Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retinal Ganglion Cells (RGC)
2.2. Preparation of Neuroretina Explants (Eyecup Preparations)
2.3. Agent Preparations
2.4. Experimental Protocol
2.5. MTT Test
2.6. ROS Production
2.7. ERK Activation Assay
2.8. p53 Activity
2.9. MMP-9 Quantification Assay
2.10. p38 Quantification Assay
2.11. Detection of JNK Activity
2.12. SOD Assay
2.13. Quantification of Retinal Layer
2.14. Western Blot
2.15. Statistical Analysis
3. Results
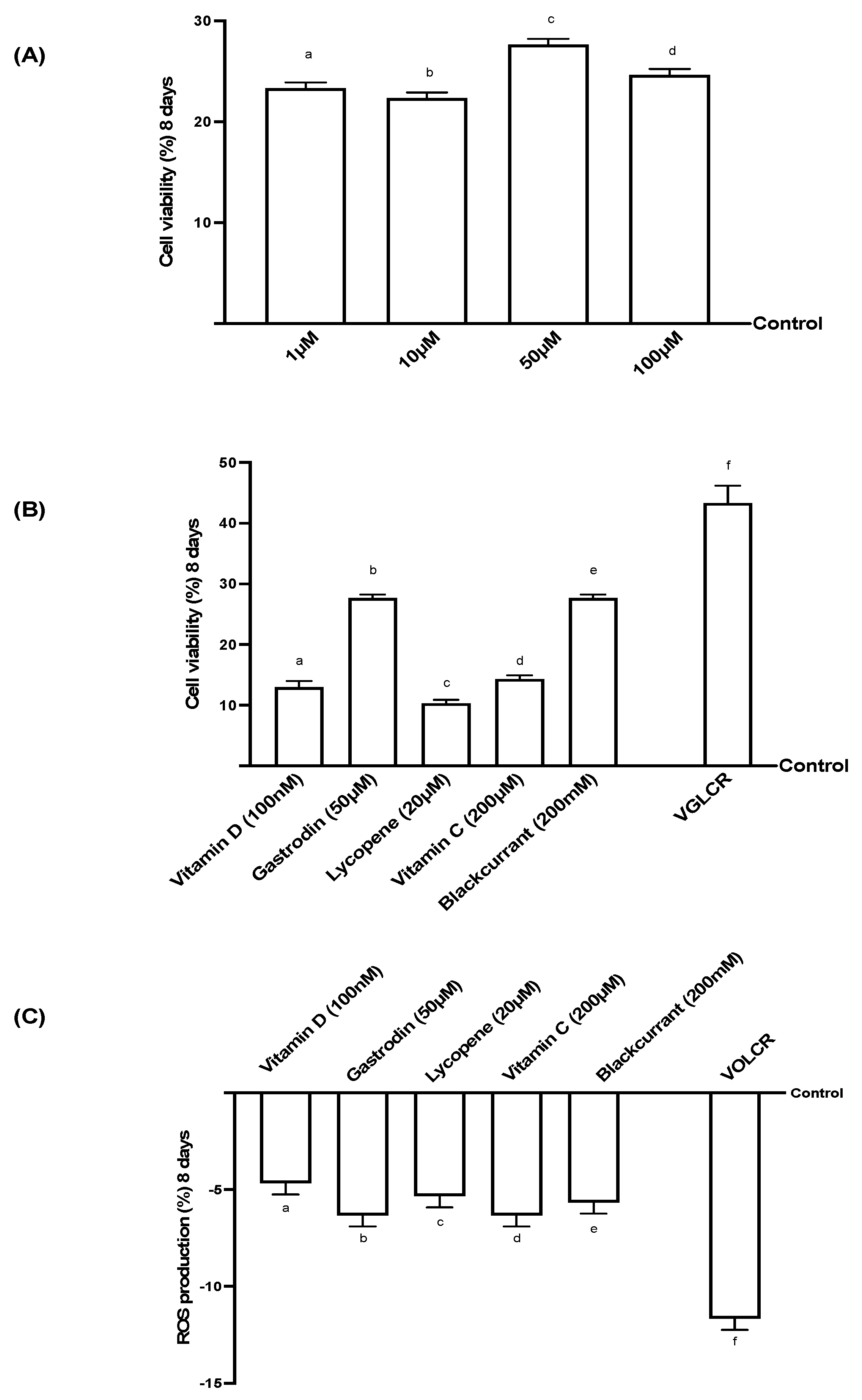
3.1. Analysis of Cell Viability and ROS on RGC Cells Treated for 8 Days
3.2. Cell Viability and ROS Production on RGC Cells Subjected to Conditions Mimicking Glaucoma
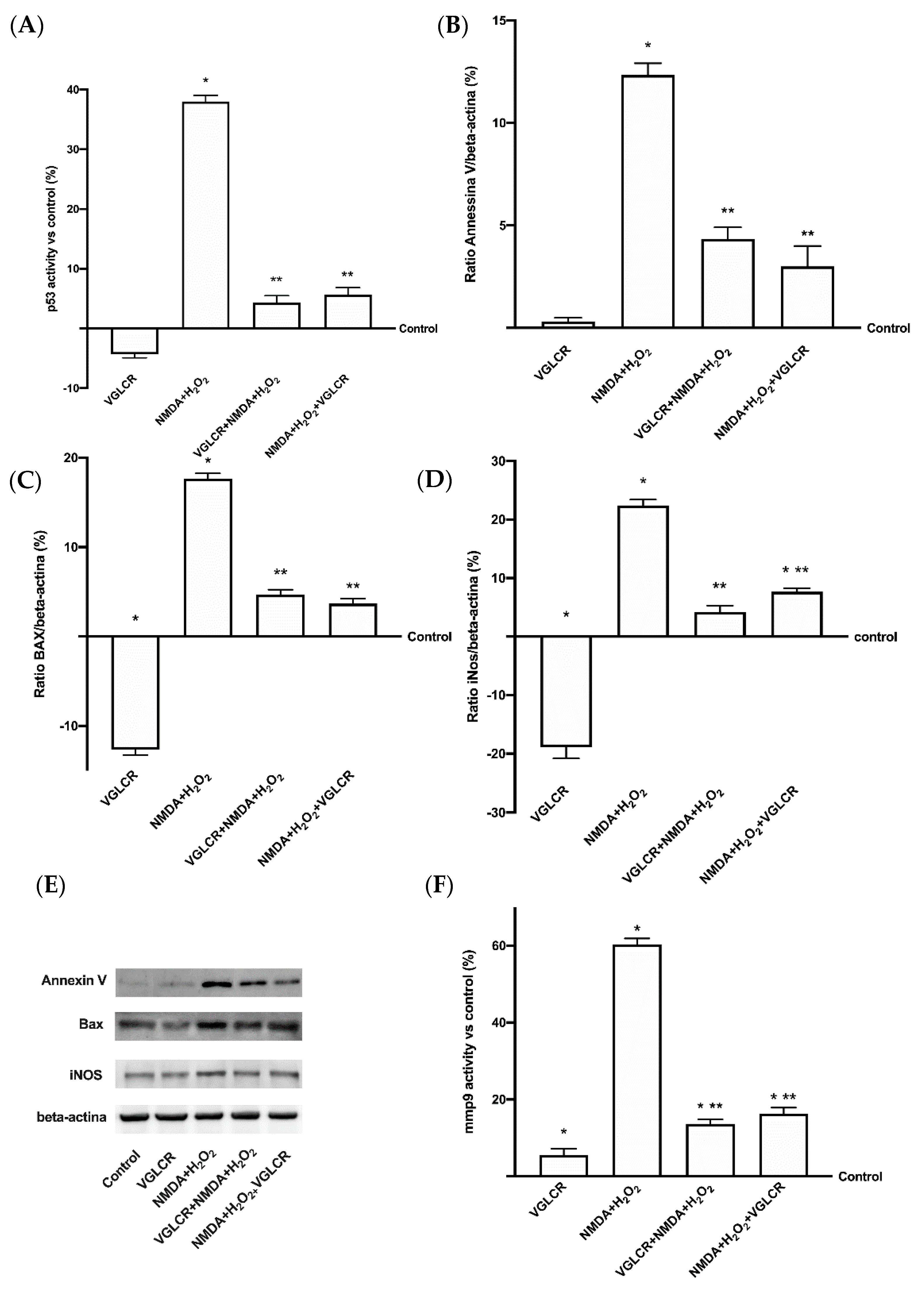
3.3. Analysis of the Main Intracellular Pathways Activated on RGC Subjected to Conditions Mimicking Glaucoma
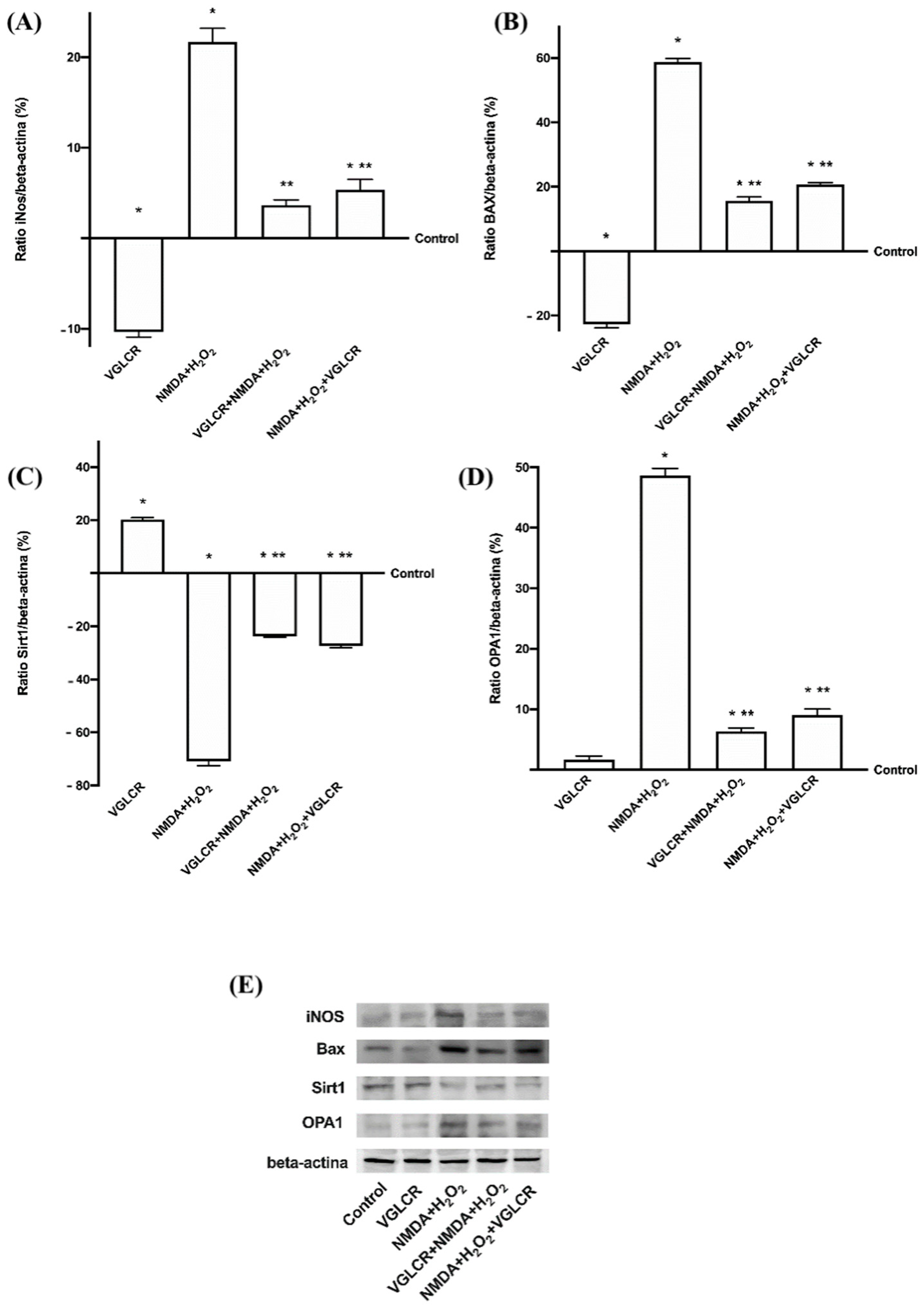
3.4. Beneficial Role of VGLCR to Prevent/Restore the Damage Caused by NMDA + H2O2 in Retinal Extracts
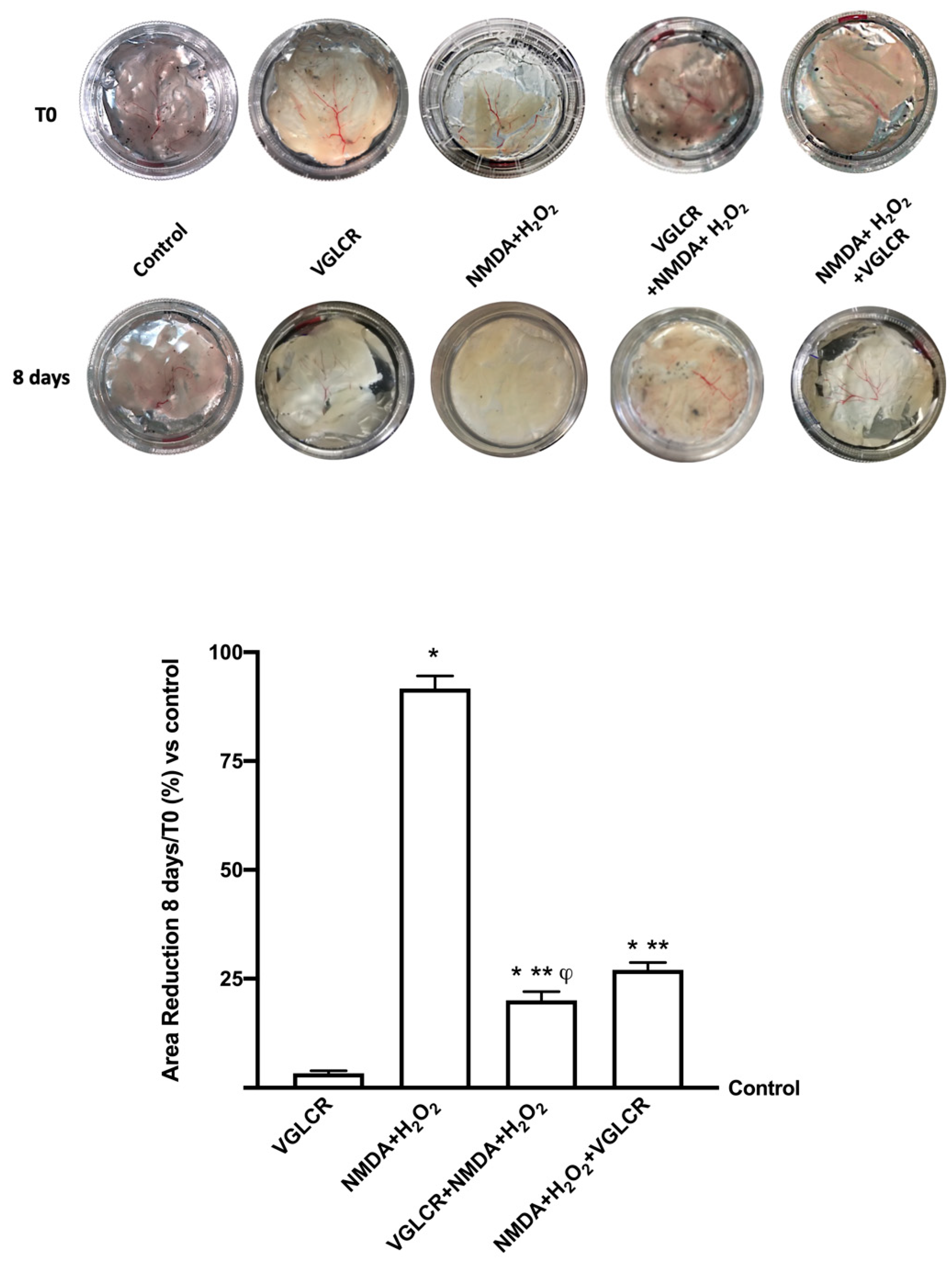
3.5. Ocular Damage Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvio, P. Global Data on Visual Impairments 2010; World Health Organization: Geneve, Switzerland, 2012. [Google Scholar]
- Song, B.J.; Aiello, L.P.; Pasquale, L.R. Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr. Diabetes Rep. 2016, 16, 124. [Google Scholar] [CrossRef]
- Song, W.; Huang, P.; Zhang, C. Neuroprotective therapies for glaucoma. Drug Des. Dev. Ther. 2015, 9, 1469–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrone, L.A.; Rombola, L.; Adornetto, A.; Corasaniti, M.T.; Russo, R. Rational Basis for Nutraceuticals in the Treatment of Glaucoma. Curr. Neuropharmacol. 2018, 16, 1004–1017. [Google Scholar] [CrossRef]
- Izzotti, A.; Di Marco, B.; De Flora, S.; Saccà, S. Glaucoma: Epidemiologia molecolare, patogenesi, prevenzione. Recenti Progressi Med. 2006, 97, 35–37. [Google Scholar] [CrossRef]
- Saccà, S.C.; Corazza, P.; Gandolfi, S.; Ferrari, D.; Sukkar, S.; Iorio, E.L.; Traverso, C.E. Substances of Interest That Support Glaucoma Therapy. Nutrients 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonne, E.U.; Ikpeazu, V.O.; Ugbogu, E.A. The potential health benefits of dietary natural plant products in age related eye diseases. Heliyon 2020, 10, e04408, Erratum in Heliyon 2021, 17, e07069. [Google Scholar] [CrossRef]
- Shen, F.; Chen, B.; Danias, J.; Lee, K.C.; Lee, H.; Su, Y.; Podos, S.M.; Mittag, T.W. Glutamate-induced glutamine synthetase expression in retinal Muller cells after short-term ocular hypertension in the rat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3107–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galassi, F.; Renieri, G.; Sodi, A.; Ucci, F.; Vannozzi, L.; Masini, E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br. J. Ophthalmol. 2004, 88, 757–760. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.S.; Harris, A.; Evans, D.W.; Kagemann, L.; Garzozi, H.J.; Martin, B. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv. Ophthalmol. 1999, 43, S43–S50. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K. Retinal venous pressure: The role of endothelin. EPMA J. 2015, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- Liu, S.T.; Zhong, S.M.; Li, X.Y.; Gao, F.; Li, F.; Zhang, M.L.; Zhu, K.; Sun, X.H.; Wang, X.; Miao, Y. EphrinB/EphB forward signaling in Müller cells causes apoptosis of retinal ganglion cells by increasing tumor necrosis factor alpha production in rat experimental glaucomatous model. Acta Neuropathol. Commun. 2018, 24, 111. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.; Iltis, J.; Yanev, P.; Sladic, J.; Huynh, C.; Nolan, D.; Singer, M. Effect of systemic antihypertensives on change in intraocular pressure after initiating topical prostaglandins for primary open-angle glaucoma. Clin. Ophthalmol. 2019, 23, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Molloy, B.L. Implications for postoperative visual loss: Steep Trendelenburg position and effects on intraocular pressure. Am. Assoc. Nurse Anesth. J. 2011, 79, 115–121. [Google Scholar]
- European Glaucoma Society. Terminology and Guidelines for Glaucoma; Dogma SRL: Savona, Italy, 2003; pp. 3–38. [Google Scholar]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M. Neuroprotection by (endo) Cannabinoids in Glaucoma and Retinal Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Barres, B.A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. 2000, 23, 579–612. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Guido, W. Agi Workshop Participants. Report on the National Eye Institute Audacious Goals Initiative: Regenerating the Optic Nerve. Investig. Ophthalmol. Vis. Sci. 2017, 57, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Buratto, L. L’occhio, le Sue Malattie e le Sue Cure; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 16, 3158. [Google Scholar] [CrossRef]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanopar-ticles for Age-Related Macular Degeneration. AAPS Pharm. Sci. Tech. 2020, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Godino, R.; Garland, D.L.; Pierce, E.A. Isolation, culture and characterization of primary mouse RPE cells. Nat. Protoc. 2016, 11, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, G.; Chen, M.; Muckersie, E.; Xu, H. Culture and characterization of microglia from the adult murine retina. Sci. World J. 2014, 2014, 894368. [Google Scholar] [CrossRef]
- Kuehn, S.; Hurst, J.; Jashari, A.; Ahrens, K.; Tsai, T.; Wunderlich, I.M.; Dick, H.B.; Joachim, S.C.; Schnichels, S. The Novel Induction of Retinal Ganglion Cell Apoptosis in Porcine Organ Culture by NMDA—An Opportunity for the Replacement of Animals in Experiment. Altern. Lab. Anim. 2016, 44, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.; Kuehn, S.; Jashari, A.; Tsai, T.; Bartz-Schmidt, K.U.; Schnichels, S.; Joachim, S.C. A novel porcine ex vivo retina culture model for oxidative stress induced by H2O2. Altern. Lab. Anim. 2017, 45, 11–25. [Google Scholar] [CrossRef]
- Yin, J.; Thomas, F.; Lang, J.C.; Chaum, E. Modulation of oxidative stress responses in the human retinal pigment epithelium following treatment with vitamin C. J. Cell Physiol. 2011, 226, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Hysi, P.G.; Khawaja, A.P.; Menni, C.; Tamraz, B.; Wareham, N.; Khaw, K.T.; Foster, P.J.; Benet, L.Z.; Spector, T.D.; Hammond, C.J. Ascorbic acid metabolites are involved in intraocular pressure control in the general population. Redox Biol. 2018, 20, 349–353. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Schouten, J.S.A.G.; Webers, C.A.B. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 16, 359. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, H.; Eperjesi, F. An ideal ocular nutritional supplement? Ophthalmic Physiol. Opt. 2004, 24, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyagari, R.; Chen, Y.I.; Zangwill, L.M. Association of severity of primary open-angle glaucoma with serum vitamin D levels in patients of African descent. Mol. Vis. 2019, 25, 438–445. [Google Scholar]
- Buomprisco, G.; Pascarella, A.; Pescosolido, N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 57, 559–565. [Google Scholar]
- Abouzeid, H.; Samer, C.F. Vitamin D and Glaucoma: A Critical Review of the Literature. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Yang, P.M.; Wu, Z.Z.; Zhang, Y.Q.; Wung, B.S. Lycopene inhibits ICAM-1 expression and NF-κB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016, 155, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Pasquale, L.R.; Willett, W.; Rosner, B.; Egan, K.M.; Faberowski, N.; Hankinson, S.E. Antioxidant intake and primary open-angle glaucoma: A prospective study. Am. J. Epidemiol. 2003, 15, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Ohguro, I.; Hiroshi, O. Black currant anthocyanins normalized abnormal levels of serum concentrations of endothelin-1 in patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013, 29, 480–487. [Google Scholar] [CrossRef]
- Ohguro, H.; Ohguro, I.; Katai, M. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Opthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances- an overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Liu, Y.M.; Zhao, X.F.; Zhang, H. Gastrodin protects retinal ganglion cells through inhibiting microglial-mediated neuroinflammation in an acute ocular hypertension model. Int. J. Ophthalmol. 2017, 10, 1483–1489. [Google Scholar]
- Zhang, T.H.; Huang, C.M.; Gao, X.; Wang, J.W.; Hao, L.L.; Ji, Q. Gastrodin inhibits high glucose induced human retinal endothelial cell apoptosis by regulating the SIRT1/TLR4/NF κBp65 signaling pathway. Mol. Med. Rep. 2018, 17, 7774–7780. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.Y.; Bian, L.G.; Yang, P.; Sui, Y.; Li, R.; Chen, Y.L.; Sun, L.; Ai, Q.L.; Zhong, L.M.; Lu, D. Gastrodin attenuates proliferation and inflammatory responses in activated microglia through Wnt/β-catenin signaling pathway. Brain Res. 2019, 15, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.; Aviles-Trigueros, M.; Villegas-Perez, M.; Vidal-Sanz, M.; Trivino, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Snook, J.D.; Zhuang, I.; Shen, G.; Frankfort, B.J. Optimized culture of retinal ganglion cells and amacrine cells from adult mice. PLoS ONE 2020, 15, e0242426. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, W.; Onishi, A.; Tu, H.Y.; Takihara, Y.; Matsumura, M.; Tsujimoto, K.; Inatani, M.; Nakazawa, T.; Takahashi, M. Culture Systems of Dissociated Mouse and Human Pluripotent Stem Cell-Derived Retinal Ganglion Cells Purified by Two-Step Immunopanning. Investig. Ophthalmol. Vis. Sci. 2018, 59, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Zhang, X.; Zheng, D.; Ge, J.; Laties, A.M.; Mitchell, C.H. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp. Eye Res. 2011, 93, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.; Reinehr, S.; Maliha, A.M.; Joachim, S.C. Immune Mediated Degeneration and Possible Protection in Glaucoma. Front. Neurosci. 2019, 2, 931. [Google Scholar] [CrossRef] [Green Version]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef] [Green Version]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.E.; Hollyfield, J.G.; LaVail, M.M. Retinal Degeneration Clinical and Laboratory Applications; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Molinari, C.; Morsanuto, V.; Ruga, S.; Notte, F.; Farghali, M.; Galla, R.; Uberti, F. The Role of BDNF on Aging-Modulation Markers. Brain Sci. 2020, 10, 285. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Bardelli, C.; Molinari, C. Highly Diluted Acetylcholine Promotes Wound Repair in an In Vivo Model. Adv. Wound Care 2018, 7, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Williamson, A.J.; Dibling, B.C.; Boyne, J.R.; Selby, P.; Burchill, S.A. Basic fibroblast growth factor-induced cell death is effected through sustained activation of p38MAPK and up-regulation of the death receptor p75NTR. J. Biol. Chem. 2004, 279, 47912–47928. [Google Scholar] [CrossRef] [Green Version]
- Uberti, F.; Morsanuto, V.; Aprile, S.; Ghirlanda, S.; Stoppa, I.; Cochis, A.; Grosa, G.; Rimondini, L.; Molinari, C. Biological effects of combined resveratrol and vitamin D3 on ovarian tissue. J. Ovarian Res. 2017, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.C.; Cassini-Vieira, P.; Souza-Fagundes, E.M.; Barcelos, L.S.; Castañon, M.C.; Scio, E. Pereskia aculeata Miller leaves accelerate excisional wound healing in mice. J. Ethnopharmacol. 2016, 194, 131–136. [Google Scholar] [CrossRef]
- Dahlmann-Noor, A.; Tailor, V.; Bunce, C.; Abou-Rayyah, Y.; Adams, G.; Brookes, J.; Khaw, P.T.; Papadopoulos, M. Quality of life and functional vision in children with glaucoma. Ophthalmology 2017, 124, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, K.A. Natural therapies for ocular disorders, part two: Cataracts and glaucoma. Altern. Med. Rev. 2001, 6, 141–166. [Google Scholar]
- Levin, L.A.; Crowe, M.E.; Quigley, H.A. The Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants. Neuroprotection for Glaucoma: Requirements for Clinical Translation. Exp. Eye Res. 2017, 157, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A.; Peeples, P. History of neuroprotection and rationale as a therapy for glaucoma. Am. J. Manag. Care 2012, 14 (Suppl. S1), S11–S14. [Google Scholar]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.A. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, I.D.; Ambrósio, A.F.; Santiago, A.R. Modeling Human Glaucoma: Lessons from the in vitro Models. Ophthalmic Res. 2017, 57, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans-A Review of the Literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delincé, M.; Di Caprio, G.; Upadhyayula, S.; de Nola, G.; Hunt, R.; Ohashi, K.G.; Gray, T.; et al. Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, J.; Park, H.; Choi, H.; Kim, J. Modelling neurodegenerative diseases with 3D brain organoids. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1497–1509. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Tang, Y.; Zhu, G.; Matragoon, S.; Khalifa, Y.; Liu, E.K.; Liu, J.Y.; Hanson, E.; Mian, S.; Fatteh, N.; et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: Critical role of p38 MAPK activation. Mol. Vis. 2008, 14, 2190–2203. [Google Scholar]
- Ishikawa, M.; Yoshitomi, T.; Zorumski, C.F.; Izumi, Y. Experimentally induced mammalian models of glaucoma. Biomed Res. Int. 2015, 2015, 281214. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.C.; Cepurna Ying Guo, W.O.; Johnson, E.C. Pathophysiology of human glaucomatous optic nerve damage: Insights from rodent models of glaucoma. Exp. Eye Res. 2011, 93, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.V.; Martin, K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3503–3512. [Google Scholar] [CrossRef]
- Seitz, R.; Tamm, E.R. N-methyl-D-aspartate (NMDA)-mediated excitotoxic damage: A mouse model of acute retinal ganglion cell damage. Methods Mol. Biol. 2013, 935, 99–109. [Google Scholar]
- Schnichels, S.; Kiebler, T.; Hurst, J.; Maliha, A.M.; Löscher, M.; Dick, H.B.; Bartz-Schmidt, K.U.; Joachim, S.C. Retinal Organ Cultures as Alternative Research Models. Altern. Lab. Anim. 2019, 47, 19–29. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [Green Version]
- Kortuem, K.; Geiger, L.K.; Levin, L.A. Differential susceptibility of retinal ganglion cells to reactive oxygen species. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3176–3182. [Google Scholar]
- Kimura, A.; Namekata, K.; Guo, X.; Noro, T.; Harada, C.; Harada, T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid. Med. Cell Longev. 2017, 2017, 2817252. [Google Scholar] [CrossRef] [PubMed]
- Varma, H.; Lo, D.C.; Stockwell, B.R. High throughput screening for neurodegeneration and complex disease phenotypes. Comb. Chem. High Throughput Screen. 2008, 11, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Al-Zidan, R.N.; Conner, M.T.; Salman, M.M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, C.; Ruga, S.; Farghali, M.; Galla, R.; Fernandez-Godino, R.; Clemente, N.; Uberti, F. Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration. Foods 2021, 10, 1885. https://doi.org/10.3390/foods10081885
Molinari C, Ruga S, Farghali M, Galla R, Fernandez-Godino R, Clemente N, Uberti F. Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration. Foods. 2021; 10(8):1885. https://doi.org/10.3390/foods10081885
Chicago/Turabian StyleMolinari, Claudio, Sara Ruga, Mahitab Farghali, Rebecca Galla, Rosario Fernandez-Godino, Nausicaa Clemente, and Francesca Uberti. 2021. "Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration" Foods 10, no. 8: 1885. https://doi.org/10.3390/foods10081885
APA StyleMolinari, C., Ruga, S., Farghali, M., Galla, R., Fernandez-Godino, R., Clemente, N., & Uberti, F. (2021). Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration. Foods, 10(8), 1885. https://doi.org/10.3390/foods10081885






