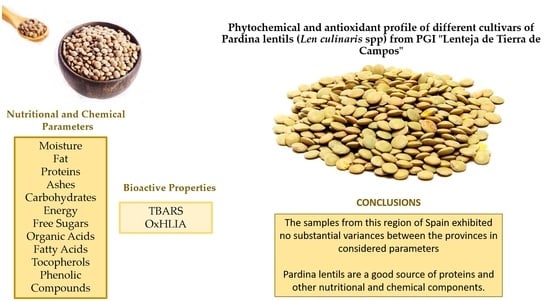
Phytochemical and Antioxidant Profile of Pardina Lentil Cultivars from Different Regions of Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Standards and Reagents
2.3. Nutritional Value
2.4. Chemical Characterization
2.4.1. Free Sugars
2.4.2. Organic Acids
2.4.3. Tocopherols
2.4.4. Fatty Acids
2.5. Non-Nutrient Composition
2.5.1. Extract Preparation
2.5.2. Phenolic Compounds
2.6. Antioxidant Activity Evaluation
2.6.1. Thiobarbituric Acid Reactive Substances (TBARS)
2.6.2. Oxidative Hemolysis Inhibition Assay (OxHLIA)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.2. Chemical Composition
3.3. Phenolic Compounds
3.4. Bioactive Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| C6:0 | 0.68 ± 0.06 | 0.15 ± 0.015 | 0.32 ± 0.01 | 0.07 ± 0.004 | 0.759 ± 0.001 | 0.79 ± 0.04 | 0.93 ± 0.04 | 0.20 ± 0.01 | 0.12 ± 0.01 | 0.21 ± 0.01 |
| C8:0 | 0.13 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.03 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.01 |
| C11:0 | 0.09 ± 0.008 | 0.091 ± 0.008 | 0.11 ± 0.01 | 0.028 ± 0.001 | 0.14 ± 0.01 | 0.043 ± 0.001 | 0.025 ± 0.001 | 0.037 ± 0.003 | 0.07 ± 0.01 | 0.054 ± 0.004 |
| C12:0 | 0.10 ± 0.004 | 0.076 ± 0.002 | 0.09 ± 0.01 | 0.039 ± 0.003 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.040 ± 0.002 | 0.04 ± 0.01 | 0.08 ± 0.01 |
| C13:0 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.15 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.01 | 0.27 ± 0.01 |
| C14:0 | 0.75 ± 0.03 | 0.76 ± 0.01 | 0.531 ± 0.003 | 0.61 ± 0.01 | 0.38 ± 0.03 | 0.57 ± 0.06 | 0.50 ± 0.03 | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.352 ± 0.004 |
| C15:0 | 0.77 ± 0.02 | 0.37 ± 0.01 | 0.55 ± 0.01 | 0.36 ± 0.03 | 0.63 ± 0.01 | 0.30 ± 0.01 | 0.44 ± 0.03 | 0.44 ± 0.01 | 0.39 ± 0.01 | 0.80 ± 0.07 |
| C16:0 | 14.14 ± 0.01 | 15.93 ± 0.04 | 14.31 ± 0.02 | 15.3 ± 0.3 | 14.2 ± 0.2 | 15.24 ± 0.07 | 14.0 ± 0.1 | 15.5 ± 0.3 | 16.1 ± 0.1 | 15.37 ± 0.09 |
| C16:1 | 0.13 ± 0.01 | 0.140 ± 0.004 | 0.18 ± 0.01 | 0.12 ± 0.01 | 0.29 ± 0.01 | 0.38 ± 0.01 | 0.47 ± 0.01 | 0.41 ± 0.01 | 0.88 ± 0.01 | 0.90 ± 0.01 |
| C17:0 | 0.40 ± 0.01 | 0.289 ± 0.004 | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.63 ± 0.01 | 0.82 ± 0.05 | 0.9 ± 0.1 | 0.14 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| C18:0 | 2.59 ± 0.17 | 1.95 ± 0.01 | 2.4 ± 0.2 | 2.4 ± 0.2 | 1.41 ± 0.01 | 2.4 ± 0.2 | 2.1 ± 0.1 | 2.27 ± 0.01 | 2.18 ± 0.01 | 2.11 ± 0.06 |
| C18:1n9c | 15.12 ± 0.04 | 14.08 ± 0.02 | 14.2 ± 0.2 | 15.7 ± 0.2 | 16.0 ± 0.1 | 14.3 ± 0.1 | 14.15 ± 0.05 | 13.65 ± 0.07 | 13.08 ± 0.08 | 14.7 ± 0.3 |
| C18:2n6c | 44.08 ± 0.06 | 45.13 ± 0.01 | 44.52 ± 0.01 | 44.2 ± 0.2 | 43.8 ± 0.2 | 43.30 ± 0.04 | 42.76 ± 0.09 | 43.99 ± 0.02 | 45.27 ± 0.06 | 43.4 ± 0.2 |
| C18:3n3 | 14.11 ± 0.07 | 14.84 ± 0.07 | 15.41 ± 0.04 | 14.24 ± 0.06 | 13.19 ± 0.06 | 14.16 ± 0.04 | 15.42 ± 0.03 | 15.37 ± 0.01 | 14.43 ± 0.01 | 14.8 ± 0.4 |
| C20:0 | 1.5 ± 0.2 | 1.35 ± 0.03 | 1.06 ± 0.07 | 1.47 ± 0.08 | 2.20 ± 0.06 | 1.69 ± 0.02 | 1.69 ± 0.09 | 1.85 ± 0.03 | 1.49 ± 0.07 | 1.61 ± 0.03 |
| C20:1 | 1.05 ± 0.07 | 0.99 ± 0.02 | 0.92 ± 0.01 | 1.00 ± 0.04 | 1.2 ± 0.1 | 1.38 ± 0.07 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.54 ± 0.02 | 1.27 ± 0.03 |
| C21:0 | 0.70 ± 0.07 | 0.84 ± 0.08 | 0.95 ± 0.04 | 0.60 ± 0.02 | 0.77 ± 0.02 | 0.91 ± 0.06 | 0.42 ± 0.04 | 0.18 ± 0.01 | 0.15 ± 0.01 | 0.22 ± 0.01 |
| C22:0 | 1.84 ± 0.08 | 1.26 ± 0.01 | 1.51 ± 0.01 | 1.40 ± 0.08 | 1.4 ± 0.1 | 1.29 ± 0.09 | 1.49 ± 0.03 | 1.07 ± 0.01 | 1.01 ± 0.01 | 1.21 ± 0.03 |
| C22:1 | 0.29 ± 0.02 | 0.25 ± 0.01 | 0.34 ± 0.02 | 0.22 ± 0.01 | 0.39 ± 0.01 | 0.28 ± 0.01 | 0.52 ± 0.03 | 0.83 ± 0.01 | 0.85 ± 0.01 | 0.84 ± 0.04 |
| C23:0 | 0.21 ± 0.01 | 0.33 ± 0.02 | 0.53 ± 0.03 | 0.70 ± 0.03 | 0.39 ± 0.01 | 0.56 ± 0.01 | 0.75 ± 0.03 | 1.01 ± 0.07 | 0.94 ± 0.05 | 0.95 ± 0.05 |
| C24:0 | 1.19 ± 0.09 | 1.07 ± 0.07 | 1.60 ± 0.06 | 1.1 ± 0.1 | 1.96 ± 0.05 | 1.33 ± 0.05 | 1.6 ± 0.1 | 0.83 ± 0.01 | 0.88 ± 0.04 | 0.77 ± 0.06 |
| SFA | 25.2 ± 0.1 | 24.6 ± 0.1 | 24.5 ± 0.1 | 24.6 ± 0.4 | 25.2 ± 0.3 | 26.2 ± 0.1 | 25.1 ± 0.1 | 24.1 ± 0.1 | 23.9 ± 0.1 | 24.1 ± 0.3 |
| MUFA | 16.6 ± 0.1 | 24.6 ± 0.1 | 15.6 ± 0.1 | 17.0 ± 0.2 | 17.9 ± 0.1 | 16.4 ± 0.2 | 16.8 ± 0.1 | 16.6 ± 0.2 | 16.4 ± 0.1 | 17.7 ± 0.3 |
| PUFA | 58.2 ± 0.1 | 60.0 ± 0.1 | 59.9 ± 0.1 | 58.5 ± 0.2 | 57.0 ± 0.3 | 57.5 ± 0.1 | 58.2 ± 0.1 | 59.4 ± 0.1 | 59.7 ± 0.1 | 58.2 ± 0.6 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| C6:0 | 0.057 ± 0.001 | 0.094 ± 0.004 | 0.087 ± 0.005 | 0.091 ± 0.002 | 0.068 ± 0.004 | 0.082 ± 0.008 | 0.075 ± 0.006 | 0.073 ± 0.001 | 0.044 ± 0.002 | 0.055 ± 0.004 |
| C8:0 | 0.054 ± 0.001 | 0.068 ± 0.004 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 | 0.15 ± 0.01 | 0.26 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| C11:0 | 0.034 ± 0.002 | 0.071 ± 0.004 | 0.032 ± 0.001 | 0.062 ± 0.004 | 0.033 ± 0.002 | 0.065 ± 0.001 | 0.092 ± 0.004 | 0.061 ± 0.003 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| C12:0 | 0.037 ± 0.004 | 0.042 ± 0.002 | 0.042 ± 0.002 | 0.07 ± 0.01 | 0.063 ± 0.006 | 0.066 ± 0.003 | 0.08 ± 0.01 | 0.097 ± 0.006 | 0.07 ± 0.01 | 0.064 ± 0.002 |
| C13:0 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.174 ± 0.002 | 0.09 ± 0.01 | 0.21 ± 0.01 | 0.27 ± 0.01 | 0.39 ± 0.02 | 0.37 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.01 |
| C14:0 | 0.39 ± 0.04 | 0.40 ± 0.02 | 0.59 ± 0.02 | 0.35 ± 0.03 | 0.72 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.092 ± 0.001 | 0.62 ± 0.03 | 0.35 ± 0.01 |
| C15:0 | 0.35 ± 0.02 | 0.36 ± 0.01 | 0.42 ± 0.01 | 0.37 ± 0.03 | 0.55 ± 0.04 | 0.68 ± 0.03 | 0.17 ± 0.01 | 0.111 ± 0.002 | 0.32 ± 0.01 | 0.33 ± 0.02 |
| C16:0 | 15.17 ± 0.07 | 14.96 ± 0.04 | 13.42 ± 0.09 | 14.2 ± 0.3 | 15.4 ± 0.2 | 15.9 ± 0.1 | 14.5 ± 0.5 | 15.0 ± 0.2 | 13.9 ± 0.2 | 13.99 ± 0.01 |
| C16:1 | 0.69 ± 0.06 | 0.65 ± 0.01 | 0.66 ± 0.01 | 0.48 ± 0.01 | 0.54 ± 0.01 | 0.33 ± 0.03 | 0.44 ± 0.02 | 0.61 ± 0.01 | 0.45 ± 0.01 | 0.23 ± 0.02 |
| C17:0 | 0.50 ± 0.01 | 0.48 ± 0.01 | 0.55 ± 0.01 | 0.50 ± 0.01 | 0.09 ± 0.01 | 0.34 ± 0.01 | 0.14 ± 0.01 | 0.79 ± 0.02 | 0.67 ± 0.01 | 0.17 ± 0.01 |
| C18:0 | 2.10 ± 0.06 | 2.03 ± 0.01 | 2.53 ± 0.03 | 2.5 ± 0.2 | 2.1 ± 0.1 | 2.05 ± 0.02 | 2.41 ± 0.11 | 2.21 ± 0.01 | 2.05 ± 0.02 | 2.28 ± 0.09 |
| C18:1n9c | 14.49 ± 0.07 | 14.2 ± 0.1 | 15.29 ± 0.05 | 15.44 ± 0.06 | 14.97 ± 0.02 | 14.95 ± 0.08 | 14.8 ± 0.2 | 13.3 ± 0.2 | 14.4 ± 0.1 | 15.4 ± 0.6 |
| C18:2n6c | 44.7 ± 0.3 | 45.1 ± 0.2 | 44.17 ± 0.02 | 43.45 ± 0.03 | 44.2 ± 0.3 | 43.8 ± 0.3 | 44.11 ± 0.01 | 44.5 ± 0.3 | 44.99 ± 0.08 | 45.4 ± 0.1 |
| C18:3n3 | 15.74 ± 0.05 | 15.85 ± 0.03 | 14.60 ± 0.03 | 15.25 ± 0.05 | 13.8 ± 0.1 | 14.76 ± 0.05 | 14.16 ± 0.07 | 14.4 ± 0.1 | 14.63 ± 0.03 | 14.48 ± 0.04 |
| C20:0 | 0.88 ± 0.08 | 0.89 ± 0.04 | 1.22 ± 0.08 | 1.07 ± 0.02 | 1.04 ± 0.03 | 1.02 ± 0.01 | 1.4 ± 0.1 | 1.05 ± 0.09 | 0.43 ± 0.02 | 0.88 ± 0.01 |
| C20:1 | 1.3 ± 0.1 | 1.41 ± 0.02 | 1.76 ± 0.04 | 1.66 ± 0.12 | 1.27 ± 0.05 | 1.6 ± 0.1 | 1.40 ± 0.03 | 1.75 ± 0.06 | 1.7 ± 0.2 | 1.11 ± 0.01 |
| C21:0 | 0.60 ± 0.01 | 0.72 ± 0.01 | 0.69 ± 0.04 | 0.42 ± 0.02 | 0.93 ± 0.06 | 0.2 ± 0.1 | 0.66 ± 0.02 | 0.70 ± 0.03 | 1.25 ± 0.03 | 1.39 ± 0.07 |
| C22:0 | 0.29 ± 0.01 | 0.335 ± 0.003 | 1.15 ± 0.07 | 1.11 ± 0.01 | 1.01 ± 0.01 | 1.25 ± 0.02 | 1.22 ± 0.01 | 1.015 ± 0.004 | 1.29 ± 0.08 | 1.12 ± 0.05 |
| C22:1 | 0.72 ± 0.02 | 0.61 ± 0.01 | 0.74 ± 0.02 | 0.81 ± 0.03 | 0.80 ± 0.06 | 0.24 ± 0.02 | 0.35 ± 0.01 | 0.52 ± 0.01 | 0.8 ± 0.1 | 0.58 ± 0.03 |
| C23:0 | 0.95 ± 0.03 | 0.87 ± 0.05 | 0.89 ± 0.01 | 0.98 ± 0.03 | 1.02 ± 0.02 | 1.14 ± 0.04 | 1.71 ± 0.03 | 1.6 ± 0.1 | 1.02 ± 0.07 | 0.95 ± 0.06 |
| C24:0 | 0.82 ± 0.05 | 0.79 ± 0.08 | 0.91 ± 0.06 | 1.0 ± 0.1 | 1.08 ± 0.01 | 1.12 ± 0.01 | 1.70 ± 0.01 | 1.48 ± 0.04 | 1.05 ± 0.06 | 0.90 ± 0.04 |
| SFA | 22.4 ± 0.1 | 22.2 ± 0.3 | 22.8 ± 0.1 | 22.9 ± 0.1 | 24.4 ± 0.3 | 24.3 ± 0.1 | 24.8 ± 0.3 | 24.9 ± 0.2 | 23.0 ± 0.1 | 22.8 ± 0.3 |
| MUFA | 17.2 ± 0.1 | 16.8 ± 0.1 | 18.4 ± 0.1 | 18.4 ± 0.1 | 17.6 ± 0.1 | 17.1 ± 0.1 | 17.0 ± 0.2 | 16.2 ± 0.1 | 17.4 ± 0.1 | 17.3 ± 0.5 |
| PUFA | 60.4 ± 0.2 | 60.9 ± 0.2 | 58.8 ± 0.1 | 58.7 ± 0.1 | 58.1 ± 0.4 | 58.6 ± 0.2 | 58.3 ± 0.1 | 58.9 ± 0.1 | 59.6 ± 0.1 | 59.9 ± 0.2 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
| C6:0 | 0.035 ± 0.003 | 0.074 ± 0.004 | 0.106 ± 0.001 | 0.18 ± 0.01 | 0.062 ± 0.001 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.62 ± 0.02 |
| C8:0 | 0.092 ± 0.003 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.38 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.40 ± 0.01 |
| C11:0 | 0.037 ± 0.002 | 0.057 ± 0.003 | 0.082 ± 0.004 | 0.08 ± 0.01 | 0.066 ± 0.001 | 0.056 ± 0.003 | 0.16 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.32 ± 0.01 |
| C12:0 | 0.053 ± 0.004 | 0.06 ± 0.01 | 0.11 ± 0.01 | 0.043 ± 0.003 | 0.059 ± 0.003 | 0.082 ± 0.004 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.14 ± 0.01 |
| C13:0 | 0.079 ± 0.005 | 0.08 ± 0.01 | 0.16 ± 0.01 | 0.164 ± 0.004 | 0.22 ± 0.01 | 0.38 ± 0.03 | 0.21 ± 0.01 | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.58 ± 0.03 |
| C14:0 | 0.43 ± 0.01 | 0.47 ± 0.02 | 0.71 ± 0.01 | 0.45 ± 0.03 | 0.656 ± 0.004 | 1.03 ± 0.04 | 0.59 ± 0.01 | 0.60 ± 0.02 | 0.41 ± 0.03 | 0.48 ± 0.02 |
| C15:0 | 0.339 ± 0.004 | 0.39 ± 0.01 | 0.45 ± 0.01 | 0.40 ± 0.02 | 0.572 ± 0.002 | 1.1 ± 0.1 | 0.49 ± 0.01 | 0.53 ± 0.02 | 0.33 ± 0.02 | 0.13 ± 0.01 |
| C16:0 | 14.1 ± 0.6 | 15.40 ± 0.06 | 13.37 ± 0.05 | 14.79 ± 0.06 | 15.3 ± 0.1 | 14.0 ± 0.1 | 13.6 ± 0.5 | 13.1 ± 0.4 | 12.63 ± 0.09 | 14.4 ± 0.5 |
| C16:1 | 0.57 ± 0.02 | 0.45 ± 0.04 | 0.77 ± 0.01 | 0.45 ± 0.04 | 0.29 ± 0.02 | 0.31 ± 0.01 | 0.26 ± 0.01 | 0.31 ± 0.03 | 0.36 ± 0.02 | 0.93 ± 0.02 |
| C17:0 | 0.54 ± 0.02 | 0.37 ± 0.01 | 0.88 ± 0.04 | 0.87 ± 0.01 | 0.55 ± 0.03 | 0.84 ± 0.02 | 0.76 ± 0.06 | 0.50 ± 0.01 | 0.34 ± 0.02 | 0.89 ± 0.04 |
| C18:0 | 2.69 ± 0.06 | 2.03 ± 0.03 | 2.82 ± 0.04 | 2.81 ± 0.03 | 2.02 ± 0.02 | 2.0 ± 0.1 | 2.2 ± 0.2 | 2.06 ± 0.04 | 1.94 ± 0.04 | 1.21 ± 0.06 |
| C18:1n9c | 15.10 ± 0.06 | 15.16 ± 0.08 | 15.6 ± 0.1 | 15.34 ± 0.04 | 15.82 ± 0.04 | 14.3 ± 0.5 | 14.45 ± 0.06 | 14.7 ± 0.1 | 15.3 ± 0.1 | 15.3 ± 0.4 |
| C18:2n6c | 45.3 ± 0.1 | 45.15 ± 0.05 | 45.7 ± 0.1 | 45.13 ± 0.02 | 44.2 ± 0.2 | 43.6 ± 0.1 | 45.1 ± 0.1 | 44.33 ± 0.01 | 45.34 ± 0.04 | 43.88 ± 0.09 |
| C18:3n3 | 14.30 ± 0.02 | 15.0 ± 0.1 | 13.68 ± 0.05 | 13.3 ± 0.1 | 12.79 ± 0.03 | 13.02 ± 0.01 | 13.38 ± 0.07 | 13.4 ± 0.4 | 14.6 ± 0.1 | 13.54 ± 0.09 |
| C20:0 | 0.63 ± 0.02 | 0.92 ± 0.01 | 1.14 ± 0.07 | 1.06 ± 0.01 | 1.38 ± 0.09 | 1.76 ± 0.16 | 1.49 ± 0.02 | 1.32 ± 0.02 | 1.04 ± 0.02 | 1.03 ± 0.02 |
| C20:1 | 1.25 ± 0.03 | 0.40 ± 0.03 | 0.58 ± 0.05 | 0.58 ± 0.04 | 0.42 ± 0.03 | 0.65 ± 0.06 | 0.8 ± 0.1 | 1.73 ± 0.04 | 2.86 ± 0.02 | 2.56 ± 0.08 |
| C21:0 | 1.8 ± 0.2 | 0.36 ± 0.03 | 1.02 ± 0.01 | 0.73 ± 0.02 | 0.58 ± 0.03 | 0.89 ± 0.08 | 1.09 ± 0.01 | 2.6 ± 0.1 | 1.02 ± 0.07 | 1.11 ± 0.01 |
| C22:0 | 0.88 ± 0.01 | 0.88 ± 0.08 | 1.07 ± 0.03 | 1.08 ± 0.02 | 1.5 ± 0.1 | 1.62 ± 0.03 | 1.46 ± 0.02 | 1.3 ± 0.1 | 0.94 ± 0.02 | 0.88 ± 0.02 |
| C22:1 | 0.40 ± 0.04 | 1.20 ± 0.07 | 0.49 ± 0.04 | 0.66 ± 0.05 | 0.80 ± 0.07 | 0.97 ± 0.05 | 1.08 ± 0.03 | 0.90 ± 0.04 | 0.97 ± 0.02 | 0.42 ± 0.01 |
| C23:0 | 0.67 ± 0.01 | 0.78 ± 0.07 | 0.88 ± 0.02 | 1.01 ± 0.06 | 1.28 ± 0.07 | 1.52 ± 0.05 | 1.19 ± 0.08 | 1.11 ± 0.07 | 0.84 ± 0.03 | 0.52 ± 0.01 |
| C24:0 | 0.80 ± 0.05 | 0.76 ± 0.06 | 0.37 ± 0.01 | 0.86 ± 0.08 | 1.46 ± 0.06 | 1.32 ± 0.01 | 1.48 ± 0.02 | 1.15 ± 0.05 | 0.79 ± 0.01 | 0.67 ± 0.02 |
| SFA | 23.1 ± 0.1 | 22.6 ± 0.1 | 23.2 ± 0.2 | 24.6 ± 0.1 | 25.7 ± 0.1 | 27.2 ± 0.3 | 25.0 ± 0.2 | 24.7 ± 0.3 | 20.5 ± 0.2 | 23.4 ± 0.5 |
| MUFA | 17.3 ± 0.1 | 17.2 ± 0.1 | 17.5 ± 0.1 | 17.0 ± 0.1 | 17.3 ± 0.1 | 16.3 ± 0.5 | 16.6 ± 0.1 | 17.6 ± 0.1 | 19.5 ± 0.1 | 19.2 ± 0.5 |
| PUFA | 59.6 ± 0.1 | 60.2 ± 0.1 | 59.3 ± 0.2 | 58.4 ± 0.1 | 57.0 ± 0.2 | 56.6 ± 0.1 | 58.5 ± 0.2 | 57.7 ± 0.4 | 59.9 ± 0.1 | 57.4 ± 0.1 |
References
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food; InTech: London, UK, 2017. [Google Scholar]
- Avrelija, C.; Walter, C. Antimicrobial Agents Deriving from Indigenous Plants. Recent Patents Food Nutr. Agric. 2010, 2, 83–92. [Google Scholar] [CrossRef]
- Balsano, C. Antioxidant Effects of Natural Bioactive Compounds. Curr. Pharm. Des. 2009, 15, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Johnson, C.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens culinaris L.): A prebiotic-rich whole food legume. Food Res. Int. 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Sabaté, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100, 476S–482S. [Google Scholar] [CrossRef]
- Joshi, M.; Timilsena, Y.; Adhikari, B. Global production, processing and utilization of lentil: A review. J. Integr. Agric. 2017, 16, 2898–2913. [Google Scholar] [CrossRef]
- Kumar, S.K.; Barpete, S.; Kumar, J.; Gupta, P.; Sarker, A. Global Lentil Production: Constraints and Strategies. SATSA Mukhapatra Annu. Tech. Issue 2013, 17, 1–13. [Google Scholar]
- Alexander, W. Lentil Trading and Marketing: Australian Grain Exports. Available online: http://grdc.com.au/Researchand-Development/GRDC-UpdatePapers/2015/08/Lentiltrading-and-marketing (accessed on 7 April 2021).
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Behall, K.; Conway, J. Short-Term Satiety and Glycemic Response after Consumption of Whole Grains Varying in the Amount of b-Glucan in Overweight Adults. In Proceedings of the North American As-sociation for the Study of Obesity 2005 Annual Meeting. Obes. Res. 2005, 13, A219. [Google Scholar]
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: An overview. Am. J. Clin. Nutr. 2003, 78, 544S–551S. [Google Scholar] [CrossRef] [Green Version]
- Philanto, A.; Korhonrn, H. Bioactive Peptides and Proteins. Adv. Food Nutr. Res. 2003, 47, 175–276. [Google Scholar]
- Tharanathan, R.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Gallaher, D.D. Whole Grain Intake and Cardiovascular Disease: A Review. Curr. Atheroscler. Rep. 2004, 6, 415–423. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Characterization and in vitro digestibility of non-conventional starches from guinea arrowroot and La Armuña lentils as potential food sources for special diet regimens. Starch 2018, 70, 1700124. [Google Scholar] [CrossRef]
- Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on Quality Schemes for Agricultural Products and Foodstuffs. Available online: http://data.europa.eu/eli/reg/2012/1151/oj (accessed on 7 April 2021).
- Tahir, M.; Vandenberg, A.; Chibbar, R.N. Influence of environment on seed soluble carbohydrates in selected lentil cultivars. J. Food Compos. Anal. 2011, 24, 596–602. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Iyda, J.H.; Fernandes, Â.; Ferreira, F.D.; Alves, M.J.; Pires, T.C.S.P.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 2019, 121, 714–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.A.; Sokovic, M.; Barros, L.; Ferreira, I.C. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Bessada, S.; Barreira, J.C.; Barros, L.; Ferreira, I.C.; Oliveira, B. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Ramdath, D.D.; Lu, Z.-H.; Maharaj, P.L.; Winberg, J.; Brummer, Y.; Hawke, A. Proximate Analysis and Nutritional Evaluation of Twenty Canadian Lentils by Principal Component and Cluster Analyses. Foods 2020, 9, 175. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, S.; Shad, M.A.; Iqbal, S.; Qayum, M.; Ahmad, A.; Luthria, D.L.; Amarowicz, R. Com-positional Studies of Lentil (Lens Culinaris Medik.) Cultivars Commonly Grown in Pakistan. Pak. J. Bot. 2011, 43, 1563–1567. [Google Scholar]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Jafari, S.M.; Faraji, K. Proximate composition, mineral content, and fatty acids profile of two varieties of lentil seeds cultivated in Iran. Chem. Nat. Compd. 2012, 47, 976–978. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.-M.; Martín-Ortega, G. Comparison of Several Procedures Used for the Extraction of Anthocyanins from Red Grapes. J. Agric. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Johnson, C.; Thavarajah, D.; Thavarajah, P.; Fenlason, A.; McGee, R.; Kumar, S.; Combs, G.F. A global survey of low-molecular weight carbohydrates in lentils. J. Food Compos. Anal. 2015, 44, 178–185. [Google Scholar] [CrossRef]
- Martinez, A.; Vargas, R.; Galano, A. Citric acid: A promising copper scavenger. Comput. Theor. Chem. 2018, 1133, 47–50. [Google Scholar] [CrossRef]
- Morales, P.; Cebadera-Miranda, L.; Cámara, R.M.; Reis, F.S.; Barros, L.; Berrios, J.D.J.; Ferreira, I.; Cámara, M. Lentil flour formulations to develop new snack-type products by extrusion processing: Phytochemicals and antioxidant capacity. J. Funct. Foods 2015, 19, 537–544. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentil (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Belury, M.A.; Cole, R.M.; Snoke, D.B.; Banh, T.; Angelotti, A. Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins, Leukot. Essent. Fat. Acids 2018, 132, 30–33. [Google Scholar] [CrossRef]
- Dhakal, K.H.; Jung, K.-H.; Chae, J.-H.; Shannon, J.G.; Lee, J.-D. Variation of unsaturated fatty acids in soybean sprout of high oleic acid accessions. Food Chem. 2014, 164, 70–73. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gomez-Serranillos, M.P.; Palomino, O.; Carretero, M. Influence of Processing in the Phenolic Composition and Health-Promoting Properties of Lentils (Lens culinaris L.). J. Food Process. Preserv. 2016, 41, e13113. [Google Scholar] [CrossRef]
- Aguilera, Y.; Dueñas, M.; Estrella, I.; Hernández, T.; Benitez, V.; Esteban, R.M.; Martin-Cabrejas, M. Evaluation of Phenolic Profile and Antioxidant Properties of Pardina Lentil as Affected by Industrial Dehydration. J. Agric. Food Chem. 2010, 58, 10101–10108. [Google Scholar] [CrossRef] [PubMed]
- Quesada, S.P.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant Activity and Phenolic Compositions of Lentil (Lens culinaris var. Morton) Extract and Its Fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Guerrero, M.C.P.; López-Lázaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Bobe, G.; Sansbury, L.B.; Albert, P.S.; Cross, A.J.; Kahle, L.; Ashby, J.; Slattery, M.L.; Caan, B.; Paskett, E.; Iber, F.; et al. Dietary Flavonoids and Colorectal Adenoma Recurrence in the Polyp Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
| Sample *1 | Moisture | Fat | Proteins | Ash | Carbohydrates |
|---|---|---|---|---|---|
| 1 | 9.39 ± 0.08 c | 0.95 ± 0.05 klmn | 22.70 ± 0.4 kl | 2.50 ± 0.03 hij | 64.50 ± 0.2 fg |
| 2 | 4.91 ± 0.01 s | 1.04 ± 0.04 fgh | 23.90 ± 0.6 g | 2.69 ± 0.03 bc | 67.50 ± 0.5 ab |
| 3 | 9.00 ± 0.1 de | 0.88 ± 0.05 qrs | 23.88 ± 0.09 g | 2.60 ± 0.1 cdef | 63.60 ± 0.2 ijk |
| 4 | 6.09 ± 0.09 qr | 1.08 ± 0.03 def | 24.80 ± 0.1 e | 2.54 ± 0.04 fghi | 65.53 ± 0.02 e |
| 5 | 7.65 ± 0.01 l | 0.96 ± 0.05 klm | 25.28 ± 0.07 bcd | 2.80 ± 0.1 a | 63.30 ± 0.1 jklm |
| 6 | 6.40 ± 0.1 op | 0.97 ± 0.02 klm | 25.11 ± 0.08 cde | 2.80 ± 0.1 a | 64.70 ± 0.2 f |
| 7 | 7.53 ± 0.03 l | 1.08 ± 0.01 de | 25.50 ± 0.1 bc | 2.60 ± 0.1 defg | 63.30 ± 0.1 jkl |
| 8 | 6.73 ± 0.08 mn | 1.10 ± 0.02 cd | 24.87 ± 0.01 de | 2.57 ± 0.02 fgh | 64.73 ± 0.02 f |
| 9 | 6.70 ± 0.4 mn | 1.10 ± 0.03 cd | 23.30 ± 0.6 hi | 2.70 ± 0.1 b | 66.20 ± 0.6 cd |
| 10 | 6.73 ± 0.01 mn | 1.15 ± 0.02 b | 23.20 ± 0.1 ij | 2.45 ± 0.02 jkl | 66.50 ± 0.1 c |
| 11 | 7.97 ± 0.03 k | 1.22 ± 0.06 a | 25.40 ± 0.7 bc | 2.40 ± 0.1 m | 63.10 ± 0.4 lmn |
| 12 | 8.00 ± 0.5 k | 1.14 ± 0.02 bc | 25.60 ± 0.4 ab | 2.90 ± 0.2 bcd | 62.60 ± 0.5 no |
| 13 | 7.50 ± 0.3 l | 1.15 ± 0.01 b | 22.00 ± 0.2 m | 2.40 ± 0.1 lm | 66.99 ± 0.03 b |
| 14 | 9.80 ± 0.1 b | 0.90 ± 0.01 opqr | 24.00 ± 0.2 fg | 2.50 ± 0.1 jkl | 62.90 ± 0.3 lmn |
| 15 | 6.70 ± 0.2 mn | 1.01 ± 0.04 hij | 25.90 ± 0.1 a | 2.60 ± 0.06 efg | 63.70 ± 0. 2hijk |
| 16 | 9.30 ± 0.3 c | 0.91 ± 0.06 nopq | 23.20 ± 0.1 ij | 2.53 ± 0.06 ghi | 64.00 ± 0.1 ghi |
| 17 | 6.30 ± 0.1 pq | 1.05 ± 0.05 efg | 23.80 ± 0.1 g | 2.62 ± 0.01 efg | 66.20 ± 0.1 cd |
| 18 | 6.90 ± 0.09 m | 1.05 ± 0.04 efg | 22.60 ± 0.5 l | 2.44 ± 0.05 jkl | 67.00 ± 0.3 b |
| 19 | 5.99 ± 0.01 r | 1.04 ± 0.01 efgh | 23.10 ± 0.2 ijk | 2.62 ± 0.05 cdef | 67.30 ± 0.1 b |
| 20 | 8.40 ± 0.2 hi | 0.98 ± 0.02 jkl | 22.50 ± 0.3 l | 2.10 ± 0.02 q | 66.10 ± 0.1 cd |
| 21 | 8.50 ± 0.1 gh | 0.82 ± 0.06 s | 21.10 ± 0.2 n | 2.10 ± 0.1 q | 67.50 ± 0.2 ab |
| 22 | 9.77 ± 0.01 b | 0.94 ± 0.01 lmno | 23.90 ± 0.5 g | 2.21 ± 0.04 no | 63.20 ± 0.4 klm |
| 23 | 9.76 ± 0.01 b | 1.01 ± 0.01 hij | 24.80 ± 0.1 e | 2.28 ± 0.04 n | 62.20 ± 0.1 o |
| 24 | 6.6 ± 0.1 no | 1.04 ± 0.04 efgh | 25.50 ± 0.6 bc | 2.63 ± 0.02 bcdef | 64.20 ± 0.6 gh |
| 25 | 10.10 ± 0.4 a | 0.93 ± 0.02 mnop | 22.90 ± 0.2 ijk | 2.30 ± 0.1 no | 63.80 ± 0.1 hij |
| 26 | 9.20 ± 0.2 cd | 0.95 ± 0.01 klm | 22.80 ± 0.3 jkl | 2.10 ± 0.1 q | 64.90 ± 0.4 f |
| 27 | 8.70 ± 0.1 fg | 0.99 ± 0.04 ijk | 25.10 ± 0.6 cde | 2.50 ± 0.1 klm | 62.80 ± 0.3 mn |
| 28 | 6.40 ± 0.4 p | 0.96 ± 0.04 klm | 24.30 ± 0.2 f | 2.30 ± 0.1 n | 66.10 ± 0.2 cd |
| 29 | 8.30 ± 0.2 hi | 1.03 ± 0.03 ghi | 20.60 ± 0.6 o | 2.18 ± 0.01 op | 67.80 ± 0.5 a |
| 30 | 7.59 ± 0.04 l | 0.85 ± 0.03 s | 23.67 ± 0.01 gh | 2.13 ± 0.03 pq | 65.76 ± 0.03 de |
| 31 | 8.01 ± 0.04 jk | 0.87 ± 0.03 rs | 23.92 ± 0.02 fg | 2.70 ± 0.1 bcde | 64.53 ± 0.01 fg |
| 32 | 9.30 ± 0.6 c | 0.93 ± 0.04 mnop | 21.40 ± 0.6 n | 2.82 ± 0.02 a | 65.56 ± 0.04 e |
| 33 | 8.80 ± 0.3 ef | 0.89 ± 0.04 pqr | 23.80 ± 0.6 g | 2.50 ± 0.1 ij | 64.00 ± 0.7 hi |
| 34 | 8.25 ± 0.08 ij | 0.88 ± 0.05 qrs | 24.80 ± 0.7 c | 2.50 ± 0.1 ijk | 63.60 ± 0.5 ijk |
| Free Sugars | |||
|---|---|---|---|
| Sample *1 | Sucrose | Raffinose | Total Sugars |
| 1 | 0.91 ± 0.01 c | 0.23 ± 0.01 klmn | 1.13 ± 0.01 kl |
| 2 | 1.04 ± 0.01 s | 0.20 ± 0.01 fgh | 1.24 ± 0.02 g |
| 3 | 0.95 ± 0.01 de | 0.19 ± 0.01 qrs | 1.14 ± 0.01 g |
| 4 | 1.03 ± 0.01 qr | 0.19 ± 0.01 def | 1.22 ± 0.01 e |
| 5 | 1.09 ± 0.01 l | 0.21 ± 0.00klm | 1.30 ± 0.01 bcd |
| 6 | 1.10 ± 0.01 op | 0.23 ± 0.01 klm | 1.33 ± 0.01 cde |
| 7 | 1.06 ± 0.01 l | 0.23 ± 0.01 de | 1.29 ± 0.02 bc |
| 8 | 1.08 ± 0.01 mn | 0.20 ± 0.01 cd | 1.28 ± 0.01 de |
| 9 | 1.03 ± 0.01 mn | 0.19 ± 0.01 cd | 1.22 ± 0.01 hi |
| 10 | 1.12 ± 0.01 mn | 0.19 ± 0.01 b | 1.31 ± 0.02 ij |
| 11 | 1.08 ± 0.01 k | 0.23 ± 0.01 a | 1.31 ± 0.01 bc |
| 12 | 1.11 ± 0.01 k | 0.23 ± 0.01 bc | 1.33 ± 0.01 ab |
| 13 | 0.92 ± 0.01 l | 0.20 ± 0.01 b | 1.13 ± 0.01 m |
| 14 | 0.90 ± 0.01 b | 0.22 ± 0.01 opqr | 1.12 ± 0.01 fg |
| 15 | 1.03 ± 0.01 mn | 0.19 ± 0.01 hij | 1.22 ± 0.01 a |
| 16 | 0.91 ± 0.01 c | 0.22 ± 0.02 nopq | 1.13 ± 0.02 ij |
| 17 | 1.03 ± 0.01 pq | 0.18 ± 0.01 efg | 1.22 ± 0.01 g |
| 18 | 1.11 ± 0.01 m | 0.18 ± 0.01 efg | 1.30 ± 0.02 l |
| 19 | 1.12 ± 0.01 l | 0.24 ± 0.01 efgh | 1.36 ± 0.02 ijk |
| 20 | 1.07 ± 0.0 hi | 0.23 ± 0.01 jkl | 1.29 ± 0.01 l |
| 21 | 1.10 ± 0.01 gh | 0.18 ± 0.01 s | 1.28 ± 0.01 n |
| 22 | 0.99 ± 0.01 b | 0.18 ± 0.01 lmno | 1.17 ± 0.01 g |
| 23 | 0.95 ± 0.01 b | 0.19 ± 0.01 hij | 1.14 ± 0.01 e |
| 24 | 0.94 ± 0.01 no | 0.19 ± 0.01 efgh | 1.12 ± 0.01 bc |
| 25 | 0.92 ± 0.04 a | 0.18 ± 0.01 mnop | 1.10 ± 0.03 ijk |
| 26 | 1.00 ± 0.01 cd | 0.23 ± 0.01 klm | 1.23 ± 0.01 jkl |
| 27 | 0.91 ± 0.01 fg | 0.18 ± 0.01 ijk | 1.09 ± 0.01 cde |
| 28 | 1.09 ± 0.03 p | 0.19 ± 0.01 klm | 1.28 ± 0.03 f |
| 29 | 1.14 ± 0.01 hi | 0.15 ± 0.01 ghi | 1.29 ± 0.01 o |
| 30 | 0.93 ± 0.01 l | 0.19 ± 0.01 s | 1.12 ± 0.01 gh |
| 31 | 0.92 ± 0.01 jk | 0.22 ± 0.01 rs | 1.14 ± 0.01 gh |
| 32 | 1.00 ± 0.01 c | 0.22 ± 0.01 mnop | 1.21 ± 0.01 n |
| 33 | 1.00 ± 0.01 ef | 0.23 ± 0.01 pqr | 1.23 ± 0.01 g |
| 34 | 1.11 ± 0.01 ij | 0.23 ± 0.01 qrs | 1.35 ± 0.01 c |
| Organic Acids | ||||
|---|---|---|---|---|
| Sample *1 | Oxalic Acid | Shikimic Acid | Citric Acid | Total Organic Acids |
| 1 | 0.26 ± 0.01 hjkl | 0.06 ± 0.01 ef | 15.30 ± 0.1 e | 15.60 ± 0.1 f |
| 2 | 0.27 ± 0.01 efghj | 0.10 ± 0.01 a | 18.10 ± 0.1 a | 18.40 ± 0.1 b |
| 3 | 0.24 ± 0.01 mnop | 0.08 ± 0.01 b | 12.60 ± 0.3 kl | 12.90 ± 0.4 lmn |
| 4 | 0.20 ± 0.01 q | 0.07 ± 0.01 d | 12.20 ± 0.5 m | 12.50 ± 0.5 o |
| 5 | 0.27 ± 0.01 efghj | 0.07 ± 0.01 c | 13.50 ± 0.4 g | 13.80 ± 0.4 h |
| 6 | 0.24 ± 0.03 op | 0.07 ± 0.01 cd | 13.08 ± 0.02 ij | 13.39 ± 0.01 jk |
| 7 | 0.27 ± 0.01 fghj | 0.08 ± 0.01 b | 13.81 ± 0.06 f | 14.20 ± 0.1 g |
| 8 | 0.25 ± 0.01 lmn | 0.06 ± 0.01 | 11.32 ± 0.01 o | 11.63 ± 0.01 q |
| 9 | 0.20 ± 0.01 q | 0.06 ± 0.01 fg | 11.00 ± 0.2 p | 11.30 ± 0.2 r |
| 10 | 0.25 ± 0.01 klm | 0.04 ± 0.01 k | 10.51 ± 0.01 q | 10.81 ± 0.02 s |
| 11 | 0.26 ± 0.01 ghjk | 0.05 ± 0.01 hi | 12.82 ± 0.01 jk | 13.14 ± 0.01 kl |
| 12 | 0.25 ± 0.01 klmn | 0.05 ± 0.01 j | 12.60 ± 0.1 kl | 12.90 ± 0.1 lmn |
| 13 | 0.20 ± 0.01 q | 0.07 ± 0.01 d | 13.20 ± 0.1 hi | 13.40 ± 0.1 ij |
| 14 | 0.28 ± 0.01 cd | 0.05 ± 0.01 hi | 13.22 ± 0.03 ghi | 13.56 ± 0.04 hij |
| 15 | 0.28 ± 0.01 def | 0.06 ± 0.01 ef | 12.60 ± 0.1 kl | 12.90 ± 0.1 lm |
| 16 | 0.23 ± 0.01 p | 0.05 ± 0.01 h | 12.60 ± 0.1 kl | 12.90 ± 0.1 lm |
| 17 | 0.30 ± 0.01 ab | 0.05 ± 0.01 gh | 12.70 ± 0.3 k | 13.10 ± 0.3 l |
| 18 | 0.31 ± 0.01 a | 0.07 ± 0.01 cde | 15.10 ± 0.1 e | 15.40 ± 0.1 f |
| 19 | 0.29 ± 0.01 bc | 0.1 0 ± 0.01 a | 15.95 ± 0.01 d | 16.34 ± 0.01 de |
| 20 | 0.28 ± 0.01 def | 0.07 ± 0.01 c | 16.00 ± 0.05 d | 16.40 ± 0.1 de |
| 21 | 0.28 ± 0.01 de | 0.06 ± 0.01 ef | 16.99 ± 0.03 b | 17.33 ± 0.03 b |
| 22 | 0.24 ± 0.01 nop | 0.03 ± 0.01 m | 15.90 ± 0.1 d | 16.20 ± 0.1 e |
| 23 | 0.24 ± 0.01 mnop | 0.06 ± 0.01 f | 16.20 ± 0.3 d | 16.50 ± 0.3 d |
| 24 | 0.27 ± 0.01 efghj | 0.05 ± 0.01 h | 16.50 ± 0.1 c | 16.90 ± 0.1 c |
| 25 | 0.21 ± 0.01 q | 0.05 ± 0.01 hi | 13.21 ± 0.02 ghi | 13.47 ± 0.1 ij |
| 26 | 0.25 ± 0.01 lmn | 0.04 ± 0.01 l | 12.30 ± 0.1 m | 12.53 ± 0.04 o |
| 27 | 0.27 ± 0.01 defgh | 0.03 ± 0.01 m | 12.22 ± 0.04 m | 12.53 ± 0.04 o |
| 28 | 0.27 ± 0.01 defgh | 0.05 ± 0.01 hij | 14.00 ± 0.1 gh | 14.00 ± 0.1 hii |
| 29 | 0.26 ± 0.01 jklm | 0.05 ± 0.01 j | 11.60 ± 0.7n | 11.90 ± 0.7p |
| 30 | 0.27 ± 0.02 dfg | 0.06 ± 0.01 f | 12.30 ± 0.1 m | 12.60 ± 0.1 no |
| 31 | 0.27 ± 0.02 defgh | 0.07 ± 0.01 cd | 13.40 ± 0.1 gh | 13.70 ± 0.1 hi |
| 32 | 0.25 ± 0.01 mno | 0.05 ± 0.01 ij | 12.39 ± 0.03 lm | 12.68 ± 0.03 mno |
| 33 | 0.27 ± 0.01 efgh | 0.06 ± 0.01 fg | 12.70 ± 0.2 kl | 13.00 ± 0.2 l |
| 34 | 0.25 ± 0.01 klmn | 0.03 ± 0.01 m | 11.47 ± 0.01 no | 11.75 ± 0.01 pq |
| Sample *1 | Tocopherols | Fatty Acids | Groups of Fatty Acids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | γ-Tocopherol | Total Tocopherols | C16:0 | C18:1n9c | C18:2n6c | C18:3n3 | SFA | MUFA | PUFA | |
| 1 | 0.28 ± 0.01 fgh | 3.89 ± 0.03 ijk | 4.17 ± 0.04 gh | 14.14 ± 0.01 hijk | 15.12 ± 0.04 fgh | 44.08 ± 0.06 ghi | 14.11 ± 0.07 kl | 25.2 ± 0.1 e | 16.6 ± 0.1 qr | 58.2 ± 0.1 m |
| 2 | 0.32 ± 0.01 bcd | 3.88 ± 0.01 ijk | 4.21 ± 0.01 fg | 15.93 ± 0.04 ab | 14.08 ± 0.02 l | 45.13 ± 0.01 bc | 14.84 ± 0.07 de | 24.6 ± 0.1 hij | 24.6 ± 0.1 u | 60.0 ± 0.1 cd |
| 3 | 0.29 ± 0.01 defg | 3.98 ± 0.04 fg | 4.28 ± 0.04 ef | 14.31 ± 0.02 ghi | 14.2 ± 0.2 l | 44.52 ± 0.01 ef | 15.41 ± 0.04 b | 24.5 ± 0.1 ij | 15.6 ± 0.1 u | 59.9 ± 0.1 d |
| 4 | 0.29 ± 0.02defg | 4.07 ± 0.01 de | 4.36 ± 0.01 d | 15.3 ± 0.3 cde | 15.7 ± 0.2 cde | 44.2 ± 0.2 fgh | 14.24 ± 0.06 ijk | 24.6 ± 0.4 hij | 17.0 ± 0.2 no | 58.5 ± 0.2 kl |
| 5 | 0.35 ± 0.01 ab | 4.15 ± 0.03 bc | 4.50 ± 0.01 b | 14.2 ± 0.2 hij | 16.0 ± 0.1 c | 43.8 ± 0.2 ijk | 13.19 ± 0.06 pqrs | 25.2 ± 0.3 ef | 17.9 ± 0.1 f | 57.0 ± 0.3 r |
| 6 | 0.32 ± 0.01 cde | 3.90 ± 0.07 hijk | 4.22 ± 0.08 fg | 15.24 ± 0.07 cde | 14.3 ± 0.1 kl | 43.30 ± 0.04 lm | 14.16 ± 0.04 jk | 26.2 ± 0.1 b | 16.4 ± 0.2 st | 57.5 ± 0.1 op |
| 7 | 0.23 ± 0.06 lmn | 4.16 ± 0.05 bc | 4.39 ± 0.01 d | 14.0 ± 0.1 hijk | 14.15 ± 0.05 l | 42.76 ± 0.09 n | 15.42 ± 0.03 b | 25.1 ± 0.1 ef | 16.8 ± 0.1 pq | 58.2 ± 0.1 m |
| 8 | 0.28 ± 0.03 fgh | 4.13 ± 0.06 bcd | 4.42 ± 0.04 cd | 15.5 ± 0.3 bcd | 13.65 ± 0.07 m | 43.99 ± 0.02 ghi | 15.37 ± 0.01 b | 24.1 ± 0.1 lm | 16.6 ± 0.2 qr | 59.4 ± 0.1 gh |
| 9 | 0.27 ± 0.02 ghij | 4.10 ± 0.06 cd | 4.37 ± 0.04 d | 16.1 ± 0.1 a | 13.08 ± 0.08 n | 45.27 ± 0.06 bc | 14.43 ± 0.01 ghij | 23.9 ± 0.1 m | 16.4 ± 0.1 st | 59.7 ± 0.1 ef |
| 10 | 0.23 ± 0.04 lmn | 4.00 ± 0.09 ef | 4.23 ± 0.05 efg | 15.37 ± 0.09 bcd | 14.7 ± 0.3 ijk | 43.4 ± 0.2 klm | 14.8 ± 0.4 def | 24.1 ± 0.3 klm | 17.7 ± 0.3 fg | 58.2 ± 0.6 m |
| 11 | 0.24 ± 0.02 ijklmn | 4.11 ± 0.04 bcd | 4.36 ± 0.02 d | 15.17 ± 0.07 cde | 14.49 ± 0.07 jkl | 44.7 ± 0.3 de | 15.74 ± 0.05 a | 22.4 ± 0.1 tu | 17.2 ± 0.1 klm | 60.4 ± 0.2 b |
| 12 | 0.36 ± 0.02 a | 4.28 ± 0.04 a | 4.65 ± 0.06 a | 14.96 ± 0.04 def | 14.2 ± 0.1 l | 45.1 ± 0.2 bc | 15.85 ± 0.03 a | 22.2 ± 0.3 u | 16.8 ± 0.1 op | 60.9 ± 0.2 a |
| 13 | 0.27 ± 0.09 ghi | 3.95 ± 0.09 fghi | 4.23 ± 0.18 efg | 13.42 ± 0.09 lm | 15.29 ± 0.05 efg | 44.17 ± 0.02 fgh | 14.60 ± 0.03 efgh | 22.8 ± 0.1 qrs | 18.4 ± 0.1 e | 58.8 ± 0.1 i |
| 14 | 0.31 ± 0.01 cdef | 4.18 ± 0.08 b | 4.50 ± 0.08 bc | 14.2 ± 0.3 hij | 15.44 ± 0.06 def | 43.45 ± 0.03 klm | 15.25 ± 0.05 bc | 22.9 ± 0.1 pqr | 18.4 ± 0.1 e | 58.7 ± 0.1 ij |
| 15 | 0.28 ± 0.07 efgh | 3.84 ± 0.04 k | 4.12 ± 0.1 h | 15.4 ± 0.2 bcd | 14.97 ± 0.02 ghi | 44.2 ± 0.3 fgh | 13.8 ± 0.1 lm | 24.4 ± 0.3 jk | 17.6 ± 0.1 ghi | 58.1 ± 0.4 m |
| 16 | 0.34 ± 0.04 abc | 3.97 ± 0.02 fgh | 4.31 ± 0.02 e | 15.9 ± 0.1ab | 14.95 ± 0.08 ghi | 43.8 ± 0.3 ijk | 14.76 ± 0.05 def | 24.3 ± 0.1 jkl | 17.1 ± 0.1 lmn | 58.6 ± 0.2 jk |
| 17 | 0.24 ± 0.02 ijklm | 3.87 ± 0.05 jk | 4.12 ± 0.07 h | 14.5 ± 0.5 fgh | 14.8 ± 0.2 hij | 44.11 ± 0.01 ghi | 14.16 ± 0.07 jk | 24.8 ± 0.3 gh | 17.0 ± 0.2 nop | 58.3 ± 0.1 lm |
| 18 | 0.26 ± 0.05 ghijk | 3.51 ± 0.05 m | 3.77 ± 0.01 j | 15.0 ± 0.2 def | 13.3 ± 0.2 mn | 44.5 ± 0.3 ef | 14.4 ± 0.1 ghijk | 24.9 ± 0.2 fg | 16.2 ± 0.1 t | 58.9 ± 0.1 i |
| 19 | 0.23 ± 0.03 klmn | 2.5 ± 0.2 s | 2.7 ± 0.2 q | 13.9 ± 0.2 ijkl | 14.4 ± 0.1 jkl | 44.99 ± 0.08 cd | 14.63 ± 0.03 efg | 23.0 ± 0.1 opq | 17.4 ± 0.1 ijk | 59.6 ± 0.1 f |
| 20 | 0.22 ± 0.01 lmn | 2.6 ± 0.2 r | 2.9 ± 0.2 p | 13.99 ± 0.01 hijk | 15.4 ± 0.6 def | 45.4 ± 0.1 ab | 14.48 ± 0.04 fghi | 22.8 ± 0.3 qrs | 17.3 ± 0.5 jk | 59.9 ± 0.2 de |
| 21 | 0.23 ± 0.01 lmn | 2.64 ± 0.05 r | 2.88 ± 0.05 p | 14.1 ± 0.6 hijk | 15.10 ± 0.06 fgh | 45.3 ± 0.1 bc | 14.30 ± 0.02 hijk | 23.1 ± 0.1 nop | 17.3 ± 0.1 jkl | 59.6 ± 0.1 fg |
| 22 | 0.23 ± 0.01 lmn | 2.83 ± 0.03 q | 3.06 ± 0.04 o | 15.40 ± 0.06 bcd | 15.16 ± 0.08 fgh | 45.15 ± 0.05 bc | 15.0 ± 0.1 cd | 22.6 ± 0.1 rs | 17.2 ± 0.1 klm | 60.2 ± 0.1 c |
| 23 | 0.24 ± 0.01 jklmn | 2.85 ± 0.02 pq | 3.09 ± 0.01 no | 13.37 ± 0.05 lm | 15.6 ± 0.1 cde | 45.7 ± 0.1 a | 13.68 ± 0.05 mn | 23.2 ± 0.2 no | 17.5 ± 0.1 hij | 59.3 ± 0.2 h |
| 24 | 0.24 ± 0.01 jklmn | 2.92 ± 0.09 p | 3.2 ± 0.1 n | 14.79 ± 0.06 efg | 15.34 ± 0.04 efg | 45.13 ± 0.02 bc | 13.3 ± 0.1 opqr | 24.6 ± 0.1 hi | 17.0 ± 0.1 mno | 58.4 ± 0.1 kl |
| 25 | 0.22 ± 0.01 mn | 3.22 ± 0.04 o | 3.44 ± 0.03 m | 15.3 ± 0.1 cde | 15.82 ± 0.04 cd | 44.2 ± 0.2 fgh | 12.79 ± 0.03 t | 25.7 ± 0.1 d | 17.3 ± 0.1 jk | 57.0 ± 0.2 r |
| 26 | 0.26 ± 0.02 hijkl | 2.82 ± 0.08 q | 3.07 ± 0.06 o | 14.0 ± 0.1 hijk | 14.3 ± 0.5 kl | 43.6 ± 0.1 jkl | 13.02 ± 0.01 rst | 27.2 ± 0.3 a | 16.3 ± 0.5 t | 56.6 ± 0.1 s |
| 27 | 0.32 ± 0.01 bcd | 3.21 ± 0.04 oq | 3.53 ± 0.04 l | 13.6 ± 0.5 jklm | 14.45 ± 0.06 jkl | 45.1 ± 0.1 bc | 13.38 ± 0.07 nop | 25.0 ± 0.2 efg | 16.6 ± 0.1 rs | 58.5 ± 0.2 kl |
| 28 | 0.21 ± 0.01 mn | 2.85 ± 0.09 pq | 3.06 ± 0.08 o | 13.1 ± 0.4 mn | 14.7 ± 0.1 ijk | 44.33 ± 0.01 efg | 13.4 ± 0.4 opq | 24.7 ± 0.3 ghi | 17.6 ± 0.1 gh | 57.7 ± 0.4 no |
| 29 | 0.27 ± 0.01 ghij | 2.79 ± 0.04 q | 2.79 ± 0.04 o | 12.63 ± 0.09 n | 15.3 ± 0.1 efg | 45.34 ± 0.04 abc | 14.6 ± 0.1 efgh | 20.5 ± 0.2 v | 19.5 ± 0.1 c | 59.9 ± 0.1 de |
| 30 | 0.23 ± 0.06 mn | 3.33 ± 0.06 n | 3.6 ± 0.1 kl | 14.4 ± 0.5 ghi | 15.3 ± 0.4 efg | 43.88 ± 0.09 hij | 13.54 ± 0.09 mno | 23.4 ± 0.5 n | 19.2 ± 0.5 d | 57.4 ± 0.1 pq |
| 31 | 0.28 ± 0.01 fgh | 3.34 ± 0.05 n | 3.62 ± 0.06 k | 13.07 ± 0.01 mn | 17.4 ± 0.2 b | 44.3 ± 0.3 fg | 12.9 ± 0.2 st | 22.8 ± 0.1 qrs | 20.0 ± 0.3 b | 57.2 ± 0.1 q |
| 32 | 0.28 ± 0.04 fgh | 3.60 ± 0.03 l | 3.60 ± 0.01 i | 14.2 ± 0.2 hi | 15.50 ± 0.02 def | 44.5 ± 0.26 ef | 13.3 ± 0.5 opqr | 22.6 ± 0.2 st | 19.6 ± 0.1 c | 57.8 ± 0.2 n |
| 33 | 0.23 ± 0.01 klmn | 3.56 ± 0.05 lm | 3.80 ± 0.04 j | 15.7 ± 0.5 abc | 19.6 ± 0.2 a | 40.0 ± 0.3 o | 13.1 ± 0.1 qrst | 25.9 ± 0.6 cd | 21.0 ± 0.2 a | 53.1 ± 0.4 t |
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative identification |
|---|---|---|---|---|---|
| 1 | 11.26 | 347 | 901 | 593(35), 285(75) | Kaempferol-deoxyhexoside-hexoside isomer I |
| 2 | 11.97 | 347 | 901 | 593(35), 285(75) | Kaempferol-deoxyhexoside-hexoside isomer II |
| 3 | 14.53 | 345 | 755 | 593(29), 285(100) | Kaempferol-O-hexoside-O-deoxyhexoside-hexoside |
| Sample *1 | Kaempferol- Deoxyhexoside- Hexoside | Kaempferol-Deoxyhexoside-Hexoside | Kaempferol-O-Hexoside-O- Deoxyhexoside-Hexoside | Total Phenolic Compounds |
|---|---|---|---|---|
| 1 | 29.6 ± 0.1 u | 25.6 ± 0.2 lm | 23.2 ± 0.1 o | 80 ± 0.01 v |
| 2 | 29.0 ± 0.1 v | 26.1 ± 0.4 k | 24.9 ± 0.1 d | 80 ± 0.01 u |
| 3 | 38.8 ± 0.5 s | 28.7 ± 0.1 e | 23.5 ± 0.1 n | 90 ± 0.01 p |
| 4 | 40.7 ± 0.9 o | 30.1 ± 0.9 c | 23.7 ± 0.1 jklmn | 90 ± 0.01 l |
| 5 | 25.6 ± 0.1 y | nd | nd | 30 ± 0.01 z |
| 6 | 39.0 ± 0.1 rs | 28.5 ± 0.1 e | 23.6 ± 0.1 mn | 90 ± 0.01 p |
| 7 | 40.8 ± 0.1 o | 29.1 ± 0.5 d | 23.6 ± 0.1 mn | 90 ± 0.01 m |
| 8 | 39.5 ± 0.1 5 pq | 28.7 ± 0.1 e | 23.5 ± 0.1 n | 90 ± 0.01 o |
| 9 | 23.0 ± 0. 1z | 24.2 ± 0.1 q | 23.0 ± 0.1 o | 70 ± 0.01 x |
| 10 | 58.1 ± 0.1 c | 34.6 ± 0.1 a | 24.0 ± 0.1 fg | 120 ± 0.01 c |
| 11 | 35.0 ± 0.1 t | 35.0 ± 0.1 j | 35.0 ± 0.1 d | 90 ± 0.01 s |
| 12 | 23.0 ± 0.1 z | 23.0 ± 0.1 q | 23.0 ± 0.1 o | 70 ± 0.01 x |
| 13 | 41.3 ± 0.1 n | 41.3 ± 0.3 f | 41.3 ± 0.1 e | 90 ± 0.01 m |
| 14 | 28.2 ± 0.2 x | 28.2 ± 0.1 r | 28.2 ± 0.2 b | 80 ± 0.01 t |
| 15 | 39.3 ± 0.5 qr | 39.3 ± 0.4 j | 39.3 ± 0.1 ghij | 90 ± 0.01 q |
| 16 | 43.1 ± 0.1 l | 43.1 ± 0.2 fg | 43.1 ± 0.1 fgh | 100 ± 0.01 jk |
| 17 | 52.3 ± 0.1 e | 52.3 ± 0.2 b | 52.3 ± 0.1 ghijk | 110 ± 0.01 f |
| 18 | 50.8 ± 0.1 f | 50.8 ± 0.1 gh | 50.8 ± 0.1 ghi | 100 ± 0.01 g |
| 19 | 48.1 ± 0.2 h | 48.1 ± 0.6 e | 48.1 ± 0.1 ghi | 100 ± 0.01 g |
| 20 | 56.3 ± 0.1 d | 56.3 ± 0.1 c | 56.3 ± 0.1 ghi | 110 ± 0.01 d |
| 21 | 71.6 ± 0.7 b | 71.6 ± 0.1 s | 71.6 ± 0.7 c | 120 ± 0.01 b |
| 22 | 57.9 ± 0.1 c | 57.9 ± 0.4 i | 57.9 ± 0.1 d | 110 ± 0.01 e |
| 23 | 43.9 ± 0.2 k | 43.9 ± 0.2 h | 43.9 ± 0.1 ghij | 100 ± 0.01 jk |
| 24 | 44.9 ± 0.2 j | 44.9 ± 0.2 j | 44.9 ± 0.1 ef | 100 ± 0.01 jk |
| 25 | 42.6 ± 0.3 m | 42.6 ± 0.1 kl | 42.6 ± 0.1 fgh | 90 ± 0.01 n |
| 26 | 48.1 ± 0.2 h | 28.7 ± 0.2 e | 76.8 ± 0.8 a | 150 ± 0.01 a |
| 27 | 46.6 ± 0.1 i | 46.6 ± 0.1 no | 46.6 ± 0.1 hijkl | 100 ± 0.01 j |
| 28 | 157.8 ± 0.2 a | 157.8 ± 0.2 op | 157.8 ± 0.1 ijklm | 50 ± 0.01 y |
| 29 | 45.0 ± 0.1 j | 45.0 ± 0.1 p | 45.0 ± 0.1 ghijk | 90 ± 0.01 m |
| 30 | 43.9 ± 0.1 k | 43.9 ± 0.2 op | 43.9 ± 0.1 ghijk | 90 ± 0.01 n |
| 31 | 46.4 ± 0.1 i | 46.4 ± 0.1 nop | 46.4 ± 0.1 lmn | 100 ± 0.01 jk |
| 32 | 48.4 ± 0.3 h | 48.4 ± 0.3 no | 48.4 ± 0.1 klmn | 100 ± 0.01 i |
| 33 | 49.5 ± 0.1 g | 49.5 ± 0.1 mn | 49.5 ± 0.1 ghijk | 100 ± 0.01 h |
| 34 | 39.7 ± 0.1 p | 39.7 ± 0.1 q | 39.7 ± 0.1 mn | 90 ± 0.01 r |
| Sample *1 | OxHLIA (IC50 Values, µg/mL) | TBARS | |
|---|---|---|---|
| Δt = 60 min | Δt = 120 min | (EC50 Values, μg/mL) | |
| 1 | 249 ± 7 e | 629 ± 7 c | 219 ± 9 fghijk |
| 2 | 132 ± 6 mn | 260 ± 12lmno | 211 ± 3 nopq |
| 3 | 211 ± 12 fgh | 904 ± 23 b | 217 ± 4 ghijklmn |
| 4 | 147 ± 6 klm | 243 ± 11 mnop | 215 ± 9 ghijklm |
| 5 | 196 ± 4 ghi | 329 ± 6 hij | 215 ± 9 hijklmn |
| 6 | 809 ± 22 a | 1312 ± 27 a | 219 ± 1 efghij |
| 7 | 303 ± 12 d | 522 ± 20 d | 213 ± 5 lmno |
| 8 | 298 ± 5 d | 453 ± 6 f | 219 ± 7 hijklmn |
| 9 | 222 ± 6 efg | 358 ± 10 gh | 214 ± 3 ijklmn |
| 10 | 236 ± 8 ef | 384 ± 14 g | 219 ± 8 efghij |
| 11 | 346 ± 10 bc | 582 ± 18 c | 214 ± 6 jklmn |
| 12 | 181 ± 11 hij | 345 ± 20 ghi | 216 ± 7 hijklmn |
| 13 | 95 ± 5 | 184 ± 10 q | 216 ± 4 hijklmn |
| 14 | 102 ± 5 n | 178 ± 9 q | 218 ± 3 ghijkl |
| 15 | 146 ± 6 klm | 253 ± 8 lmnop | 212 ± 8 mnop |
| 16 | 146 ± 3 klm | 269 ± 13 klmn | 211 ± 6 nopq |
| 17 | 176 ± 5 ijk | 301 ± 6 ijkl | 224 ± 5 abcdef |
| 18 | 244 ± 9 e | 392 ± 14 g | 220 ± 9 efghi |
| 19 | 291 ± 9 d | 467 ± 12 ef | 228 ± 4 a |
| 20 | 132 ± 7 mn | 316 ± 10 hijk | 228 ± 8 a |
| 21 | 143 ± 4 lm | 237 ± 7 nop | 220 ± 5 efghi |
| 22 | 187 ± 8 hi | 329 ± 11 hij | 225 ± 7 abcde |
| 23 | 193 ± 8 ghi | 317 ± 13 hijk | 220 ± 3 defgh |
| 24 | 131 ± 4 mn | 288 ± 3 jklm | 226 ± 2 abcd |
| 25 | 85 ± 4 | 204 ± 8 pq | 222 ± 3 bcdefg |
| 26 | 63 ± 4 | 214 ± 11 opq | 222 ± 5 bcdefg |
| 27 | 169 ± 6 ijkl | 290 ± 9 jklm | 226 ± 4 abc |
| 28 | 244 ± 7 e | 428 ± 15 f | 227 ± 4 ab |
| 29 | 321 ± 12 cd | 519 ± 21 de | 213 ± 4 klmno |
| 30 | 362 ± 12 b | 587 ± 21 c | 221 ± 8 cdefgh |
| 31 | 142 ± 8 b | 253 ± 16 c | 206 ± 6 q |
| 32 | 152 ± 4 lm | 300 ± 6 lmnop | 212 ± 3 mnop |
| 33 | 140 ± 5 jklm | 260 ± 7 ijkl | 206 ± 1 pq |
| 34 | 145 ± 6 klm | 288 ± 9 jklm | 208 ± 1 opq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberal, Â.; Fernandes, Â.; Dias, M.I.; Pinela, J.; Vivar-Quintana, A.M.; Ferreira, I.C.F.R.; Barros, L. Phytochemical and Antioxidant Profile of Pardina Lentil Cultivars from Different Regions of Spain. Foods 2021, 10, 1629. https://doi.org/10.3390/foods10071629
Liberal Â, Fernandes Â, Dias MI, Pinela J, Vivar-Quintana AM, Ferreira ICFR, Barros L. Phytochemical and Antioxidant Profile of Pardina Lentil Cultivars from Different Regions of Spain. Foods. 2021; 10(7):1629. https://doi.org/10.3390/foods10071629
Chicago/Turabian StyleLiberal, Ângela, Ângela Fernandes, Maria Inês Dias, José Pinela, Ana María Vivar-Quintana, Isabel C. F. R. Ferreira, and Lillian Barros. 2021. "Phytochemical and Antioxidant Profile of Pardina Lentil Cultivars from Different Regions of Spain" Foods 10, no. 7: 1629. https://doi.org/10.3390/foods10071629