Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract
Abstract
:1. Introduction
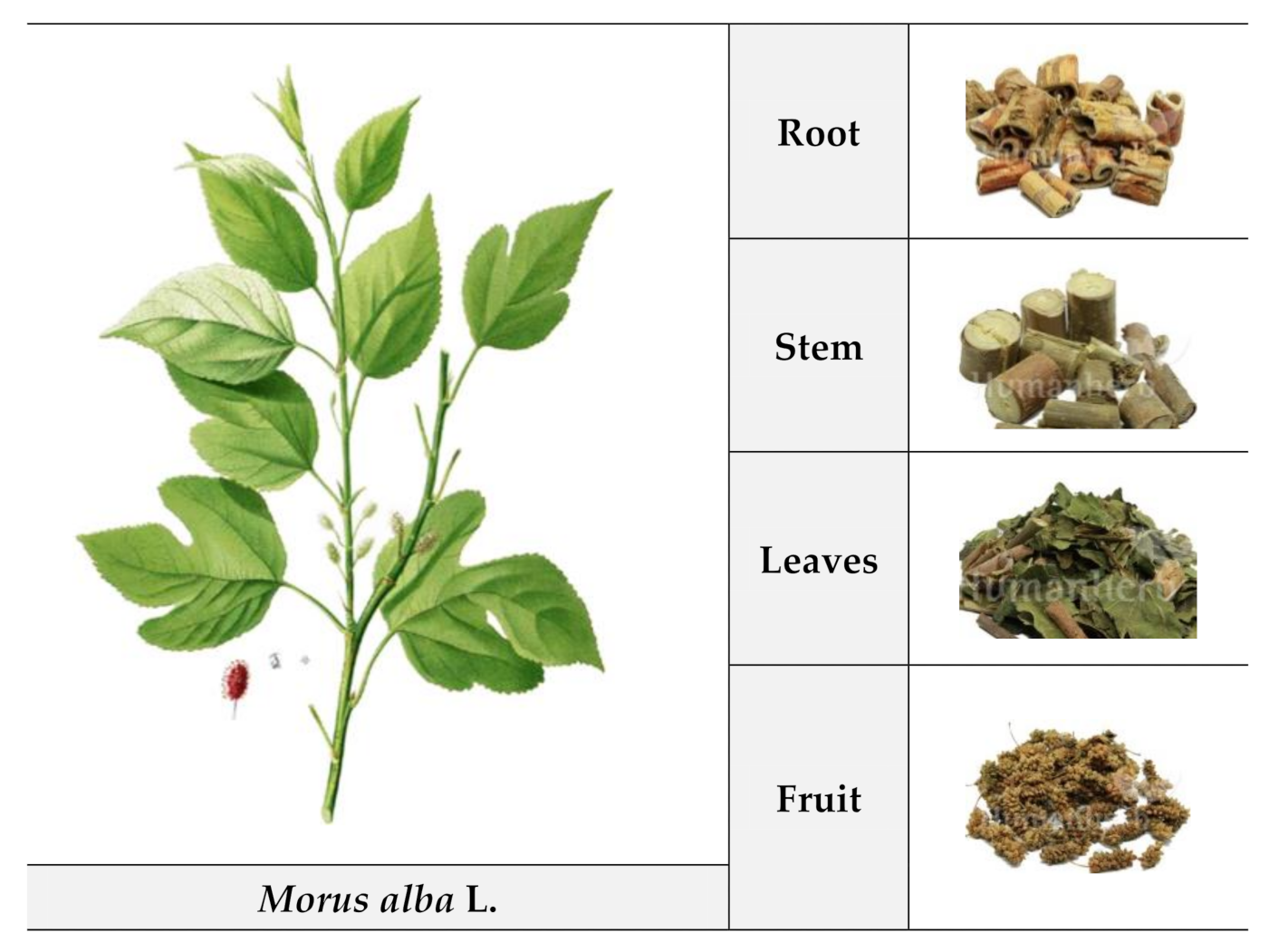
2. M. alba L. Plant Parts: Pharmacological Potential and Bioactive Phytochemical Composition
2.1. Root
2.2. Stem
2.3. Leaf
2.4. Fruit
3. M. alba L. Extraction Solvents and Their Pharmacological Potential
4. Immune Stimulation by Maturity of M. alba Fruits
5. Immune Stimulation by M. alba Bioactive Phytochemicals
5.1. Polysaccharides
5.2. Alkaloids
6. The Mechanism of Action of M. alba Fruit Extract on the Immune System
6.1. The Immune Stimulation
6.2. Host Defense
6.3. Inflammatory Response
7. Safety of M. alba Extracts
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farshid, M.A.; Fazeli, M.; Shomali, T.; Nazifi, S.; Namazi, F. Protective effect of black mulberry (Morus nigra L.) fruit hydroalcoholic extract against testosterone-induced benign prostatic hyperplasia in rats. Rev. Int. Androl. 2021, 19, 53–61. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Dhanyalakshmi, K.H.; Nataraja, K.N. Mulberry (Morus spp.) has the features to treat as a potential perennial model system. Plant Signal. Behav. 2018, 13, e1491267. [Google Scholar] [CrossRef]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-N.; Park, S.; Jeon, H.-J.; Kim, H.-B.; Nam, J.-H. Reduction of adenovirus 36-induced obesity and inflammation by mulberry extract. Microbiol. Immunol. 2014, 58, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, H.J.; Bucheli, P.; Zhang, P.F.; Wei, D.Z.; Lu, Y.H. Phytochemical profiles of different mulberry (Morus sp.) species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.R.; Kim, H.S.; Yun, M.J.; Lee, H.K.; Kim, Y.J.; Kim, S.Y.; Lee, M.K.; Hong, J.T.; Kim, Y.; Han, S.B. Promoting effect of polysaccharide isolated from Mori fructus on dendritic cell maturation. Food Chem. Toxicol. 2013, 51, 411–418. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry Fruit Extract Affords Protection against Ethyl Carbamate-Induced Cyto-toxicity and Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of Maturity on Phenolics (Phenolic Acids and Flavonoids) Profile of Strawberry Cultivars and Mulberry Species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2014, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Jun, H. Principles and Practice of Eastern Medicine; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2009. [Google Scholar]
- Yoon, S.; Kim, H. Donguibogam; Donguibogam Publishing Company: Seoul, Korea, 2006; pp. 297–2189. [Google Scholar]
- Kwon, O.-M.; Cha, W.-S.; Park, S.-Y.; Oh, J.-H.; Ahn, S.-W. The appropriation of donguibogam and bencao gangmu and the shaping of distinctive Korean medicine in the late joseon dynasty. Korean J. Orient. Med. 2011, 17, 17–24. [Google Scholar]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef] [PubMed]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-P.; Ou, T.-T.; Wang, C.-J. Mulberry (Sang Shèn Zǐ) and Its Bioactive Compounds, the Chemoprevention Effects and Molecular Mechanisms In Vitro and In Vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Słonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef]
- Wu, J.N. Chinese Materia Medica; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.D.C.A.; Freitas, K.D.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Li, S.-W.; Yan, Q.; Hu, X.-P.; Li, L.-Y.; Zhou, H.; Pan, L.-X.; Li, J.; Shen, C.-P.; Xu, T. Natural products, extracts and formulations comprehensive therapy for the improvement of motor function in alcoholic liver disease. Pharmacol. Res. 2019, 150, 104501. [Google Scholar] [CrossRef]
- Abakushina, E.V.; Gelm, Y.V.; Pasova, I.A.; Bazhin, A.V. Immunotherapeutic Approaches for the Treatment of Colorectal Cancer. Biochemistry 2019, 84, 720–728. [Google Scholar] [CrossRef]
- Chang, B.Y.; Kim, S.B.; Lee, M.K.; Park, H.; Kim, S.Y. Improved Chemotherapeutic Activity by Morus alba Fruits through Immune Response of Toll-Like Receptor 4. Int. J. Mol. Sci. 2015, 16, 24139–24158. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, H.; Li, B.; Ma, R.; Zhang, N.; Zhang, X.; Jiao, L.; Wu, W. Systematic fractionation and immunoenhancement of water-soluble polysaccharides isolated from fruit of Morus alba L. Int. J. Biol. Macromol. 2018, 116, 1056–1063. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.-B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2012, 145, 393–396. [Google Scholar] [CrossRef]
- Chen, X.; Sheng, Z.; Qiu, S.; Yang, H.; Jia, J.; Wang, J.; Jiang, C. Purification, characterization and in vitro and in vivo immune enhancement of polysaccharides from mulberry leaves. PLoS ONE 2019, 14, e0208611. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cheon, J.M.; Choi, E.-O.; Jeong, J.W.; Lee, K.W.; Kim, K.Y.; Kim, S.G.; Kim, S.; Hong, S.H.; Park, C.; et al. The Immunomodulatory Activity of Mori folium, the Leaf of Morus alba L. in RAW 264.7 Macrophages In Vitro. J. Cancer Prev. 2016, 21, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yang, H.; Jia, J.; Chen, Y.; Wang, J.; Chen, H.; Jiang, C. Mulberry leaf polysaccharide supplementation contributes to enhancing the respiratory mucosal barrier immune response in Newcastle disease virus—Vaccinated chicks. Poult. Sci. 2020, 100, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Han, S.B.; Lee, K.H.; Lee, C.W.; Kim, C.Y.; Lee, E.J.; Huh, H. Immunomodulating activity of a polysaccharide isolated from mori cortex radicis. Arch. Pharmacal Res. 2000, 23, 240–242. [Google Scholar] [CrossRef]
- Yu, J.S.; Lim, S.H.; Lee, S.R.; Choi, C.I.; Kim, K.H. Antioxidant and Anti-Inflammatory Effects of White Mulberry (Morus alba L.) Fruits on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2021, 26, 920. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Nascimento, M.F.; Ferreira, M.R.; Moura, D.F.; Souza, T.G.; Silva, G.C.; Ramos, E.H.; Paiva, P.M.; Medeiros, P.L.; Silva, T.G.; et al. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. J. Ethnopharmacol. 2016, 194, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Jiménez, F.J.; Ruiz-Malagón, A.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Lozano-Sánchez, J.; Arráez-Román, D.; Cenis, J.L.; Lozano-Pérez, A.A.; et al. Comparative Study of the Antioxidant and Anti-Inflammatory Effects of Leaf Extracts from Four Different Morus alba Genotypes in High Fat Diet-Induced Obesity in Mice. Antioxidants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kim, J.E.; Park, J.J.; Choi, J.Y.; Song, B.R.; Choi, Y.W.; Kim, D.S.; Kim, K.M.; Song, H.K.; Hwang, D.Y. Protective role of fermented mulberry leave extract in LPS-induced inflammation and autophagy of RAW264.7 macrophage cells. Mol. Med. Rep. 2020, 22, 4685–4695. [Google Scholar] [CrossRef] [PubMed]
- Bharani, S.E.R.; Asad, M.; Dhamanigi, S.S.; Chandrakala, G.K. Immunomodulatory activity of methanolic extract of Morus alba Linn. (mulberry) leaves. Pak. J. Pharm. Sci. 2010, 23, 63–68. [Google Scholar]
- Soonthornsit, N.; Pitaksutheepong, C.; Hemstapat, W.; Utaisincharoen, P.; Pitaksuteepong, T. In Vitro Anti-Inflammatory Activity of Morus alba L. Stem Extract in LPS-Stimulated RAW 264.7 Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, S.; Devi, U.; Kumar, V.R.; Kumar, V.; Anwar, F.; Kaithwas, G. Dual inhibition of arachidonic acid pathway by mulberry leaf extract. Inflammopharmacology 2015, 23, 65–70. [Google Scholar] [CrossRef]
- Yiemwattana, I.; Kaomongkolgit, R.; Wirojchanasak, S.; Chaisomboon, N. Morus alba Stem Extract Suppresses Matrix Metalloproteinases (MMP)-1, MMP-9, and Tissue Inhibitors of Metalloproteinase (TIMP)-1 Expression via Inhibition of Ikappa Balpha Degradation Induced by Porphyromonas gingivalis LPS Signal in THP-1 Cells. Eur. J. Dent. 2019, 13, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Tien, Y.J.; Chen, C.H.; Beltran, F.N.; Amor, E.C.; Wang, R.J.; Wu, D.J.; Mettling, C.; Lin, Y.L.; Yang, W.C. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 2013, 13, 45. [Google Scholar] [CrossRef] [Green Version]
- Wongwat, T.; Srihaphon, K.; Pitaksutheepong, C.; Boonyo, W.; Pitaksuteepong, T. Suppression of inflammatory mediators and matrix metalloproteinase (MMP)-13 by Morus alba stem extract and oxyresveratrol in RAW 264.7 cells and C28/I2 human chondrocytes. J.Tradit. Complementary Med. 2020, 10, 132–140. [Google Scholar] [CrossRef]
- Kavitha, Y.; Geetha, A. Anti-inflammatory and preventive activity of white mulberry root bark extract in an experimental model of pancreatitis. J. Tradit. Complement. Med. 2018, 8, 497–505. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Fu, X.; Liu, R.H. A novel polysaccharide isolated from mulberry fruits (Murus alba L.) and its selenide derivative: Structural characterization and biological activities. Food Funct. 2016, 7, 2886–2897. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Fu, X. Fructus mori L. polysaccharide-iron chelates formed by self-embedding with iron(iii) as the core exhibit good antioxidant activity. Food Funct. 2019, 10, 3150–3160. [Google Scholar] [CrossRef]
- Gui, Z.; Raman, S.T.; Ganeshan, A.K.P.G.; Chen, C.; Jin, C.; Li, S.-H.; Chen, H.-J. In vitro and In vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.). Pharmacogn. Mag. 2016, 12, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and Antioxidant Effects and Phytochemicals of Mulberry Fruit (Morus alba L.) Polyphenol Enhanced Extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef]
- Liao, B.-Y.; Zhu, D.-Y.; Thakur, K.; Li, L.; Zhang, J.-G.; Wei, Z.-J. Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.). Molecules 2017, 22, 2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ann, J.-Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Lu, T.; Wang, M.; Zou, X.; Zhang, Y.; Yang, X.; Dong, Y.; Zhou, H. Flavonoids from Morus alba L. Leaves: Opti-mization of Extraction by Response Surface Methodology and Comprehensive Evaluation of Their Antioxidant, Antimi-crobial, and Inhibition of alpha-Amylase Activities through Analytical Hierarchy Process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, B.; Hossain, M.; Rashid, M.; Sadik, G.; Nasrin, S.; et al. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Res. Notes 2013, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-S.; Ji, H.D.; Rhee, M.H.; Sung, Y.-Y.; Yang, W.-K.; Kim, S.H.; Kim, H.-K. Antiplatelet Activity ofMorus albaLeaves Extract, Mediated via Inhibiting Granule Secretion and Blocking the Phosphorylation of Extracellular-Signal-Regulated Kinase and Akt. Evid. Based Complement. Altern. Med. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Park, S.W.; Shin, K.C.; Yoou, S.-K.; Park, H.J.; Eun, S.H.; Bae, Y.M.; Lee, H.M.; Chae, H.-J.; Chae, S.-W.; Choi, B.H. Effects of an ethanolic extract of mulberry fruit on blood pressure and vascular remodeling in spontaneous hypertensive rats. Clin. Exp. Hypertens. 2018, 41, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Qian, L.; Zhou, X.; Gao, Q.; Bruce, I.; Xia, Q. Endothelium-independent relaxation and contraction of rat aorta induced by ethyl acetate extract from leaves of Morus alba (L.). J. Ethnopharmacol. 2008, 120, 442–446. [Google Scholar] [CrossRef]
- Arraki, K.; Totoson, P.; Attia, R.; Zedet, A.; Pudlo, M.; Messaoud, C.; Demougeot, C.; Girard, C. Arginase inhibitory properties of flavonoid compounds from the leaves of Mulberry (Morus alba, Moraceae). J. Pharm. Pharmacol. 2020, 72, 1269–1277. [Google Scholar] [CrossRef]
- Zheng, X.-K.; Cao, Y.-G.; Ke, Y.-Y.; Zhang, Y.-L.; Li, F.; Gong, J.-H.; Zhao, X.; Kuang, H.-X.; Feng, W.-S. Phenolic constituents from the root bark of Morus alba L. and their cardioprotective activity in vitro. Phytochemistry 2017, 135, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Paudel, K.R. Vascular Protection by Ethanol Extract of Morus alba Root Bark: Endothelium-Dependent Relaxation of Rat Aorta and Decrease of Smooth Muscle Cell Migration and Proliferation. Evid. Based Complement. Altern. Med. 2018, 2018, 7905763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamatake, Y.; Shibata, M.; Nagai, M. Pharmacological studies on root bark of mulberry tree (Morus alba L.). Jpn. J. Pharmacol. 1976, 26, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Yang, H.; Yoo, Y.-M.; Hong, S.S.; Lee, N.; Lee, H.-J.; Lee, H.-J.; Myung, C.-S.; Choi, K.-C.; Jeung, E.-B. Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease. J. Atheroscler. Thromb. 2012, 19, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarikaphuti, A.; Nararatwanchai, T.; Hashiguchi, T.; Ito, T.; Thaworanunta, S.; Kikuchi, K.; Oyama, Y.; Maruyama, I.; Tancharoen, S. Preventive effects of Morus alba L. anthocyanins on diabetes in Zucker diabetic fatty rats. Exp. Ther. Med. 2013, 6, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.-S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef]
- Hunyadi, A.; Veres, K.; Danko, B.; Kele, Z.; Wéber, E.; Hetenyi, A.; Zupko, I.; Hsieh, T.-J. In Vitro Anti-diabetic Activity and Chemical Characterization of an Apolar Fraction of Morus alba Leaf Water Extract. Phytother. Res. 2012, 27, 847–851. [Google Scholar] [CrossRef]
- Assiri, A.M.; Elbeeh, M.; Amin, A.H.; Ramadan, M.F. Ameliorative impact of Morus alba leaves’ aqueous extract against embryonic ophthalmic tissue malformation in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017, 95, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Bong, H.Y.; Jeong, H.I.; Kim, Y.K.; Kim, J.Y.; Kwon, O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr. Res. Pr. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo an-ti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; El-Twab, S.M.A.; Abdel-Reheim, E.S. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: The underlying mechanism. Eur. J. Nutr. 2016, 56, 1671–1684. [Google Scholar] [CrossRef]
- Król, E.; Jeszka-Skowron, M.; Krejpcio, Z.; Flaczyk, E.; Wójciak, R.W. The Effects of Supplementary Mulberry Leaf (Morus alba) Extracts on the Trace Element Status (Fe, Zn and Cu) in Relation to Diabetes Management and Antioxidant Indices in Diabetic Rats. Biol. Trace Element Res. 2016, 174, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunyadi, A.; Liktor-Busa, E.; Márki, A.; Martins, A.; Jedlinszki, N.; Hsieh, T.-J.; Báthori, M.; Hohmann, J.; Zupkó, I. Metabolic Effects of Mulberry Leaves: Exploring Potential Benefits in Type 2 Diabetes and Hyperuricemia. Evid. Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singab, A.N.B.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef]
- Kim, E.-S.; Park, S.-J.; Lee, E.-J.; Kim, B.-K.; Huh, H.; Lee, B.-J. Purification and characterization of Moran 20K from Morus alba. Arch. Pharm. Res. 1999, 22, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kongstad, K.T.; Jager, A.K.; Nielsen, J.; Staerk, D. Quadruple high-resolution al-pha-glucosidase/alpha-amylase/PTP1B/radical scavenging profiling combined with high-performance liquid chromatography-high-resolution mass spectrometry-solid-phase extraction-nuclear magnetic resonance spectroscopy for identification of antidiabetic constituents in crude root bark of Morus alba L. J. Chromatogr. A 2018, 1556, 55–63. [Google Scholar]
- Salimi, F.; Moradi, M.; Tajik, H.; Molaei, R. Optimization and characterization of eco-friendly antimicrobial nanocellulose sheet prepared using carbon dots of white mulberry (Morus alba L.). J. Sci. Food Agric. 2020, 101, 3439–3447. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Mesquita Mda, S.; da Silva, G.C.; de Oliveira Lima, E.; de Medeiros, P.L.; Paiva, P.M.; de Souza, I.A.; Napoleao, T.H. Evaluation of Toxicity and Antimicrobial Activity of an Ethanolic Extract from Leaves of Morus alba L. (Moraceae). Evidence-based complementary and alternative medicine. eCAM 2015, 2015, 513978. [Google Scholar]
- Bauomy, A.A. The Potential Role of Morus alba Leaves Extract on the Brain of Mice Infected with Schistosoma Mansoni. CNS Neurol. Disord. Drug Targets 2014, 13, 1513–1519. [Google Scholar] [CrossRef]
- Sheikhlar, A.; Alimon, A.R.; Daud, H.; Saad, C.R.; Webster, C.D.; Meng, G.Y.; Ebrahimi, M. White Mulberry (Morus alba) Foliage Methanolic Extract Can Alleviate Aeromonas hydrophila Infection in African Catfish (Clarias gariepinus). Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, B.; Khan, S.N.; Haque, I.; Alam, M.; Mushfiq, M.; Khan, A.U. Novel anti-adherence activity of mulberry leaves: Inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J. Antimicrob. Chemother. 2008, 62, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Yu, J.S.; Lee, S.R.; Hwang, G.S.; Kang, K.S.; Park, J.G.; Kim, H.Y.; Kim, K.H.; Yamabe, N. Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2018, 19, 1117. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Park, J.Y.; Hyun, K.K.; Lee, S.O.; Ryu, J.-Y.; Choi, S.-Z.; Kang, K.S.; Yamabe, N.; Kim, K.H. Odisolane, a Novel Oxolane Derivative, and Antiangiogenic Constituents from the Fruits of Mulberry (Morus alba L.). J. Agric. Food Chem. 2016, 64, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Dabili, S.; Fallah, S.; Aein, M.; Vatannejad, A.; Panahi, G.; Fadaei, R.; Moradi, N.; Shojaii, A. Survey of the effect of doxorubicin and flavonoid extract of white Morus alba leaf on apoptosis induction in a-172 GBM cell line. Arch. Physiol. Biochem. 2019, 125, 136–141. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Inoue, T.; Chan, H.T. Phenolic constituents and anticancer properties of Morus alba (white mulberry) leaves. J. Integr. Med. 2020, 18, 189–195. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- Min, T.R.; Park, H.J.; Park, M.N.; Kim, B. The Root Bark of Morus alba L. Suppressed the Migration of Human Non-Small-Cell Lung Cancer Cells through Inhibition of Epithelial-Mesenchymal Transition Mediated by STAT3 and Src. Int. J. Mol. Sci. 2019, 20, 2244. [Google Scholar] [CrossRef] [Green Version]
- Eo, H.J.; Park, J.H.; Park, G.H.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Anti-inflammatory and anti-cancer activity of mulberry (Morus alba L.) root bark. BMC Complement. Altern. Med. 2014, 14, 200. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Nihei, M.; Nagai, H.; Fukushi, H.; Tabata, K.; Suzuki, T.; Akihisa, T. Albanol A from the Root Bark of Morus alba L. Induces Apoptotic Cell Death in HL60 Human Leukemia Cell Line. Chem. Pharm. Bull. 2010, 58, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.-L.; Park, S.-Y.; Kang, S.; Park, D.; Kim, S.-H.; Um, J.-Y.; Jang, H.-J.; Lee, J.-H.; Jeong, C.-H.; Jang, J.-H.; et al. Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells. Am. J. Cancer Res. 2014, 5, 289–299. [Google Scholar]
- Hu, D.; Bao, T.; Lu, Y.; Su, H.; Ke, H.; Chen, W. Polysaccharide from Mulberry Fruit (Morus alba L.) Protects against Palmitic-Acid-Induced Hepatocyte Lipotoxicity by Activating the Nrf2/ARE Signaling Pathway. J. Agric. Food Chem. 2019, 68, 13016–13024. [Google Scholar] [CrossRef] [PubMed]
- Pel, P.; Chae, H.-S.; Nhoek, P.; Kim, Y.-M.; Chin, Y.-W. Chemical Constituents with Proprotein Convertase Subtilisin/Kexin Type 9 mRNA Expression Inhibitory Activity from Dried Immature Morus alba Fruits. J. Agric. Food Chem. 2017, 65, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Ignatowicz, E.; Flaczyk, E.; Przeor, M.; Kurpik, M.; Liebert, J.J. Protective Effect of Morus alba Leaf Extract on N-Nitrosodiethylamine-induced Hepatocarcinogenesis in Rats. In Vivo 2016, 30, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.-W.; Yang, T.-Y.; Teng, C.-S.; Lee, Y.-J.; Yu, M.-H.; Lee, H.-J.; Hsu, L.-S.; Wang, C.-J. Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals. Int. J. Med. Sci. 2021, 18, 53–64. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Fermented mulberry (Morus alba) leaves suppress high fat diet-induced hepatic steatosis through amelioration of the inflammatory response and autophagy pathway. BMC Complement. Med. Ther. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, J.; Chen, Q.; Liu, M.; Wang, S.; Yu, H.; Zhang, Y.; Wang, T. Regulation effects of total flavonoids in Morus alba L. on hepatic cholesterol disorders in orotic acid induced NAFLD rats. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Lin, W.L.; Chang, C.K.; Lee, K.C.; Tung, S.Y.; Kuo, H.C. Protective Effects of Morus Root Extract (MRE) Against Lipopolysaccharide-Activated RAW264.7 Cells and CCl4-Induced Mouse Hepatic Damage. Cell. Physiol. Biochem. 2018, 51, 1376–1388. [Google Scholar] [CrossRef]
- Seo, K.H.; Lee, D.Y.; Jeong, R.H.; Lee, D.S.; Kim, Y.E.; Hong, E.K.; Kim, Y.C.; Baek, N.I. Neuroprotective effect of prenylated arylbenzofuran and flavonoids from Morus alba fruits on glutamate-induced oxidative injury in HT22 hippo-campal cells. J. Med. Food 2015, 18, 403–408. [Google Scholar] [CrossRef]
- Liu, D.; Du, D. Mulberry Fruit Extract Alleviates Cognitive Impairment by Promoting the Clearance of Amyloid-β and Inhibiting Neuroinflammation in Alzheimer’s Disease Mice. Neurochem. Res. 2020, 45, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Yoo, J.-M.; Li, F.Y.; Baek, S.Y.; Kim, M.R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr. Neurosci. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhang, T.; Wang, X.; Hamann, M.T.; Kang, J.; Yu, D.-Q.; Chen, R.-Y. A Chemical Investigation of the Leaves of Morus alba L. Molecules 2018, 23, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.V.; Nade, V.S. Anti-dopaminergic effect of the methanolic extract of Morus alba L. leaves. Indian J. Pharmacol. 2008, 40, 221–226. [Google Scholar] [PubMed] [Green Version]
- Kang, T.H.; Oh, H.R.; Jung, S.M.; Ryu, J.H.; Park, M.W.; Park, Y.K.; Kim, S.Y. Enhancement of Neuroprotection of Mulberry Leaves (Morus alba L.) Prepared by the Anaerobic Treatment against Ischemic Damage. Biol. Pharm. Bull. 2006, 29, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebai, O.; Belkhir, M.; Boujelben, A.; Fattouch, S.; Amri, M. Morus alba leaf extract mediates neuroprotection against glyphosate-induced toxicity and biochemical alterations in the brain. Environ. Sci. Pollut. Res. 2017, 24, 9605–9613. [Google Scholar] [CrossRef] [Green Version]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharmacal Res. 2017, 40, 338–349. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef]
- Peng, C.-H.; Lin, H.-T.; Chung, D.-J.; Huang, C.-N.; Wang, C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; You, J.; Lee, H.; Baek, N.; Hwang, J. Kuwanon G: An antibacterial agent from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003, 84, 181–185. [Google Scholar] [CrossRef]
- Jung, H.W.; Kang, S.Y.; Kang, J.S.; Kim, A.R.; Woo, E.-R.; Park, Y.-K. Effect of Kuwanon G Isolated from the Root Bark of Morus alba on Ovalbumin-induced Allergic Response in a Mouse Model of Asthma. Phytother. Res. 2014, 28, 1713–1719. [Google Scholar] [CrossRef]
- Yiemwattana, I.; Chaisomboon, N.; Jamdee, K. Antibacterial and Anti-inflammatory Potential of Morus alba Stem Extract. Open Dent. J. 2018, 12, 265–274. [Google Scholar] [CrossRef]
- Kuete, V.; Fozing, D.; Kapche, W.; Mbaveng, A.; Kuiate, J.; Ngadjui, B.; Abegaz, B. Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J. Ethnopharmacol. 2009, 124, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zu, Y.; Fu, Y.; Efferth, T. In Vitro Antioxidant and Antimicrobial Activity of Extracts from Morus alba L. Leaves, Stems and Fruits. Am. J. Chin. Med. 2012, 40, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, M. New triterpenoids from Morus alba L. stem bark. Nat. Prod. Res. 2013, 27, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Khunakornvichaya, A.; Lekmeechai, S.; Pham, P.P.; Himakoun, W.; Pitaksuteepong, T.; Morales, N.P.; Hemstapat, W. Morus alba L. Stem Extract Attenuates Pain and Articular Cartilage Damage in the Anterior Cruciate Ligament Transection-Induced Rat Model of Osteoarthritis. Pharmacology 2016, 98, 209–216. [Google Scholar] [CrossRef]
- Riviere, C.; Krisa, S.; Pechamat, L.; Nassra, M.; Delaunay, J.C.; Marchal, A.; Badoc, A.; Waffo-Teguo, P.; Merillon, J.M. Polyphenols from the stems of Morus alba and their inhibitory activity against nitric oxide production by lipopolysaccha-ride-activated microglia. Fitoterapia 2014, 97, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Herke, I.; Veres, K.; Erdei, A.; Simon, A.; Tóth, G. Volatile Glycosides from the Leaves of Morus alba with a Potential Contribution to the Complex Anti-diabetic Activity. Nat. Prod. Commun. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.M.; Thomas, J. Polyphenolic constituents in mulberry leaf extract (M. latifolia L. cv. BC259) and its antidiabetic effect in streptozotocin induced diabetic rats. Pak. J. Pharm. Sci. 2019, 32, 69–74. [Google Scholar]
- Nakagawa, K.; Ogawa, K.; Higuchi, O.; Kimura, T.; Miyazawa, T.; Hori, M. Determination of iminosugars in mulberry leaves and silkworms using hydrophilic interaction chromatography–tandem mass spectrometry. Anal. Biochem. 2010, 404, 217–222. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Terashima, S.; Shimizu, M.; Namba, T. Two New 2-Arylbenzofuran Derivatives from Hypoglycemic Activity-Bearing Fractions of Morus insignis. Chem. Pharm. Bull. 1993, 41, 1238–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Techa, S.; Chung, J.S. Ecdysteroids Regulate the Levels of Molt-Inhibiting Hormone (MIH) Expression in the Blue Crab, Callinectes sapidus. PLoS ONE 2015, 10, e0117278. [Google Scholar] [CrossRef]
- Eo, H.; Lim, Y. Combined Mulberry Leaf and Fruit Extract Improved Early Stage of Cutaneous Wound Healing in High-Fat Diet-Induced Obese Mice. J. Med. Food 2016, 19, 161–169. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef]
- Cheigh, C.-I.; Yoo, S.-Y.; Ko, M.-J.; Chang, P.-S.; Chung, M.-S. Extraction characteristics of subcritical water depending on the number of hydroxyl group in flavonols. Food Chem. 2015, 168, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Somekawa, L.; Maegawa, H.; Tsukada, S.; Nakamura, T. Establishment of Application Guidance for OTC non-Kampo Crude Drug Extract Products in Japan. J. Intercult. Ethnopharmacol. 2017, 6, 333–338. [Google Scholar] [CrossRef]
- Amakura, Y. Characterization of Phenolic Constituents from Ephedra Herb Extract. Yakugaku zasshi. J. Pharm. Soc. Jpn 2017, 137, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, B.; Manipal, S.; Duraisamy, P.; Ahmed, A.; Mohanaganesh, S.; Jeevika, C. Role of Aqueous Extract of Morinda Citrifolia (Indian Noni) Ripe Fruits in Inhibiting Dental Caries-Causing Streptococcus Mutans and Streptococcus Mitis. J. Dent. (Tehran, Iran) 2014, 11, 703–710. [Google Scholar]
- Polumackanycz, M.; Sledzinski, T.; Goyke, E.; Wesolowski, M.; Viapiana, A. A Comparative Study on the Phenolic Composition and Biological Activities of Morus alba L. Commercial Samples. Molecules 2019, 24, 3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Chen, J.; Liu, D.; Zhang, Y.; Jiang, P.; Ye, X. Minerals, Phenolic Compounds, and Antioxidant Capacity of Citrus Peel Extract by Hot Water. J. Food Sci. 2007, 73, C11–C18. [Google Scholar] [CrossRef]
- Fung, F.M.; Su, M.; Feng, H.-T.; Li, S.F.Y. Extraction, separation and characterization of endotoxins in water samples using solid phase extraction and capillary electrophoresis-laser induced fluorescence. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, V. Initial and Bulk Extraction of Natural Products Isolation. Nat. Prod. Isol. 2012, 864, 27–41. [Google Scholar] [CrossRef]
- Chang, B.; Koo, B.; Lee, H.; Oh, J.S.; Kim, S. Activation of macrophage mediated host defense against Salmonella typhi-murium by Morus alba L. Food Nutr. Res. 2018, 62, 1289. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-Y.; Park, G.-S.; Lee, M.H.; Chang, I.A.; Kim, Y.C.; Kim, S.Y.; Lee, J.Y.; Yun, Y.G.; Park, H. Toll-like receptor 4-mediated immunoregulation by the aqueous extract of Mori Fructus. Phytother. Res. 2009, 23, 1713–1720. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, G.; Chen, W.; Xu, Y.; Wu, J. Quantification and Purification of Mulberry Anthocyanins with Macroporous Resins. J. Biomed. Biotechnol. 2004, 2004, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, H.; Han, Q.; Lan, J.; Chen, G.; Cao, G.; Yang, C. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2019, 104, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Cai, Z.; Song, L.; Liu, Y.; Wang, Q.; Feng, X. Gynostemma pentaphyllum Attenuates the Progression of Nonalcoholic Fatty Liver Disease in Mice: A Biomedical Investigation Integrated with In Silico Assay. Evid. Based Complement. Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Lee, J.; Jung, S.; Kim, J.W.; Shin, J.-H.; Lee, H.-J. The involvement of ginseng berry extract in blood flow via regulation of blood coagulation in rats fed a high-fat diet. J. Ginseng Res. 2016, 41, 120–126. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramudya, M.; Wahyuningsih, S.P.A. Immunomodulatory potential of polysaccharides from Coriolus versicolor against intracellular bacteria Neisseria gonorrhoeae. Veter. World 2019, 12, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2015, 7, 530–539. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, bioac-tivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Synytsya, A.; Capek, P.; Bleha, R.; Pohl, R.; Park, Y.I. Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr. Polym. 2016, 146, 187–196. [Google Scholar] [CrossRef]
- Seydoux, E.; Liang, H.; Cauwelaert, N.D.; Archer, M.; Rintala, N.D.; Kramer, R.; Carter, D.; Fox, C.B.; Orr, M.T. Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways to Promote Potent Adaptive Immune Responses. J. Immunol. 2018, 201, 98–112. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Molteni, M.; Bosi, A.; Rossetti, C. Natural Products with Toll-Like Receptor 4 Antagonist Activity. Int. J. Inflamm. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Carrillo, J.L.; Contreras-Cordero, J.F.; Gutiérrez-Coronado, O.; Villalobos-Gutiérrez, P.T.; Ramos-Gracia, L.G.; Hernández-Reyes, V.E. Cytokine Profiling Plays a Crucial Role in Activating Immune System to Clear Infectious Pathogens. In Immune Response Activation and Immunomodulation; Tyagi, R., Bisen, P.S., Eds.; IntechOpen: London, UK, 2019; pp. 1–30. [Google Scholar] [CrossRef] [Green Version]
- Paulina Pathria, T.L.L.; Varner, J.A. Targeting Tumor—Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef]
- Juhas, U.; Ryba-Stanislawowska, M.; Szargiej, P.; Mysliwska, J. Different pathways of macrophage activation and polarization. Postepy higieny i medycyny doswiadczalnej. Postep. Hig. Med. Dosw. 2015, 69, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, K.; Mosser, D.M. Purinergic Signaling to Terminate TLR Responses in Macrophages. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Lennartz, M.; Drake, J. Molecular mechanisms of macrophage Toll-like receptor—Fc receptor synergy. F1000 Res. 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stunault, M.I.; Bories, G.; Guinamard, R.R.; Ivanov, S. Metabolism Plays a Key Role during Macrophage Activation. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Balasubramanian, N.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds. Molecules 2019, 24, 1809. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.A.; Cunningham, D.G.; Marles, R. Assessment of herbal medicinal products: Challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010, 243, 198–216. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nature reviews. Immunology 2015, 15, 104–116. [Google Scholar]
- Marx, T.K.; Glávits, R.; Endres, J.R.; Palmer, P.A.; Clewell, A.E.; Murbach, T.S.; Hirka, G.; Pasics, I. A 28-Day Repeated Dose Toxicological Study of an Aqueous Extract of Morus alba L. Int. J. Toxicol. 2016, 35, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.Y.; Kim, S.B.; Lee, M.K.; Park, H.; Kim, S.Y. Nonclinical Safety Assessment of Morus alba L. Fruits: Study of 90-D Toxicity in Sprague Dawley Rats and Genotoxicity in Salmonella. J. Food Sci. 2016, 81, T1328–T1335. [Google Scholar] [CrossRef] [PubMed]




| Composition | M. alba (White Mulberry) | M. nigra (Black Mulberry) | Reference |
|---|---|---|---|
| Ash (mg/100 g DW) | 0.57 ± 0.11 | 0.50 ± 0.08 | [10,16] |
| Protein(mg/100 g DW) | 1.55 ± 0.30 | 0.96 ± 0.16 | [10] |
| Total sugar (g/100 g FW) | 7.55 ± 1.01 | 6.64 ± 1.12 | [10] |
| Reducing sugar (g/100 g FW) | 5.90 ± 0.92 | 4.94 ± 0.73 | [10] |
| Pectin (g/100 g FW) | Not detected | 0.76 ± 0.03 | [10] |
| Riboflavin (g/100 g FW) | 0.88 ± 0.00 | 0.04 ± 0.00 | [10] |
| Niacin (mg/100 g FW) | 3.10 ± 0.60 | 1.60 ± 0.10 | [10] |
| Total phenols (mg/100 g FW) | 1650 ± 12.25 | 880 ± 7.20 | [10,11] |
| Alkaloid (mg/100 g FW) | 660 ± 5.25 | 630 ± 5.93 | [10] |
| Total anthocyanins content(C3G µg/g frozen weight) | 911.8 | 719 | [17] |
| Pharmacological Effect | Part | Solvents | Extract or Phytochemical | Reference | Type (Species) |
|---|---|---|---|---|---|
| Immune enhancing | Fruit | Water | Crude extract | [25] | in vitro and in vivo (mouse) |
| Polysaccharide | [7,26] | in vitro | |||
| Methanol | Pyrrol alkaloid Morrole A | [27] | in vitro | ||
| Leaf | Water | Polysaccharide | [28,29,30] | in vitro and in vivo (mouse, chicken) | |
| Root | Water | Polysaccharide | [31] | in vitro | |
| Immune inhibiting | Fruit | Ethanol | Crude extract | [32] | in vitro |
| Leaf | Ethanol | Crude extract | [32,33,34,35] | in vivo | |
| Methanol | Crude extract | [36] | in vivo (mouse) | ||
| Stem | Ethanol | Crude extract | [37,38,39] | in vitro | |
| Oxyresveratrol | [40] | in vitro | |||
| Root | Methanol | Kuwanon G | [41] | in vivo (mouse) | |
| Cudraflavone B | [42] | in vivo (mouse) | |||
| Antioxidant | Fruit | Water | Polysaccharide | [43,44] | in vitro |
| Ethanol | Crude extract | [45] | in vitro | ||
| Methanol | Hydroxycinnamic acid esters, Flavonol glycosides, andAnthocyanins | [46] | in vitro | ||
| Ethyl acetate | Crude extract | [47] | in vitro | ||
| Leaf | Water | Polysaccharide | [48] | in vitro | |
| Ethanol | Crude extract | [29,34,49,50] | in vitro | ||
| Root | Methanol | Phenolic contents | [51] | in vitro | |
| Cardiovascular system protection | Leaf | Ethanol | Crude extract | [52,53] | in vitro, in vivo (Rat) |
| Ethyl acetate | Crude extract | [54] | Ex vivo (Rat) | ||
| petroleum ether, dichloromethane, ethanol | Crude extract | [55] | in vitro | ||
| Root | Water | Moracinoside C, Moracin O, Moracin P | [56] | in vitro | |
| Ethanol | Crude extract | [57] | in vitro, in vivo (Rat) | ||
| Methanol | Crude extract | [58] | in vitro | ||
| Morusinol | [59] | in vitro, in vivo (Rat) | |||
| Antidiabetic | Fruit | Water | Crude extract | [43] | in vitro |
| Ethanol | Crude extract | [47,60] | in vitroin vivo (Rat) | ||
| Methanol | Crude extractantocyanins | [61] | in vitro, in vivo (Mouse) | ||
| Leaf | Water | Crude extract | [62,63,64] | in vitro, in vivo (Mouse) | |
| Ethanol | Crude extract | [34,65,66,67] | in vitro, in vivo (Rat, Mouse) | ||
| Methanol | chlorogenic acid, rutin, isoquercitrin loliolide,1-deoxynojirimycin, fagomine 2-O-alpha-D-galactopyranosyl-1-deoxynojirimycin | [68] | in vitro, in vivo (Rat, Mouse) | ||
| Root | Ethanol | Crude extract | [69] | in vitro, in vivo (Rat) | |
| Methanol | Moran K | [70] | in vitro | ||
| Ethyl acetate | Crude extract | [71] | in vitro | ||
| Antibacterial | Fruit | Water | Crude extract | [72] | in vitro |
| Leaf | Ethanol | Crude extract | [73,74,75] | in vitro, in vivo (Rat) | |
| 1-deoxynojirimycin | [76] | in vitro | |||
| Stem | Ethanol | Crude extract | [38] | in vitro | |
| Anticancer | Fruit | Ethanol | Butyl pyroglutamate quercetin, 3-O-β-D-glucoside kaempferol, 3-O-β-D-rutinoside, rutin, and 2-phenylethyl d-rutinoside | [77] | in vitro |
| Odisolane | [78] | in vitro | |||
| Leaf | Ethanol | Flavonoid | [79,80] | in vitro | |
| Methanol | Morin | [81] | in vitro | ||
| Root | Methylene chloride | Crude extract | [82] | in vitro | |
| Methanol | Crude extract | [83] | in vitro | ||
| Albanol A | [84] | in vitro | |||
| Morusin | [85] | in vitro | |||
| Hepato-protection | Fruits | Water | Polysaccharide | [86] | in vitro |
| Chloroform | Benzofuran, Isomoracin, N-(N-benzoyl-l-phenylalanyl)-l-phenylalanol | [87] | in vitro | ||
| Leaf | Water | Crude extract | [88,89] | in vivo (Rat) | |
| Ethanol | Crude extract | [49,90] | in vivo (mouse) | ||
| Methanol | Crude extract | [91] | in vivo (mouse) | ||
| Root | Ethanol | Crude extract | [92] | in vitro and in vivo (mouse) | |
| Neuroprotection | Fruit | Ethanol | Flavonoid | [93,94,95] | in vitro and in vivo (mouse) |
| Leaf | Ethanol | sesquiterpenoid glucoside, aromatic glucoside, farnesylacetone derivative, flavan, and (9R)-hydroxyl-(10E, 12Z,15Z)-octadecatrienoic acid | [96] | in vitro | |
| Methanol | Crude extract | [97,98] | in vivo (mouse) | ||
| Acetone | Crude extract | [99] | in vivo (Rat) | ||
| Root | Methanol | mulberrofuran G, albanol B, kuwanon G | [100] | in vitro | |
| Antiobesity | Fruit | Water | Pyrrol alkaloid | [101] | in vitro |
| Leaf | Water | Crude extract | [102] | in vivo (mouse) | |
| Ethanol | Crude extract | [49] | in vivo (mouse) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.-Y.; Koo, B.-S.; Kim, S.-Y. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods 2021, 10, 1966. https://doi.org/10.3390/foods10081966
Chang B-Y, Koo B-S, Kim S-Y. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods. 2021; 10(8):1966. https://doi.org/10.3390/foods10081966
Chicago/Turabian StyleChang, Bo-Yoon, Bong-Seong Koo, and Sung-Yeon Kim. 2021. "Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract" Foods 10, no. 8: 1966. https://doi.org/10.3390/foods10081966
APA StyleChang, B.-Y., Koo, B.-S., & Kim, S.-Y. (2021). Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods, 10(8), 1966. https://doi.org/10.3390/foods10081966





