Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Lupin Seed Protein Extract
2.2. Flours Composition
2.3. Cookies Preparation
2.4. Dough Rheology
2.5. Colour Analysis
2.6. Texture Analysis
2.6.1. Dough Texture
2.6.2. Cookie Texture
2.7. Water Activity Determination
2.8. Chemical Compositions of the Cookies
2.9. Bioactivities of the Cookies
2.9.1. Protein Extraction
2.9.2. Specific MMP-9 Inhibition
2.9.3. Wound Healing Assay
2.10. Statistical Analyses
3. Results
3.1. Physical Characteristics of the Cookie Dough
3.2. Physical Properties of Cookies
3.3. Chemical Composition of Cookies
3.4. Anti-MMP-9 Activities of Cookies
3.5. Protein Cookie Extracts Inhibit Colon Cancer Cell Migration
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A. Nutraceuticals: Facts and fiction. Phytochem. 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Cookies and Crackers Europe Statista Market Forecast. Available online: https://www.statista.com/outlook/40100300/102/cookies-crackers/europe (accessed on 20 June 2021).
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Lupin Seed Protein Extract Can Efficiently Enrich the Physical Properties of Cookies Prepared with Alternative Flours. Foods 2020, 9, 1064. [Google Scholar] [CrossRef]
- Çakir, Ö.; Uçarli, C.; Tarhan, Ç.; Pekmez, M.; Turgut-Kara, N. Nutritional and health benefits of legumes and their distinctive genomic properties. Food Sci. Technol. 2019, 39, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem. 2006, 98, 291–299. [Google Scholar] [CrossRef]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- Guerreiro, J.P. Deflamin Bioactivities: A Novel Inhibitory Protein of MMP-9 from Lupinus albus (Master Dissertation). 2016. Available online: https://run.unl.pt/handle/10362/19541?mode=full (accessed on 15 May 2021).
- O’Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediators Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef]
- Chen, T.; Shi, N.; Afzali, A. Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients 2019, 11, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chango, A.; Villaume, C.; Bau, H.M.; Nicolas, J.P.; Méjean, L. Fractionation by thermal coagulation of lupin proteins: Physicochemical characteristics. Food Res. Int. 1995, 28, 91–99. [Google Scholar] [CrossRef]
- Pihlanto, A.; Mattila, P.; Mäkinen, S.; Pajari, A.M. Bioactivities of alternative protein sources and their potential health benefits. Food Funct. 2017, 8, 3443–3458. [Google Scholar] [CrossRef]
- Giarnetti, M.; Paradiso, V.M.; Caponino, F.; Summo, C.; Pasqualone, A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT Food Sci. Technol. 2015, 63, 339–345. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Xiao, H.; McClements, D.J. Delivery of lipophilic bioactives: Assembly, disassembly, and reassembly of lipid nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The texturometer—a new instrument for objective texture measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Nutritional and functional added value of oat, Kamut®, spelt, rye and buckwheat versus common wheat in breadmaking. J. Sci. Food Agric. 2011, 91, 1283–1292. [Google Scholar] [CrossRef]
- Elgeti, D.; Jekle, M.; Becker, T. Strategies for the aeration of gluten-free bread–A review. Trends Food Sci. Technol. 2015, 46, 75–84. [Google Scholar] [CrossRef]
- Pareyt, B.; Wilderjans, E.; Goesaert, H.; Brijs, K.; Delcour, J.A. The role of gluten in a sugar-snap cookie system: A model approach based on gluten–starch blends. J. Cereal Sci. 2008, 48, 863–869. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Effect of composition of gluten proteins and dough rheological properties on the cookie-making quality. Br. Food J. 2013, 115, 564–574. [Google Scholar] [CrossRef]
- Boucheham, N.; Galet, L.; Patry, S.; Zidoune, M.N. Physicochemical and hydration properties of different cereal and legume gluten-free powders. Food Sci. Nutr. 2019, 7, 3081–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traynham, T.L.; Myers, D.J.; Carriquiry, A.L.; Johnson, L.A. Evaluation of Water-Holding Capacity for Wheat–Soy Flour Blends. J. Am. Oil Chem. Soc. 2007, 84, 151–155. [Google Scholar] [CrossRef]
- Fradinho, P.; Nunes, M.C.; Raymundo, A. Developing consumer acceptable biscuits enriched with Psyllium fibre. J. Food Sci. Technol. 2015, 52, 4830–4840. [Google Scholar] [CrossRef] [Green Version]
- Mesías, M.; Morales, F.J. Effect of different flours on the formation of hydroxymethylfurfural, furfural, and dicarbonyl compounds in heated glucose/flour systems. Foods 2017, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahagún, M.; Gómez, M. Influence of protein source on characteristics and quality of gluten-free cookies. J. Food Sci. Technol. 2018, 55, 4131–4138. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2020, 38, 283–292. [Google Scholar] [CrossRef]
- Uysal, H.; Bilgiçli, N.; Elgün, A.; İbanoğlu, S.; Herken, E.N.; Demir, M.K. Effect of dietary fibre and xylanase enzyme addition on the selected properties of wire-cut cookies. J. Food Eng. 2007, 78, 1074–1078. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and In Vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT Food Sci. Technol. 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Alomari, D.Z.; Abdul-Hussain, S.S. Effect of lupin flour supplementation on chemical, physical and sensory properties of Mediterranean flat bread. Int. J. Food Sci. Nutr. 2013, 3, 49–54. [Google Scholar] [CrossRef]
- Bilgiçli, N.; Levent, H. Utilization of lupin (Lupinus albus L.) flour and bran with xylanase enzyme in cookie production. Legume Res. 2014, 37, 264–271. [Google Scholar] [CrossRef]
- Kouris-Blazos, A.; Belski, R. Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pac. J. Clin. Nutr. 2016, 25, 1–17. [Google Scholar] [CrossRef]
- Johnson, S.; Chua, V.; Hall, R.; Baxter, A. Lupin kernel fibre foods improve bowel function and beneficially modify some putative faecal risk factors for colon cancer in men. Br. J. Nutr. 2006, 95, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Cai, X.; Yan, J.; Luo, Y.; Shao, M.; Lu, Y.; Sun, Z.; Cao, P. In Vivo and In Vitro Antinociceptive effect of Fagopyrum cymosum (Trev.) Meisn extracts: A possible action by recovering intestinal barrier dysfunction. Evid. Based Complement. Alternat. Med. 2012, 2012, 983801. [Google Scholar] [CrossRef] [Green Version]
- Giménez-Bastida, J.A.; Laparra-Llopis, J.M.; Baczek, N.; Zielinski, H. Buckwheat and buckwheat enriched products exert an anti-inflammatory effect on the myofibroblasts of colon CCD-18Co. Food Funct. 2018, 9, 3387–3397. [Google Scholar] [CrossRef]
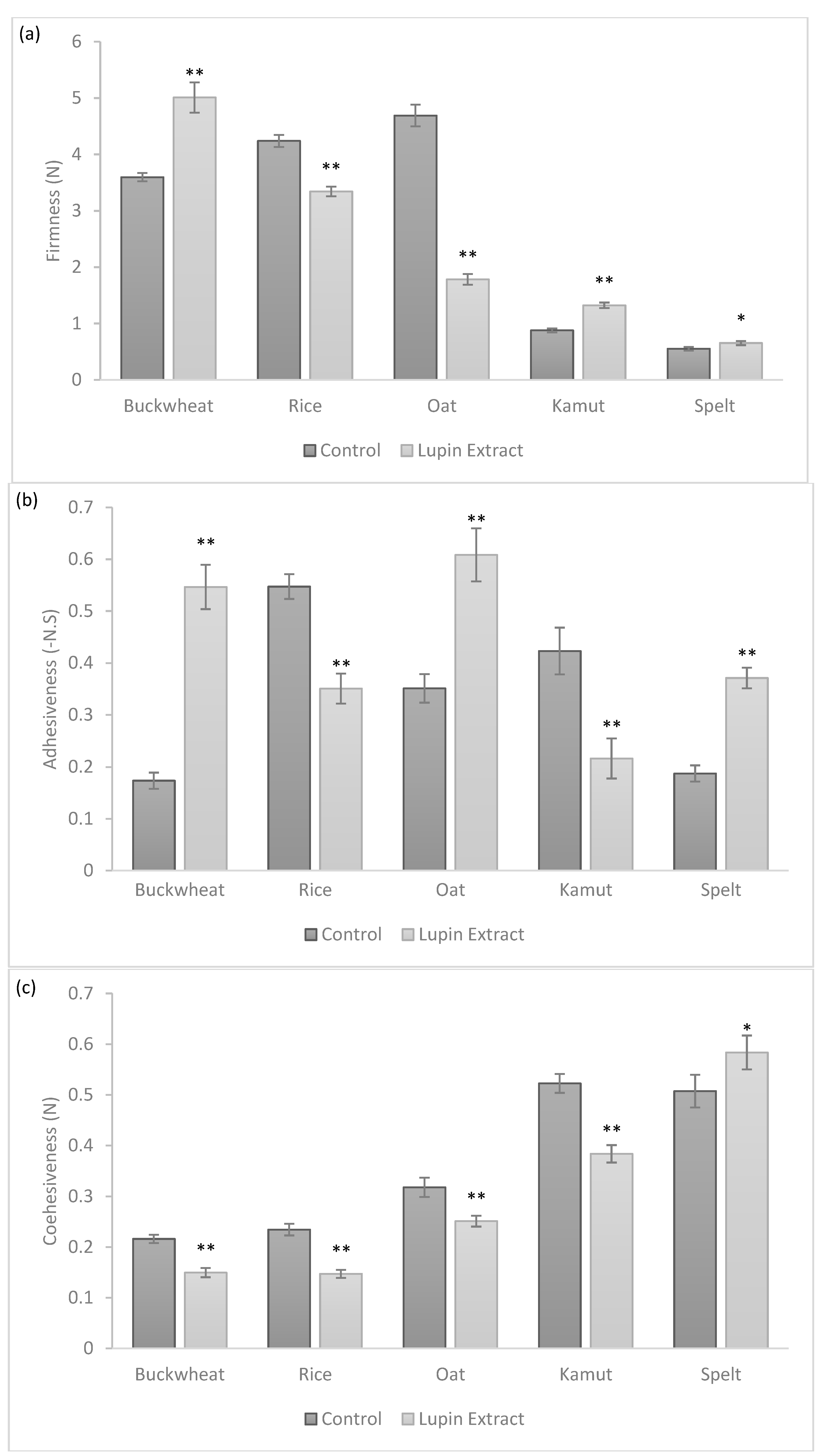


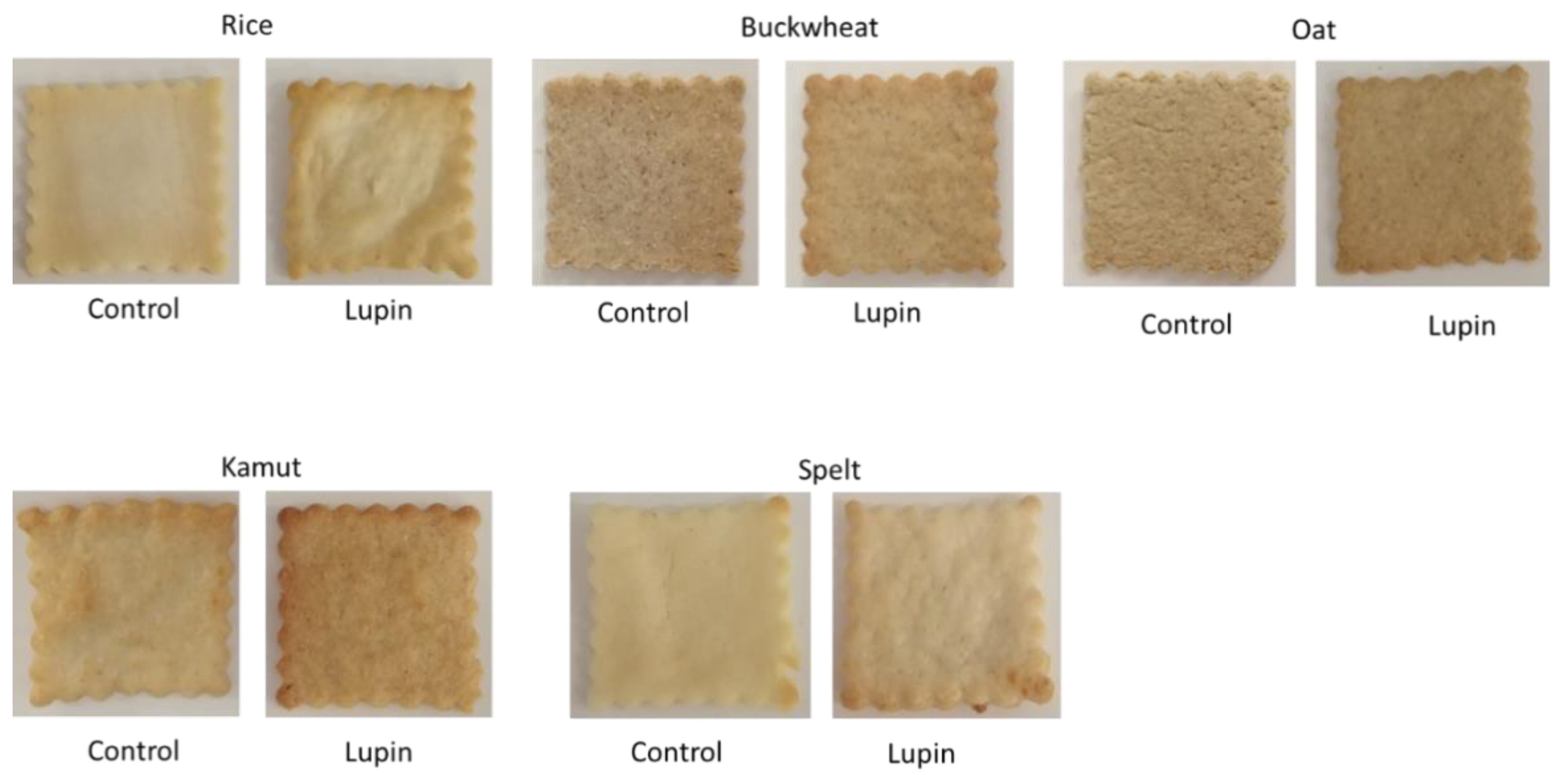


| Buckwheat | Rice | Oat | Kamut | Spelt | ||
|---|---|---|---|---|---|---|
| ΔE* | 5.98 | 17.13 | 5.20 | 2.88 | 14.64 | |
| L* | Control | 63.71 ± 1.94 | 78.63 ± 1.30 | 66.33 ± 2.72 | 68.93 ± 1.33 | 81.82 ± 2.08 |
| Lupin | 60.75 ± 2.8 * | 71.04 ± 1.99 ** | 65.76 ± 2.03 | 68.13 ± 1.96 | 71.86 ± 5.93 ** | |
| a* | Control | 7.70 ± 0.65 | −1.04 ± 0.09 | 4.20 ± 0.49 | 5.64 ± 0.62 | 2.13 ± 0.36 |
| Lupin | 5.51 ± 0.53 ** | 4.01 ± 0.55 ** | 6.53 ± 0.54 ** | 8.75 ± 0.97 ** | 9.45 ± 0.79 ** | |
| b* | Control | 26.08 ± 2.62 | 16.66 ± 1.93 | 25.94 ± 0.46 | 33.10 ± 2.04 | 28.15 ± 2.72 |
| Lupin | 25.25 ± 1.22 | 29.36 ± 1.67 ** | 30.21 ± 2.13 ** | 34.25 ± 2.77 | 34.38 ± 1.28 ** | |
| aw | Control | 0.09 ± 0.01 | 0.19 ± 0.01 | 0.07 ± 0.00 | 0.53 ± 0.01 | 0.57 ± 0.01 |
| Lupin | 0.54 ± 0.02 ** | 0.38 ± 0.00 ** | 0.28 ± 0.01 ** | 0.67 ± 0.00 ** | 0.48 ± 0.00 ** | |
| Total Ash (%) | Crude Fat (%) | Crude Protein (%) | Moisture (%) | Carbohydrates # (%) | ||
|---|---|---|---|---|---|---|
| Buckwheat | Control | 4.15 ± 0.10 | 12.32 ± 0.06 | 10.14 ± 0.06 | 3.22 ± 0.05 | 70.18 |
| Lupin | 4.78 ± 0.04 ** | 10.89 ± 0.03 ** | 13.85 ± 0.16 ** | 3.99 ± 0.12 ** | 67.75 | |
| Rice | Control | 3.16 ± 0.13 | 7.45 ± 0.13 | 5.22 ± 0.06 | 6.65 ± 0.07 | 77.52 |
| Lupin | 3.81 ± 0.10 * | 9.18 ± 0.26 ** | 10.18 ± 0.25 ** | 6.80 ± 0.11 | 70.03 | |
| Oat | Control | 4.14 ± 0.18 | 14.45 ± 0.13 | 8.03 ± 0.06 | 6.22 ± 0.21 | 67.16 |
| Lupin | 5.15 ± 0.09 ** | 14.02 ± 0.04 * | 12.42 ± 0.06 ** | 4.27 ± 0.20 ** | 64.15 | |
| Kamut | Control | 3.48 ± 0.13 | 9.96 ± 0.22 | 10.99 ± 0.09 | 10.06 ± 0.11 | 65.51 |
| Lupin | 4.31 ± 0.26 * | 9.66 ± 0.12 | 14.53 ± 0.31 ** | 5.29 ± 0.08 ** | 66.21 | |
| Spelt | Control | 3.42 ± 0.11 | 10.81 ± 0.06 | 8.60 ± 0.25 | 7.19 ± 0.05 | 69.98 |
| Lupin | 4.54 ± 0.10 ** | 11.74 ± 0.12 ** | 12.60 ± 0.31 ** | 4.60 ± 0.23 ** | 66.52 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods 2021, 10, 1929. https://doi.org/10.3390/foods10081929
Mota J, Lima A, Ferreira RB, Raymundo A. Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods. 2021; 10(8):1929. https://doi.org/10.3390/foods10081929
Chicago/Turabian StyleMota, Joana, Ana Lima, Ricardo B. Ferreira, and Anabela Raymundo. 2021. "Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies" Foods 10, no. 8: 1929. https://doi.org/10.3390/foods10081929
APA StyleMota, J., Lima, A., Ferreira, R. B., & Raymundo, A. (2021). Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods, 10(8), 1929. https://doi.org/10.3390/foods10081929







