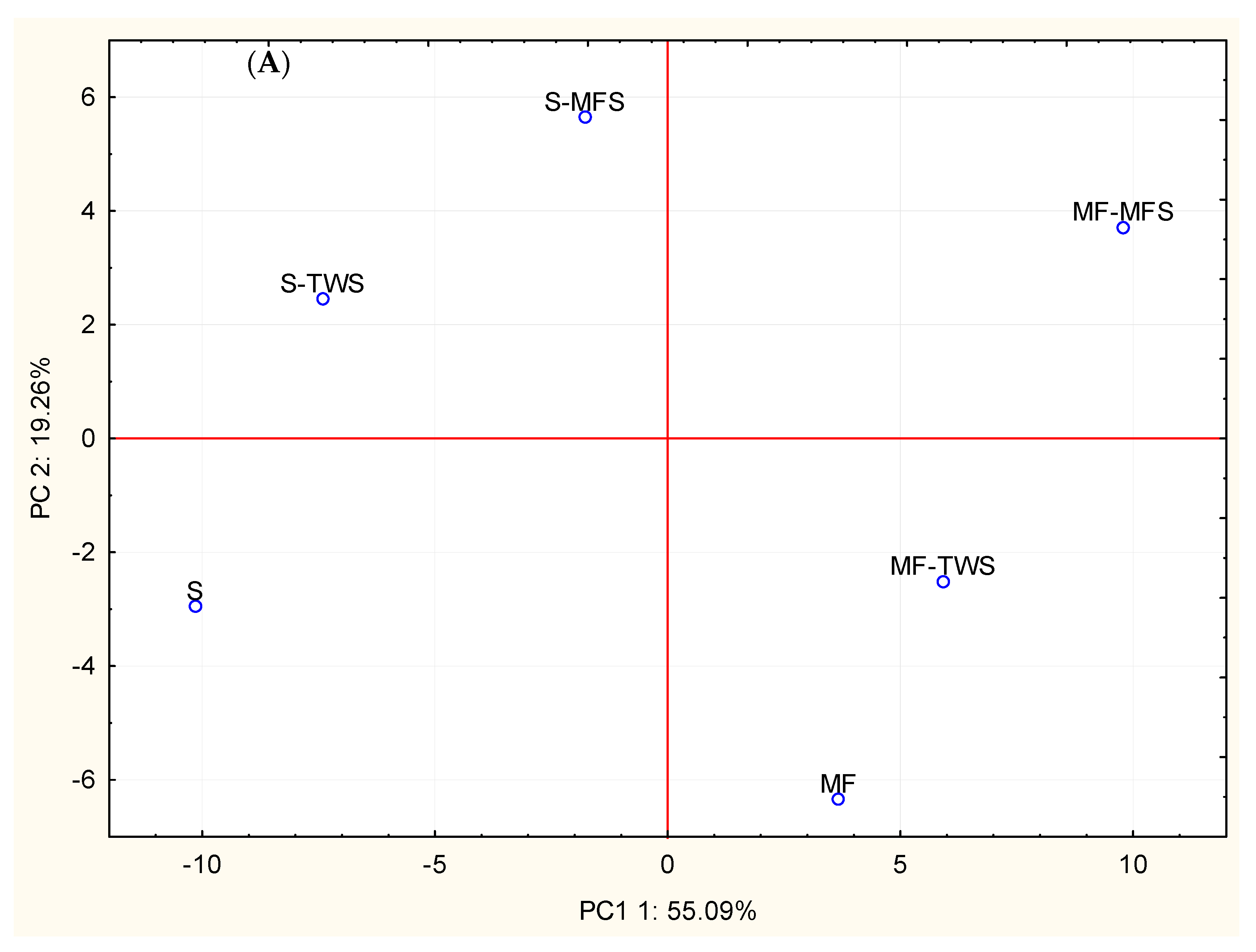
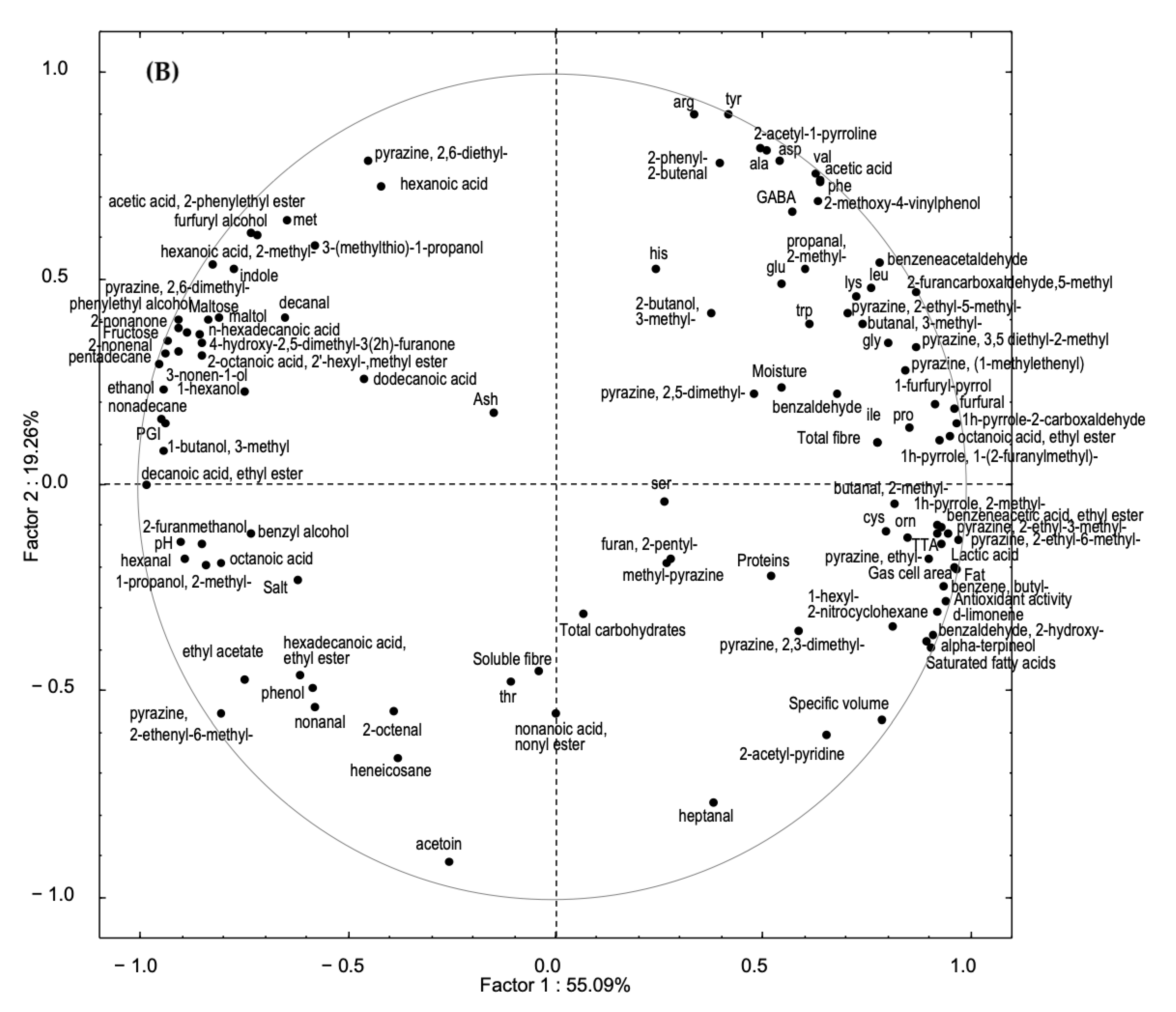
3.5. Principal Components Analysis
3.6. Sensory Analysis of Breads
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, P.M.; Monro, J.A.; Alvarez, L.; Gallagher, E. Glycemic impact and health: New horizons in white bread formulations. Crit. Rev. Food Sci. Nutr. 2011, 51, 965–982. [Google Scholar] [CrossRef]
- Hoseney, R. The Interactions that Produce Unique Products from Wheat Flour; AACC International: St. Paul, MN, USA, 1989; pp. 595–606. [Google Scholar]
- Jones, J.M.; Peña, R.J.; Korczak, R.; Braun, H.J. CIMMYT series on carbohydrates, wheat, grains, and health: Carbohydrates, grains, and wheat in nutrition and health: An overview. Part I. Role of carbohydrates in health. Cereal Foods World 2015, 60, 224–233. [Google Scholar] [CrossRef]
- Jones, J.M.; García, C.G.; Braun, H.J. Perspective: Whole and refined grains and health—Evidence supporting “make half your grains whole”. Adv. Nutr. 2020, 11, 492–506. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A. Italian durum wheat breads. In Bread Consumption and Health; Pedrosa Silva Clerici, M.T., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2012; pp. 57–79. [Google Scholar]
- Cauvain, S.P. Other cereals in breadmaking. In Technology of Breadmaking, 3rd ed.; Cauvain, S.P., Ed.; Springer Science and Business Media: New York, NY, USA, 2015; pp. 377–397. ISBN 978-3-319-14687-4. [Google Scholar]
- Cluskey, J.; Wu, Y.V.; Wall, J.; Inglett, G. Food applications of oat, sorghum, and triticale protein products. J. Am. Oil Chem. Soc. 1979, 56, 481–483. [Google Scholar] [CrossRef]
- Piperno, D.R.; Weiss, E.; Holst, I.; Nadel, D. Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis. Nature 2004, 430, 670–673. [Google Scholar] [CrossRef]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT Food Sci. Technol. 2007, 40, 860–870. [Google Scholar] [CrossRef]
- Newman, R.K.; Newman, C.W. Barley for Food and Health: Science, Technology, and Products; John Wiley & Sons: New York, NY, USA, 2008; ISBN 978-0-470-10249-7. [Google Scholar]
- Burluc, R.; Vizireanu, C.; Barna, O. Researches on buckwheat flour utilisation in bread making. J. Environ. Prot. Ecol. 2011, 12, 930–934. [Google Scholar]
- Atalay, M.H.; Bılgıçlı, N.; Elgün, A.; Demır, M.K. Effects of buckwheat (Fagopyrum esculentum Moench) milling products, transglutaminase and sodium stearoyl-2-lactylate on bread properties. J. Food Process. Preserv. 2013, 37, 1–9. [Google Scholar] [CrossRef]
- Campo, E.; del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016, 67, 75–82. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Devos, F. Traditional versus modern leavening systems. Cereal Foods World 2018, 63. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Sajdakowska, M.; Gębski, J.; Żakowska-Biemans, S.; Jeżewska-Zychowicz, M. Willingness to eat bread with health benefits: Habits, taste and health in bread choice. Public Health 2019, 167, 78–87. [Google Scholar] [CrossRef] [PubMed]
- De Boni, A.; Pasqualone, A.; Roma, R.; Acciani, C. Traditions, health and environment as bread purchase drivers: A choice experiment on high-quality artisanal Italian bread. J. Clean. Prod. 2019, 221, 249–260. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Ferreira, E.; Heleno, S.; Rodrigues, P.; Barros, L.; Ferreira, I.C. Comparison of different bread types: Chemical and physical parameters. Food Chem. 2020, 310, 125954. [Google Scholar] [CrossRef] [PubMed]
- AACC Method 10-05.01 Guidelines for Measurement of Volume by Rapeseed Displacement; AACC International: St. Paul, MN, USA, 1998.
- Minervini, F.; Pinto, D.; Di Cagno, R.; De Angelis, M.; Gobbetti, M. Scouting the application of sourdough to frozen dough bread technology. J. Cereal Sci. 2011, 54, 296–304. [Google Scholar] [CrossRef]
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Analytical methods used in food chemical control. Rapp. ISTISAN 1996, 96, 34. [Google Scholar]
- AOAC. Official Method of Analysis of the AOAC, Method 993.19; AOAC International: Arlington, VA, USA, 1996. [Google Scholar]
- Weiss, W.; Vogelmeier, C.; Görg, A. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef]
- De Angelis, M.; Di Cagno, R.; Gallo, G.; Curci, M.; Siragusa, S.; Crecchio, C.; Parente, E.; Gobbetti, M. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 2007, 114, 69–82. [Google Scholar] [CrossRef]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: A randomised, double-blind, cross-over study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Liljeberg, H.; Åkerberg, A.; Björck, I. Resistant starch formation in bread as influenced by choice of ingredients or baking conditions. Food Chem. 1996, 56, 389–394. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Haglund, Å.; Johansson, L.; Dahlstedt, L. Sensory evaluation of wholemeal bread from ecologically and conventionally grown wheat. J. Cereal Sci. 1998, 27, 199–207. [Google Scholar] [CrossRef]
- Filipčev, B.; Šimurina, O.; Bodroža-Solarov, M.; Obreht, D. Comparison of the bread-making performance of spelt varieties grown under organic conditions in the environment of northern Serbia and their responses to dough strengthening improvers. Hem. Ind. 2013, 67, 443–453. [Google Scholar] [CrossRef]
- Minervini, F.; Di Cagno, R.; Lattanzi, A.; De Angelis, M.; Antonielli, L.; Cardinali, G.; Cappellle, S.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: Interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 2012, 78, 1251–1264. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Minervini, F.; Siragusa, S.; Rizzello, C.G.; Gobbetti, M. Wholemeal wheat flours drive the microbiome and functional features of wheat sourdoughs. Int. J. Food Microbiol. 2019, 302, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Decock, P.; Cappelle, S. Bread technology and sourdough technology. Trends Food Sci. Technol. 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Thiele, C.; Gänzle, M.G.; Vogel, R.F. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 2002, 79, 45–51. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with Baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [Green Version]
- Diana, M.; Rafecas, M.; Quílez, J. Free amino acids, acrylamide and biogenic amines in gamma-aminobutyric acid enriched sourdough and commercial breads. J. Cereal Sci. 2014, 60, 639–644. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent developments on umami ingredients of edible mushrooms—A review. Trends Food Sci. Technol. 2013, 33, 78–92. [Google Scholar] [CrossRef]
- Zhao, C.J.; Kinner, M.; Wismer, W.; Gänzle, M.G. Effect of glutamate accumulation during sourdough fermentation with Lactobacillus reuteri on the taste of bread and sodium-reduced bread. Cereal Chem. 2015, 92, 224–230. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste enhancements between various amino acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Sugino, T.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr. Res. 2008, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.P.; Fishel, R.S.; Efron, D.T.; Williams, J.Z.; Fishel, M.H.; Barbul, A. Effect of supplemental ornithine on wound healing. J. Surg. Res. 2002, 106, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Bussière, F.I.; Le Boucher, J.; Farges, M.C.; Cynober, L.A.; Field, C.J.; Baracos, V.E. Amino acid nutrition and immune function in tumour-bearing rats: A comparison of glutamine-, arginine-and ornithine 2-oxoglutarate-supplemented diets. Clin. Sci. 1999, 97, 657–669. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef]
- Kanehira, T.; Nakamura, Y.; Nakamura, K.; Horie, K.; Horie, N.; Furugori, K.; Sauchi, Y.; Yokogoshi, H. Relieving occupational fatigue by consumption of a beverage containing γ-amino butyric acid. J. Nutr. Sci. Vitaminol. 2011, 57, 9–15. [Google Scholar] [CrossRef] [Green Version]
- WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Grosch, W.; Schieberle, P. Flavor of cereal products—A review. Cereal Chem. 1997, 74, 91–97. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Comasio, A.; Harth, H.; De Vuyst, L. Impact of starter culture, ingredients, and flour type on sourdough bread volatiles as monitored by selected ion flow tube-mass spectrometry. Food Res. Int. 2018, 106. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Humayun, S.; Park, S.; Oh, H.; Lim, S.; Mok, I.K.; Li, Y.; Pal, K.; Kim, D. Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem. 2021, 345, 128787. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and L. helveticus for sourdough bread making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- Harvard Health Publishing. A Good Guide to Good Carbs: The Glycemic Index. Available online: https://www.health.harvard.edu/healthbeat/a-good-guide-to-good-carbs-the-glycemic-index (accessed on 23 March 2021).
- Takahama, U.; Hirota, S. Fatty acids, epicatechin-dimethylgallate, and rutin interact with buckwheat starch inhibiting its digestion by amylase: Implications for the decrease in glycemic index by buckwheat flour. J. Agric. Food Chem. 2010, 58, 12431–12439. [Google Scholar] [CrossRef]
- Björck, I.; Liljeberg, H.; Östman, E. Low glycaemic-index foods. Br. J. Nutr. 2000, 83, S149–S155. [Google Scholar] [CrossRef]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.A.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Canas, X.; Lozano, C.; Pumarola, S.; Clapés, P. D-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Sen, L.; Nan, Y.; Hou, Z.-H.; Yang, Y.; Li, L.; Zhou, X.-R.; Ren, G.-X. Antioxidant effects of oats avenanthramides on human serum. Agric. Sci. China 2011, 10, 1301–1305. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I.; Alrababah, M.A.; Almajwal, A.; Masadeh, N.; Alhamad, M.N. Effects of barley flour and barley protein isolate on chemical, functional, nutritional and biological properties of Pita bread. Food Hydrocoll. 2012, 26, 135–143. [Google Scholar] [CrossRef]
- Verardo, V.; Glicerina, V.; Cocci, E.; Frenich, A.G.; Romani, S.; Caboni, M.F. Determination of free and bound phenolic compounds and their antioxidant activity in buckwheat bread loaf, crust and crumb. LWT Food Sci. Technol. 2018, 87, 217–224. [Google Scholar] [CrossRef]
- Söderholm, P.; Alfthan, G.; Tikkanen, M.; Adlercreutz, H. Rye bread intake improves oxidation resistance of LDL in healthy humans. Atherosclerosis 2012, 221, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Peterson, D.G. Chemistry of bread aroma: A review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Sohn, M.; Ho, C.T. Ammonia generation during thermal degradation of amino acids. J. Agric. Food Chem. 1995, 43, 3001–3003. [Google Scholar] [CrossRef]
- Rosso, M.C.; Mazzucotelli, M.; Bicchi, C.; Charron, M.; Manini, F.; Menta, R.; Fontana, M.; Reichenbach, S.E.; Cordero, C. Adding extra-dimensions to hazelnuts primary metabolome fingerprinting by comprehensive two-dimensional gas chromatography combined with time-of-flight mass spectrometry featuring tandem ionization: Insights on the aroma potential. J. Chromatogr. A 2020, 1614, 460739. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]

| Soft Wheat | Semolina | Barley | Oat | Rye | Buckwheat | Water | Sourdough | |
|---|---|---|---|---|---|---|---|---|
| S | / | 61.6 | / | / | / | / | 36.9 | / |
| S-TWS | / | 55.7 | / | / | / | / | 33.0 | 9.8 |
| S-MFS | / | 55.7 | / | / | / | / | 33.0 | 9.8 |
| MF | 36.8 | 6.2 | 6.2 | 6.2 | 3.1 | 3.1 | 36.9 | / |
| MF-TWS | 33.4 | 5.5 | 5.5 | 5.5 | 2.8 | 2.8 | 33.2 | 9.8 |
| MF-MFS | 33.4 | 5.5 | 5.5 | 5.5 | 2.8 | 2.8 | 33.2 | 9.8 |
| Semolina with Traditional Sourdough (S-TWS) | Semolina with Mixed Flours Sourdough (S-MFS) | Mixed Flours (MF) | Mixed Flours with Traditional Sourdough (MF-TWS) | Mixed Flours with Mixed Flours Sourdough (MF-MFS) | Semolina (S) | |
|---|---|---|---|---|---|---|
| Specific volume (cm3/g) | 2.9 ± 0.10 b | 2.8 ± 0.16 b | 3.3 ± 0.13 a | 3.2 ± 0.15 a | 3.2 ± 0.10 a | 2.9 ± 0.11 b |
| Gas cell area (%) | 33.2 ± 1.70 d | 37.0 ± 1.77 c | 56.7 ± 2.85 b | 52.3 ± 2.60 b | 61.7 ± 3.10 a | 30.6 ± 1.53 d |
| Ash (%) | 1.75 ± 0.035 a | 1.77 ± 0.030 a | 1.74 ± 0.026 a | 1.75 ± 0.024 a | 1.70 ± 0.034 a | 1.71 ± 0.020 a |
| Moisture (%) | 32 ± 1.3 a | 33 ± 1.0 a | 33 ± 0.9 a | 32 ± 1.2 a | 33 ± 1.3 a | 32 ± 1.0 a |
| Fat (%) | 1.1 ± 0.13 b | 1.2 ± 0.15 b | 1.6 ± 0.19 a | 1.7 ± 0.25 a | 1.8 ± 0.21 a | 1.1 ± 0.10 b |
| Total saturated fatty acids (%) | 0.21 ± 0.100 a | 0.22 ± 0.140 a | 0.32 ± 0.110 a | 0.30 ± 0.120 a | 0.32 ± 0.140 a | 0.22 ± 0.130 a |
| Total carbohydrates (%) | 52 ± 2.8 a | 51 ± 3.0 a | 52 ± 2.5 a | 52 ± 2.4 a | 50 ± 2.0 a | 50 ± 2.5 a |
| Fructose (%) | 0.12 ± 0.020 a | 0.10 ± 0.022 a | 0.00 b | 0.00 b | 0.00 b | 0.12 ± 0.019 a |
| Maltose (%) | 0.52 ± 0.022 a | 0.51 ± 0.025 a | 0.00 b | 0.00 b | 0.00 b | 0.52 ± 0.023 a |
| Proteins (%) | 10.4 ± 0.75 a | 10.4 ± 0.68 a | 10.5 ± 0.70 a | 10.6 ± 0.75 a | 10.7 ± 0.66 a | 10.6 ± 0.69 a |
| Salt (%) | 1.3 ± 0.19 a | 1.2 ± 0.15 a | 1.3 ± 0.20 a | 1.1 ± 0.18 a | 1.2 ± 0.19 a | 1.3 ± 0.18 a |
| Soluble dietary fiber (%) | 1.11 ± 0.54 a | 1.09 ± 0.47 a | 1.11 ± 0.50 a | 1.10 ± 0.55 a | 1.09 ± 0.43 a | 1.09 ± 0.50 a |
| Total dietary fiber (%) | 3.8 ± 0.60 a | 3.8 ± 0.50 a | 3.8 ± 0.62 a | 3.9 ± 0.59 a | 3.9 ± 0.57 a | 3.8 ± 0.55 a |
| pH | 5.2 ± 0.06 b | 5.1 ± 0.05 b | 5.0 ± 0.02 c | 4.9 ± 0.02 d | 4.9 ± 0.03 d | 5.6 ± 0.04 a |
| TTA (ml of NaOH 0.1 M) | 1.4 ± 0.10 c | 1.3 ± 0.08 c | 1.9 ± 0.04 b | 2.0 ± 0.03 a | 2.0 ± 0.03 a | 0.9 ± 0.04 d |
| Lactic acid (mmol/kg) | 13 ± 0.7 b | 14 ± 0.8 b | 19 ± 0.7 a | 19 ± 0.6 a | 20 ± 0.7 a | 10 ± 0.5 c |
| Acetic acid (mmol/kg) | 3 ± 0.4 b | 3 ± 0.5 b | 0.00 c | 3 ± 0.3 b | 6 ± 0.4 a | 0.00 c |
| S-TWS | S-MFS | MF | MF-TWS | MF-MFS | S | |
|---|---|---|---|---|---|---|
| asp | 473 ± 14 d | 586 ± 15 b | 286 ± 20 e | 519 ± 16 c | 612 ± 24 a | 200 ± 13 f |
| thr | 66 ± 9 ab | 54 ± 7 c | 71 ± 8 a | 54 ± 5 c | 60 ± 6 bc | 60 ± 7 bc |
| ser | 47 ± 6 c | 58 ± 8 b | 47 ± 5 c | 74 ± 10 a | 37 ± 5 d | 37 ± 6 d |
| glu | 1008 ± 22 b | 890 ± 10 c | 706 ± 17 d | 1104 ± 34 a | 1023 ± 19 b | 537 ± 15 e |
| gly | 105 ± 10 bc | 109 ± 6 b | 94 ± 8 c | 146 ± 11 a | 139 ± 8 a | 56 ± 6 d |
| ala | 285 ± 5 d | 343 ± 12 b | 169 ± 8 e | 307 ± 10 c | 370 ± 7 a | 147 ± 5 f |
| cys | 72 ± 6 c | 84 ± 8 b | 84 ± 5 b | 96 ± 6 a | 84 ± 6 b | 72 ± 7 c |
| val | 59 ± 7 d | 123 ± 9 b | 35 ± 9 e | 94 ± 10 c | 141 ± 10 a | 23 ± 10 e |
| met | 82 ± 8 a | 75 ± 8 ab | 52 ± 6 c | 67 ± 7 b | 67 ± 9 b | 75 ± 9 ab |
| ile | 0 b | 0 b | 0 b | 157 ± 10 a | 157 ± 7 a | 0 b |
| leu | 0 d | 72 ± 8 c | 0 d | 98 ± 7 b | 125 ± 8 a | 0 d |
| tyr | 63 ± 5 b | 100 ± 6 a | 27 ± 7 c | 63 ± 7 b | 100 ± 8 a | 27 ± 5 c |
| phe | 33 ± 8 d | 91 ± 7 b | 25 ± 8 ef | 58 ± 10 c | 116 ± 8 a | 17 ± 8 f |
| GABA | 227 ± 16 cd | 211 ± 14 d | 134 ± 9 e | 242 ± 18 bc | 278 ± 12 a | 113 ± 8 f |
| his | 23 ± 8 a | 0 b | 0 b | 0 b | 31 ± 7 a | 0 b |
| trp | 0 b | 0 b | 0 b | 0 b | 41 ± 6 a | 0 b |
| orn | 59 ± 7 c | 33 ± 7 d | 99 ± 6 b | 205 ± 13 a | 192 ± 15 a | 26 ± 7 d |
| lys | 80 ± 6 b | 80 ± 7 b | 58 ± 5 c | 110 ± 9 a | 110 ± 12 a | 44 ± 8 d |
| arg | 105 ± 9 c | 226 ± 10 a | 0 d | 113 ± 8 c | 174 ± 13 b | 0 d |
| pro | 184 ± 9 b | 150 ± 6 c | 178 ± 7 b | 276 ± 12 a | 276 ± 16 a | 92 ± 5 d |
| total FAA | 2971 ± 155 d | 3284 ± 148 c | 2068 ± 128 e | 3784 ± 203 b | 4131 ± 206 a | 1526 ± 121 f |
| S-TWS | S-MFS | MF | MF-TWS | MF-MFS | S | |
|---|---|---|---|---|---|---|
| PGI (%) | 80 ± 3 a | 72 ± 3 b | 68 ± 4 b | 68 ± 4 b | 68 ± 5 b | 79 ± 4 a |
| DPPH• scavenging activity (%) | 48.0 ± 1.80 b | 46.0 ± 2.04 b | 58.5 ± 2.20 a | 61.0 ± 2.51 a | 63.5 ± 2.02 a | 42.0 ± 2.10 c |
| S-TWS | S-MFS | MF | MF-TWS | MF-MFS | S | |
|---|---|---|---|---|---|---|
| Crust color | 3.7 ± 0.49 a | 2.9 ± 0.90 b | 3.0 ± 0.58 b | 2.7 ± 0.76 b | 2.3 ± 0.76 c | 2.6 ± 0.53 b |
| Odor | 3.4 ± 0.79 a | 3.0 ± 0.82 a | 2.9 ± 0.69 a | 2.9 ± 0.69 a | 2.9 ± 0.69 a | 3.6 ± 0.98 a |
| Crispness | 3.4 ± 1.13 a | 3.7 ± 0.95 a | 3.0 ± 0.82 a | 3.1 ± 1.21 a | 2.9 ± 1.21 a | 2.9 ± 1.21 a |
| Elasticity | 3.7 ± 0.76 a | 3.3 ± 0.95 a | 3.7 ± 0.76 a | 3.3 ± 1.38 a | 3.3 ± 1.25 a | 3.1 ± 1.21 a |
| Masticability | 4.0 ± 0.58 a | 3.9 ± 0.38 a | 3.4 ± 0.79 a | 3.4 ± 0.98 a | 3.6 ± 0.79 a | 3.9 ± 0.38 a |
| Acid taste | 2.0 ± 1.15 ab | 2.4 ± 0.98 a | 2.1 ± 0.90 a | 2.6 ± 1.27 a | 2.9 ± 1.07 a | 1.1 ± 0.90 b |
| Sweet taste | 3.1 ± 0.90 a | 2.9 ± 0.90 a | 2.6 ± 1.27 a | 2.6 ± 1.27 a | 2.4 ± 1.27 a | 2.9 ± 0.69 a |
| Salty taste | 2.7 ± 1.11 a | 2.6 ± 1.27 a | 2.4 ± 1.27 a | 2.4 ± 1.51 a | 2.9 ± 1.57 a | 2.4 ± 0.79 a |
| Aroma | 3.7 ± 0.76 a | 3.6 ± 0.79 a | 2.7 ± 0.76 b | 3.0 ± 1.15 ab | 2.9 ± 1.07 ab | 3.4 ± 0.98 ab |
| Overall acceptability | 3.7 ± 0.76 a | 3.7 ± 0.76 a | 3.0 ± 0.82 a | 3.1 ± 0.90 a | 3.1 ± 1.07 a | 3.0 ± 0.82 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfetta, M.; Celano, G.; Minervini, F. Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads. Foods 2021, 10, 1613. https://doi.org/10.3390/foods10071613
Molfetta M, Celano G, Minervini F. Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads. Foods. 2021; 10(7):1613. https://doi.org/10.3390/foods10071613
Chicago/Turabian StyleMolfetta, Mariagrazia, Giuseppe Celano, and Fabio Minervini. 2021. "Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads" Foods 10, no. 7: 1613. https://doi.org/10.3390/foods10071613
APA StyleMolfetta, M., Celano, G., & Minervini, F. (2021). Functional, Nutritional, and Sensory Quality of Mixed Flours-Based Breads as Compared to Durum Wheat Semolina-Based Breads. Foods, 10(7), 1613. https://doi.org/10.3390/foods10071613






