Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases
Abstract
:1. Introduction
2. Oxidative Stress and Body Health
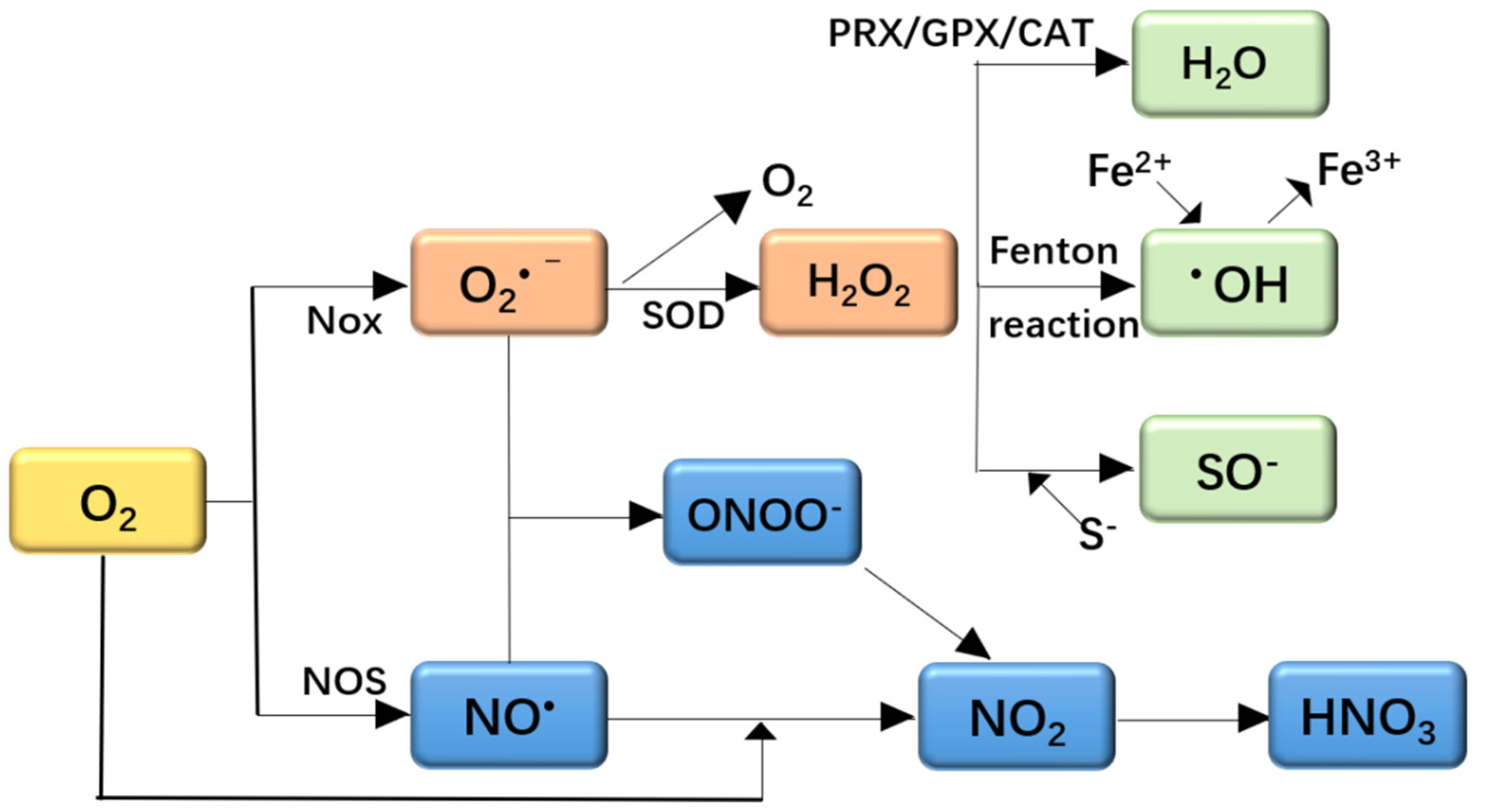
2.1. Oxidative Stress and ROS
2.2. Oxidative Stress and Obesity
2.3. Oxidative Stress and Neurodegenerative Diseases
2.4. Oxidative Stress and Immune Inflammation
3. Diet and Oxidative Stress
3.1. Dietary Pattern in Healthy People and Oxidative Stress
3.1.1. Western Diet
3.1.2. Mediterranean Diet
3.1.3. Oral Dietary Control of Oxidative Stress
3.2. Diet for Elderly People and Oxidative Stress
3.3. Diet for Athletes and Oxidative Stress
3.4. Dietary Pattern in Chronic Diseases and Oxidative Stress
3.4.1. Diet and Mitochondrial Dysfunction
3.4.2. Antioxidant Diet and Oxidative Stress
3.4.3. Diet in Obesity
3.4.4. Diet in Non-Alcoholic Steatohepatitis (NASH)
3.4.5. Diet in Type 2 Diabetes Mellitus (T2DM)
3.4.6. Diet in Cardiovascular Disease (CVD)
3.5. Diet in Neurodegenerative Diseases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Babel, R.A.; Dandekar, M.P. A review on cellular and molecular mechanisms linked to the development of diabetes complications. Curr. Diabetes Rev. 2020, 17, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H., Jr.; Newsholme, P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxid. Med. Cell Longev. 2015, 15, 181643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, J.; Bostick, B. Western diet induced obesity increases oxidative stress in the heart by impairing the Nrf2 antioxidant response pathway. J. Am. Coll. Cardiol. 2019, 73, 896. [Google Scholar] [CrossRef]
- Youn, D.Y.; Xiaoli, A.M.; Kwon, H.; Yang, F.; Pessin, J.E. The subunit assembly state of the Mediator complex is nutrient-regulated and is dysregulated in a genetic model of insulin resistance and obesity. J. Biol. Chem. 2019, 23, 9076–9083. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Homko, C.; Barrero, C.A.; Stein, T.P.; Chen, X.H.; Cheung, P.; Fecchio, C.; Koller, S.; Merali, S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 2015, 7, 304re7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batatinha, H.A.P.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, immune system, and exercise: Where will it take us? Nutrition 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2018, 134, 104621. [Google Scholar] [CrossRef]
- Tuncay, C.; Ergoren, M.C. A systematic review of precision nutrition and Mediterranean Diet: A personalized nutrition approaches for prevention and management of obesity related disorders. Clin. Nutr. ESPEN 2020, 38, 61–64. [Google Scholar] [CrossRef]
- Bjrklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail. Oxid. Med. Cell Longev. 2015, 2014, 427318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Nijhawan, P.; Arora, S.; Behl, T. Intricate role of oxidative stress in the progression of obesity. Obes. Med. 2019, 15, 100125. [Google Scholar] [CrossRef]
- Isei, M.O.; Stevens, D.; Kamunde, C. Temperature rise and copper exposure reduce heart mitochondrial reactive oxygen species scavenging capacity. Comp. Biochem. Phys. C 2021, 243, 108999. [Google Scholar]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age-related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.; Fang, S.; Kim, W.; Kim, H.J.; Kim, J.W. GPx7 ameliorates non-alcoholic steatohepatitis by regulating oxidative stress. BMB Rep. 2020, 53, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural. Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Roberts, L.J.; Milne, G.L. Isoprostanes. J. Lipid Res. 2009, 50, S219–S223. [Google Scholar] [CrossRef] [Green Version]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol. Immunol. 2014, 12, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.C. How the phagocyte NADPH oxidase regulates innate immunity. Free Radic. Biol. Med. 2018, 125, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Cécile, M.; Botzanowski, T.; Nikolaev, A.; Hellwig, P.; Cianférani, S.; Lescop, E.; Bouton, C.; Golinelli-Cohen, M.P. The H2O2-resistant Fe–S redox switch MitoNEET acts as a pH sensor to repair stress-damaged Fe–S protein. Biochemistry 2018, 57, 5616–5628. [Google Scholar]
- Groitl, B.; Jakob, U. Thiol-based redox switches. Biochim. Biophys. Acta 2014, 1844, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, D.N.; Kim, M.M.; Qian, Z.J.; Jung, W.K.; Lee, S.H.; Kim, S.K. Free radical-scavenging activities of low molecular weight chitin oligosaccharides lead to antioxidant effect in live cells. J. Food Biochem. 2010, 34, 161–177. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assay. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Serra-Majem, L.; Roman-Vinas, B.; Sanchez-Villegas, A.; Guasch-Ferre, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Aspects Med. 2019, 67, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.; Sharma, A.; Zhi, W.B.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-α and IL-6 pathways are elevated in serum of Type-1 diabetes patients with microalbuminuria. Front. Immunol. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Circulating Interleukin-6 level, dietary antioxidant capacity, and risk of colorectal cancer. Antioxidants 2019, 8, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef] [Green Version]
- Salinaro, A.T.; Cornelius, C.; Koverech, G.; Koverech, A.; Scuto, M.; Lodato, F.; Fronte, V.; Muccilli, V.; Reibaldi, M.; Longo, A.; et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder linked to Alzheimer’s disease. Front. Pharmacol. 2014, 5, 129. [Google Scholar]
- Grián-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Pallàs, M. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Burai, R.; Ait-Bouziad, N.; Chiki, A.; Lashuel, H.A. Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of Parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 2015, 137, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Liu, J.L.; Wu, Y.R.; Chen, Y.C.; Cheng, H.S.; Cheng, M.L.; Chiu, D.T.Y. Increased oxidative damage in peripheral blood correlates with severity of Parkinson’s disease. Neurobiol. Dis. 2009, 33, 429–435. [Google Scholar] [CrossRef]
- Valishkevych, B.V.; Vasylkovska, R.A.; Lozinska, L.M.; Semchyshyn, H.M. Fructose-induced carbonyl/oxidative stress in S. cerevisiae: Involvement of TOR. Biochem. Res. Int. 2016, 2016, 8917270. [Google Scholar] [CrossRef] [Green Version]
- Ling, W.A.; Zheng, K.; Zhang, D.; Gao, Y.F.; Ying, M.Z.; Wang, T.J. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar]
- Salzano, S.; Checconi, P.; Hanschmann, E.M. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.L.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, D.X.; Zhou, H.L.; Hu, Y.D.; Ying, X.; Chen, Y. Interplay of pro-inflammatory cytokines, pro-inflammatory microparticles and oxidative stress and recurrent ventricular arrhythmias in elderly patients after coronary stent implantations. Cytokine 2021, 137, 155345. [Google Scholar] [CrossRef] [PubMed]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Al-Khami, A.A.; Rodriguez, P.C.; Ochoa, A.C. Energy metabolic pathways control the fate and function of myeloid immune cells. J. Leukoc. Biol. 2017, 102, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.Y.; Xu, H.; Wang, C.G.; Sun, D.J. Dietary glutamine supplementation improves tissue antioxidant status and serum non-specific immunity of juvenile hybrid sturgeon (Acipenser schrenckii ♀ × Huso dauricus♂). J. Appl. Ichthy. 2011, 27, 715–720. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Vakili, A.Z.; Azizi, F. Western dietary pattern increases risk of cardiovascular disease in Iranian adults: A prospective population-based study. Appl. Physiol. Nutr. Metab. 2017, 42, 326–332. [Google Scholar] [CrossRef]
- Delgado, I.; Dexpert, S.; Sauvant, J.; Cryan, J.F.; Capuron, L. Influence of pro-obesogenic dietary habits on stress-induced cognitive alterations in healthy adult volunteers. Neurobiol. Stress 2021, 15, 100353. [Google Scholar] [CrossRef]
- Rai, S.K.; Fung, T.T.; Lu, N.; Keller, S.F.; Curhan, G.C.; Choi, H.K. The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: Prospective cohort study. BMJ 2017, 357, j1794. [Google Scholar] [CrossRef] [Green Version]
- Felice, N.; Cherbuin, J.N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015, 13, 215. [Google Scholar]
- Markussen, M.S.; Veierod, M.B.; Ursin, G.; Andersen, L.F. The effect of under-reporting of energy intake on dietary patterns and on the associations between dietary patterns and self-reported chronic disease in women aged 50–69 years. Br. J. Nutr. 2016, 116, 547–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, S.; Nicholas, B.; Andrew, H. Dietary pattern and its association with the prevalence of obesity, hypertension and other cardiovascular risk factors among Chinese older adults. Int. J. Environ. Res. Public Health 2014, 11, 3956–3971. [Google Scholar]
- Shang, X.W.; Li, Y.P.; Liu, A.L.; Zhang, Q.; Hu, X.Q.; Du, S.M.; Ma, J.; Xu, G.F.; Li, Y.; Guo, H.W.; et al. Dietary pattern and its association with the prevalence of obesity and related cardiometabolic risk factors among Chinese children. PLoS ONE 2017, 7, e43183. [Google Scholar] [CrossRef] [PubMed]
- Prashant, C.; Bhaskar, S. Metabolic regulation of infection and inflammation. Cytokine 2018, 112, 1–11. [Google Scholar]
- Christ, A.; Guenther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Bassler, K.; et al. Western diet triggers nlrp3-dependent innate immune reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J.; Maclauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Singer, K.; DelProposto, J.; Morris, D.L.; Zamarron, B.; Mergian, T.; Maley, N.; Cho, K.W.; Geletka, L.; Subbaiah, P.; Muir, L. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab. 2014, 3, 664–675. [Google Scholar] [CrossRef]
- Colca, J.R.; Mcdonald, W.G.; Mccommis, K.S.; Finck, B.N. Treating fatty liver disease by modulating mitochondrial pyruvate metabolism. Hepatol. Commun. 2017, 1, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.H.; Kang, E.B. Effects of treadmill exercise on the regulatory mechanisms of mitochondrial dynamics and oxidative stress in the brains of high-fat diet fed rats. J. Exerc. Nutr. Biochem. 2019, 23, 28–35. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.D.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef]
- Tapias, V.; Hu, X.; Luk, K.C.; Sanders, L.H.; Lee, V.M.; Greenamyre, J.T. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol. Life. Sci. 2017, 74, 2851–2874. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Biagi, C.; Nunzio, M.D.; Bordoni, A.; Gori, D.; Lanari, M.J.N. Effect of adherence to Mediterranean diet during pregnancy on children’s health: A systematic review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoia, M.L.; Triche, E.W.; Michaud, D.S.; Baylin, A.; Hogan, J.W.; Neuhouser, M.L.; Tinker, L.F.; Van, H.L.; Waring, M.E.; Li, W. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am. J. Clin. Nutr. 2014, 99, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Hoevenaar-Blom, M.P.; Spijkerman, A.M.; Boshuizen, H.C.; Boer, J.M.; Kromhout, D.; Verschuren, W.M. Effect of using repeated measurements of a Mediterranean style diet on the strength of the association with cardiovascular disease during 12 years: The Doetinchem Cohort Study. Eur. J. Nutr. 2014, 53, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R. PREDIMED Study Investigators: Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenech, M.; Roman, P.; Lapetra, J.; García de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Wade, A.T.; Elias, M.F.; Murphy, K.J. Adherence to a Mediterranean diet is associated with cognitive function in an older non-Mediterranean sample: Findings from the Maine-Syracuse Longitudinal Study. Nutr. Neurosci. 2021, 24, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, tria. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome axis. Cell Mol. Gastroenter. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, R.; Krycer, J.R.; Fazakerley, D.J.; Fisher-Wellman, K.H.; Su, Z.; Hoehn, K.L.; Yang, J.Y.H.; Kuncic, Z.; Vafaee, F.; James, D.E. The transcriptional response to oxidative stress is part of, but not sufficient for, insulin resistance in adipocytes. Sci. Rep. 2018, 8, 1774. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, F.; Disse, E.; Laville, M.; Mari, A.; Hojlund, K.; Anderwald, C.H.; Piatti, P.; Balkau, B. Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 2012, 55, 3228–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruzel, M.L.; Olszewska, P.; Pazdrak, B.; Krupinska, A.M.; Actor, J.K. New insights into the systemic effects of oral lactoferrin: Transcriptome profiling. Biochem. Cell Biol. 2020, 3, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, T.; Firley, W.; Kruzel, M.L. An LC-MS/MS method for quantifying lactoferrin in plasma. In Proceedings of the 14th International Conference on Lactoferrin Structure, Function and Applications, Lima, Peru, 4–8 November 2019. [Google Scholar]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lonnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The potential for lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Oguchi, S.; Shinohara, K.; Shimizu, T.; Yamashiro, Y. Effects of iron unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr. Res. 2007, 61, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.; Viana, S.D.; Reis, F. Gut microbiota dysbiosis-immune hyper response inflammation triad in coronavirus disease 2019 (COVID-19): Impact of pharmacological and nutraceutical approaches. Microorganisms 2020, 8, 1514. [Google Scholar] [CrossRef]
- Gao, X.; Gao, X.; Zhang, Y.; Holleczek, B.; Schottker, B.; Brenner, H. Oxidative stress and epigenetic mortality risk score: Associations with all-cause mortality among elderly people. Eur. J. Epidemiol. 2019, 34, 451–462. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A. Association of changes in diet quality with total and cause-specific mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef]
- Matsuyama, S.; Zhang, S.; Tomata, Y.; Abe, S.; Tanji, F.; Sugawara, Y.; Tsuji, I. Association between improved adherence to the Japanese diet and incident functional disability in older people: The ohsaki cohort 2006 study. Clin. Nutr. 2020, 39, 2238–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Dikalova, A.E. Crosstalk between mitochondrial hyperacetylation and oxidative stress in vascular dysfunction and hypertension. Antioxid. Redox Signal. 2019, 31, 710–721. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, I.H.; Weerasekera, A.; Ahmed, T.; Callaerts-Vegh, Z.; Himmelreich, U.; Hooge, R.D.; Balschun, D.; Saido, T.C.; De Strooper, B.; Dotti, C.G. High fat diet treatment impairs hippocampal long-term potentiation without alterations of the core neuropathological features of Alzheimer disease. Neurobiol. Dis. 2018, 113, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Stukas, R.; Tubelisa, L.; Zagminas, K.; Surkienė, G.; Svedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravicius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and supplement update for the endurance athlete: Review and recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaszczyk, A.; Juszczak, G. Glucocorticoids, metabolism and brain activity. Neurosci. Biobehav. Rev. 2021, 126, 113–145. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014, 44, S87–S96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getzin, A.R.; Milner, C.; Harkins, M. Fueling the triathlete: Evidence-based practical advice for athletes of all levels. Curr. Sports Med. Rep. 2017, 16, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; Desbrow, B.; Sabapathy, S.; Bellinger, P.; McCartney, D.; Irwin, C. The effect of consuming carbohydrate with and without protein on the rate of muscle glycogen resynthesis during short-term post-exercise recovery: A systematic review and meta-analysis. Sports Med. Open 2021, 7, 2891. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Close, G.L. Current controversies in sports nutrition. Eur. J. Sport Sci. 2015, 15, 1–84. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Institute of medicine of the national academies. dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Assar, M.E.; Angulo, J.; Rodríguez-Mañas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2019, 149, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I. Dietary supplements and elite athletes: When nature becomes high risk. Curr. Opin. Endocr. Metab. Res. 2019, 9, 66–73. [Google Scholar] [CrossRef]
- Garthe, I.; Maughan, R.J. Athletes and supplements: Prevalence and perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef]
- Frank, M.; Duvezin-Caubet, S.; Koob, S.; Occhipinti, A.; Jagasia, R.; Petcherski, A.; Ruonala, M.O.; Priault, M.; Salin, B.; Reichert, A.S. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim. Biophys. Acta 2012, 1823, 2297–2310. [Google Scholar] [CrossRef]
- Na, J.H.; Kim, H.D.; Lee, Y.M. Effective and safe diet therapies for Lennox-Gastaut syndrome with mitochondrial dysfunction. Ther. Adv. Neurol. Disord. 2020, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. BBA Mol. Basis Dis. 2012, 1822, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabkowski, E.R.; O’Connell, K.A.; Xu, W.H.; Ribeiro, R.F.; Hecker, P.A.; Shekar, K.C.; Daneault, C.; Rosiers, C.D.; Stanley, W.C. Docosahexaenoic acid supplementation alters key properties of cardiac mitochondria and modestly attenuates development of left ventricular dysfunction in pressure overload-induced heart failure. Cardiovasc. Drugs Ther. 2013, 27, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pino-García, R.; Rivero-Pérez, M.D.; González-Sanjosé, M.L.; Castilla-Camina, P.; Croft, K.D.; Muñiz, P. Attenuation of oxidative stress in Type 1 diabetic rats supplemented with a seasoning obtained from winemaking by-products and its effect on endothelial function. Food Funct. 2016, 7, 4410–4421. [Google Scholar] [CrossRef]
- Del Pino-García, R.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Croft, K.D.; Muñiz, P. Antihypertensive and antioxidant effects of supplementation with red wine pomace in spontaneously hypertensive rats. Food Funct. 2017, 8, 2444–2454. [Google Scholar] [CrossRef]
- Gerardi, G.; Pino-García, R.D.; Cavia-Saiz, M.; Rivero-Perez, M.D.; Muiz, P. Wine pomace product ameliorates hypertensive and diabetic aorta vascular remodeling through antioxidant and anti-inflammatory actions. J. Funct. Foods 2020, 66, 103794. [Google Scholar] [CrossRef]
- Yang, H.; Su, W.; Wang, L.; Wang, C.; Wang, C. Molecular structures of nonvolatile components in the Haihong fruit wine and their free radical scavenging effect. Food Chem. 2021, 353, 129298. [Google Scholar]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Gougeon, R.D. Exploring the chemical space of white wine anti-oxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Klotz, L.O.; Anchez-Ramos, C.; Prieto-Arroyo, I.; Urbanek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Yao, Z.L.; Yang, D.C.; Jiang, X.W.; Sun, J.X.; Tian, L.M.; Bi, J.H.; Wu, Y.; Bai, W.B. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef] [PubMed]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., III; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chi, K.T.; Lan, Y.W.; Chan, J.C.; Ma, Y.S.; Wei, Y.H. Depletion of Sirt3 leads to the impairment of adipogenic differentiation and insulin resistance via interfering mitochondrial function of adipose-derived human mesenchymal stem cells. Free Radic. Res. 2018, 5, 1398–1415. [Google Scholar] [CrossRef] [PubMed]
- Ulla, A.; Alam, M.A.; Sikder, B.; Sumi, F.A.; Rahman, M.M.; Habib, Z.F.; Mohammed, M.K.; Subhan, N.; Hossain, H.; Reza, H.M. Supplementation of syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complement. Altern. Med. 2017, 17, 289. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.A.; De Almeida, M.M.; Da Rocha, C.P.D.; Fassarella, L.B.; De Souza, L.L.; De Souza, A.F.P.; De Andrade, C.B.V.; Fortunato, R.S.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring. Sci. Rep. 2018, 8, 14751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [Green Version]
- Polanco-Briceno, S.; Glass, D.; Stuntz, M.; Caze, A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res. Notes 2016, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Orru, C.; Giordano, S.; Columbano, A. Nrf2 in neoplastic and non-neoplastic liver diseases. Cancers 2020, 12, 2932. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Frankenfeld, S.P.; Oliveira, L.P.; Ortenzi, V.H.; Rego-Monteiro, I.C.C.; Chaves, E.A.; Ferreira, A.C.; Leitão, A.C.; Carvalho, D.P.; Fortunato, R.S. The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male wistar rats. PLoS ONE 2014, 9, e102699. [Google Scholar] [CrossRef]
- Skinner, R.C.; Warren, D.C.; Naveed, M.; Agarwal, G.; Benedito, V.A.; Tou, J.C. Apple pomace improves liver and adipose inflammatory and antioxidant status in young female rats consuming a Western diet. J. Funct. Foods 2019, 61, 103471. [Google Scholar] [CrossRef]
- De Freitas Carvalho, M.M.; Lage, N.N.; de Souza Paulino, A.H.; Pereira, R.R.; de Almeida, L.T.; da Silva1, T.F.; de Brito Magalhães, C.L.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L.; et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci. Rep. 2019, 9, 8107. [Google Scholar] [CrossRef] [Green Version]
- Jeyapal, S.; Kona, S.R.; Mullapudi, S.V.; Putcha, U.K.; Gurumurthy, P.; Ibrahim, A. Substitution of linoleic acid with α-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 10953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.L.; Tan, Q.; Luo, Y.; Chen, W.; Yu, M.X.; Tu, J.C. The correlation for C1q and T2DM with dyslipidemia and insulin resistance. J. Lab. Clin. Med. 2017, 14, 2167–22169. [Google Scholar]
- Taskinen, M.R.; Borén, J.J.A. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Golizeh, M.; Kashyap, S. Glycation and deamidation result in HDL dysfunction in patients with type 2 diabetes. Diabetes 2018, 67, 330. [Google Scholar] [CrossRef]
- Weickert, M.O. High fiber intake, dietary protein, and prevention of type 2 diabetes. J. Clin. Endocr. Metab. 2018, 13, 223–224. [Google Scholar] [CrossRef] [Green Version]
- Yarar-Fisher, C.; Li, J.; McLain, A.; Gower, B.; Oster, R.; Morrow, C. Utilizing a low-carbohydrate/high-protein diet to improve metabolic health in individuals with spinal cord injury (DISH): Study protocol for a randomized controlled trial. Trials 2019, 466, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivovarova-Ramich, O.; Markova, M.; Weber, D.; Sucher, S.; Hornemann, S.; Rudovich, N.; Raila, J.; Sunaga-Franze, D.; Sauer, S.; Rohn, S.; et al. Effects of diets high in animal or plant protein on oxidative stress in individuals with type 2 diabetes: A randomized clinical trial. Redox Biol. 2020, 29, 101397. [Google Scholar] [CrossRef]
- Calvin, N.M.D.; Lisa, G.M.S.; Laura, F.M.D.; Kathleen, W.; Yu, G.M.A.; Victor, A.M.S.; Eugenia, G.M.D.; James, S.M.D.; Joseph, B.M.D.; Binita, S.M.D. Comparison of dietary patterns, perceptions of health, and perceived barriers to a heart healthy diet before and after coronary artery angiography. Am. J. Cardiol. 2019, 123, 865–873. [Google Scholar]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, B.I.; Khan, M.S.; Kaluski, E.; et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes an umbrella review and evidence map. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.G.; Khaleel, E.F.; Badi, R.M.; Abdel-Aleem, G.A.; Abdeen, H.M. Rutin hydrate inhibits apoptosis in the brains of cadmium chloride-treated rats via preserving the mitochondrial integrity and inhibiting endoplasmic reticulum stress. Neurol. Res. 2019, 41, 594–608. [Google Scholar] [CrossRef]
- Kim, S.M.; Lim, S.M.; Yoo, J.A.; Woo, M.J.; Cho, K.H. Consumption of high-dose vitamin C (1250 mg per day) enhances functional and structural properties of serum lipoprotein to improve anti-oxidant, anti-atherosclerotic, and anti-aging effects via regulation of anti-inflammatory microRNA. Food Funct. 2015, 6, 3604–3612. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.Q.; Han, Y.; Du, J.; Liu, R.Z.; Jin, K.T.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenguer-Varea, A.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martinez-Reig, M.; Mas-Bargues, C.; Ingles, M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural. Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Korejo, N.A.; Jiang, J.; Xu, M.; Zheng, K.; Mao, D.; Shi, F. Mitigation of stress from gastric mucosal injuries by mulberry extract may occur via nitric oxide synthase signaling in mice. Tissue Cell 2018, 54, 59–64. [Google Scholar] [CrossRef]
- Mehfooz, A.; Wei, Q.; Zheng, K.; Fadlalla, M.B.; Maltasic, G.; Shi, F. Protective roles of Rutin against restraint stress on spermatogenesis in testes of adult mice. Tissue Cell 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.; Chen, Y.; Li, S.; Zhang, Y.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Resveratrol protects against renal damage via attenuation of inflammation and oxidative stress in high-fat-diet-induced obese mice. Inflammation 2019, 42, 937–945. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. https://doi.org/10.3390/foods10081854
Jiang S, Liu H, Li C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods. 2021; 10(8):1854. https://doi.org/10.3390/foods10081854
Chicago/Turabian StyleJiang, Shuai, Hui Liu, and Chunbao Li. 2021. "Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases" Foods 10, no. 8: 1854. https://doi.org/10.3390/foods10081854
APA StyleJiang, S., Liu, H., & Li, C. (2021). Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods, 10(8), 1854. https://doi.org/10.3390/foods10081854





