Roles of Proteins/Enzymes from Animal Sources in Food Quality and Function
Abstract
:1. Introduction
2. Proteins in Meat
2.1. Myofibrillar Protein
2.2. Hemoglobin
2.3. Interactions between Meat Protein and Other Factors Affecting the Quality of Meat Product
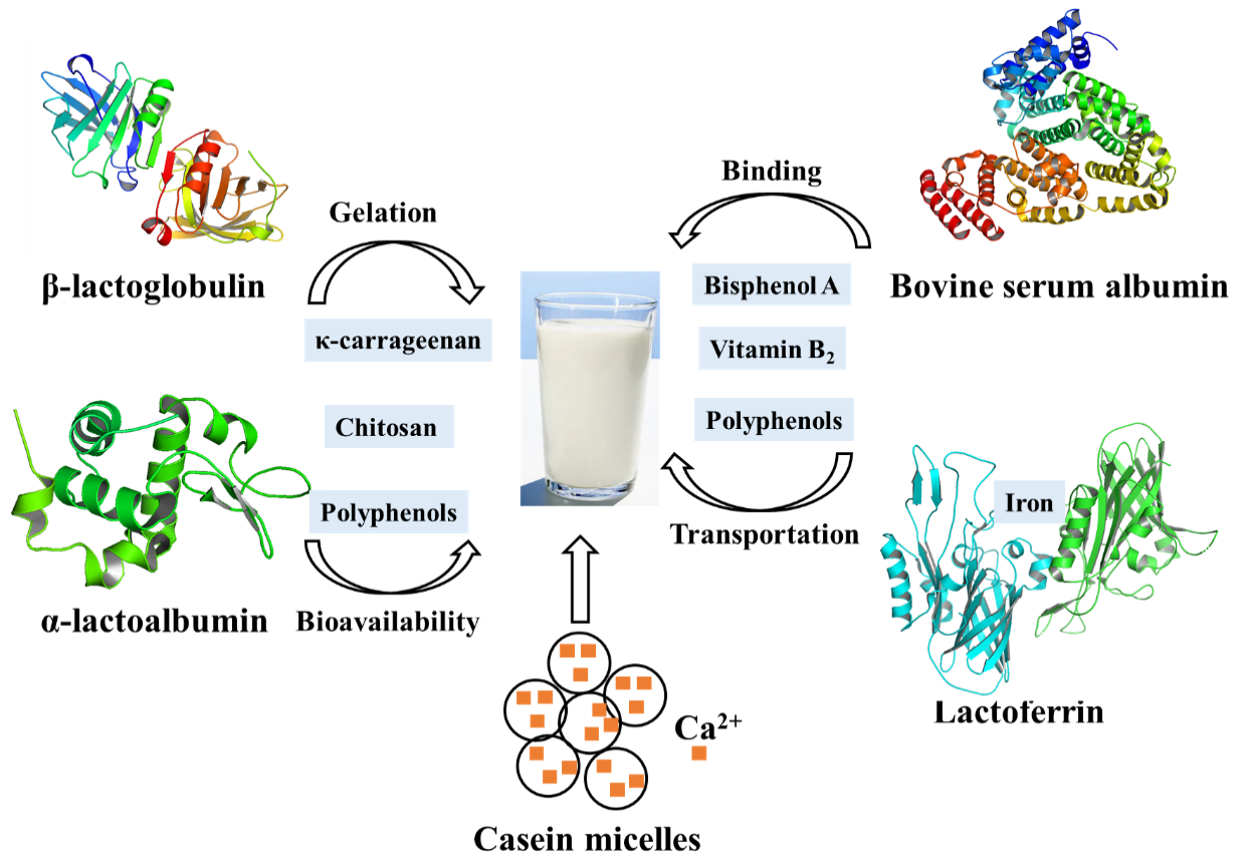
3. Proteins in Milk
3.1. Whey Protein
3.2. Bovine Serum Albumin
3.3. Casein
3.4. Lactoferrin
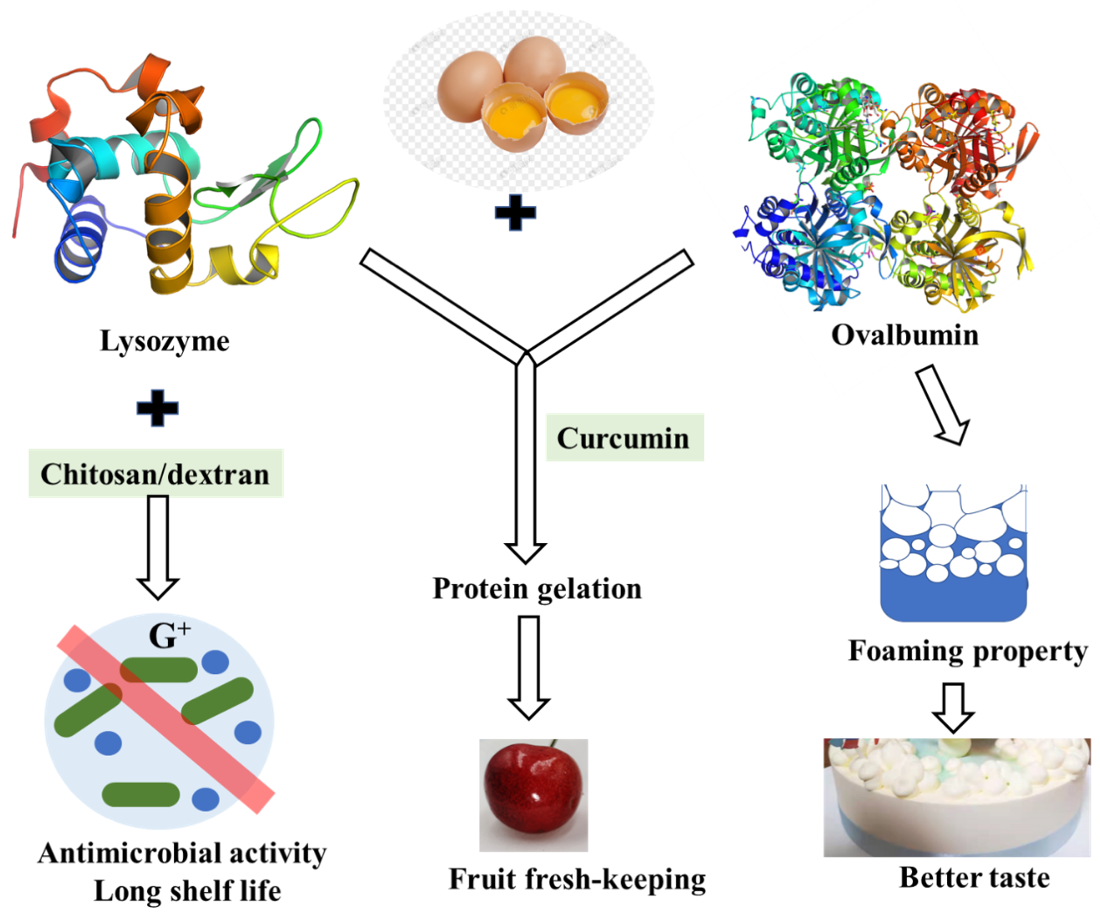
4. Proteins in Egg
4.1. Lysozyme
4.2. Ovalbumin
5. Protein in Fishery Products
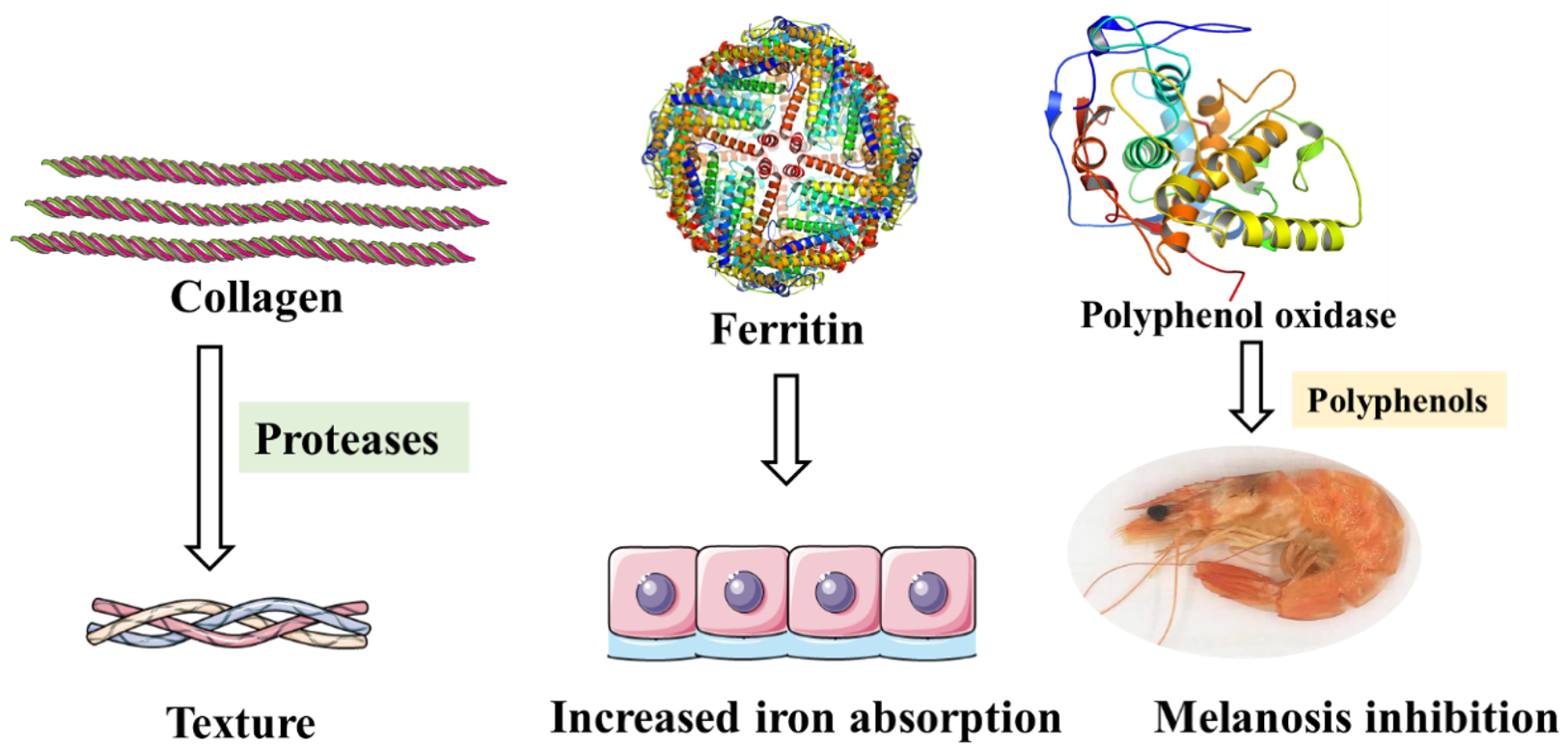
5.1. Collagen
5.2. Polyphenol Oxidase
5.3. Ferritin
5.4. Crustacyanin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barness, L.A. Dietary guidelines. Arch. Pediatr. Adolesc. Med. 1979, 133, 868. [Google Scholar] [CrossRef]
- Abbate, J.M.; Macrì, F.; Capparucci, F.; Iaria, C.; Briguglio, G.; Cicero, L.; Salvo, A.; Arfuso, F.; Ieni, A.; Piccione, G.; et al. Administration of protein hydrolysates from anchovy (Engraulis encrasicolus) waste for twelve weeks decreases metabolic dysfunction—Associated fatty liver disease severity in ApoE−/−mice. Animals 2020, 10, 2303. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Macrì, F.; Arfuso, F.; Iaria, C.; Capparucci, F.; Anfuso, C.; Ieni, A.; Cicero, L.; Briguglio, G.; Lanteri, G. Anti-Atherogenic Effect of 10% Supplementation of Anchovy (Engraulis encrasicolus) Waste Protein Hydrolysates in ApoE-Deficient Mice. Nutrients 2021, 13, 2137. [Google Scholar] [CrossRef] [PubMed]
- Monteverde, V.; Congiu, F.; Vazzana, I.; Dara, S.; Pietro, S.D.; Piccione, G. Serum lipid profile modification related to polyunsaturated fatty acid supplementation in thoroughbred horses. J. Appl. Anim. Res. 2017, 45, 615–618. [Google Scholar] [CrossRef]
- Piccione, G.; Arfuso, F.; Fazio, F.; Bazzano, M.; Giannetto, C. Serum Lipid Modification Related to Exercise and Polyunsaturated Fatty Acid Supplementation in Jumpers and Thoroughbred Horses. J. Equine. Vet. Sci. 2014, 34, 1181–1187. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhou, G. Covalent chemical modification of myofibrillar proteins to improve their gelation properties: A systematic review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 924–959. [Google Scholar] [CrossRef]
- Chen, B.; Guo, J.; Xie, Y.; Zhou, K.; Li, P.; Xu, B. Modulating the aggregation of myofibrillar protein to alleviate the textural deterioration of protein gels at high temperature: The effect of hydrophobic interactions. Food Chem. 2021, 341, 128274. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- De Souza, H.K.; Bai, G.; Gonçalves, M.D.P.; Bastos, M. Whey protein isolate–chitosan interactions: A calorimetric and spectroscopy study. Thermochim. Acta 2009, 495, 108–114. [Google Scholar] [CrossRef]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of human lactoferrin: Crystallographic structure analysis and refinement at 2.8 Å resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Fei, X.; Wang, S.; Gao, H. Binding of bisphenol A and acrylamide to BSA and DNA: Insights into the comparative interactions of harmful chemicals with functional biomacromolecules. J. Hazard. Mater. 2010, 182, 877–885. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Yang, K.; Wu, D.; Ma, J.; Wang, S.; Zhang, Y.; Sun, W. Radio frequency heating improves water retention of pork myofibrillar protein gel: An analysis from water distribution and structure. Food Chem. 2021, 350, 129265. [Google Scholar] [CrossRef]
- Dai, Y.; Miao, J.; Yuan, S.; Liu, Y.; Li, X.; Dai, R. Colour and sarcoplasmic protein evaluation of pork following water bath and ohmic cooking. Meat Sci. 2013, 93, 898–905. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Guo, J.; Huang, H.; Wang, L.; Wu, W.; Xiong, G.; Sun, W. Water distribution and textual properties of heat-induced pork myofibrillar protein gel as affected by sarcoplasmic protein. LWT—Food Sci. Technol. 2019, 103, 308–315. [Google Scholar] [CrossRef]
- Astruc, T. Connective tissue: Structure, function, and influence on meat quality. In Encyclopedia of Meat Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 321–328. [Google Scholar]
- Toldrà, M.; Lynch, S.A.; Couture, R.; Álvarez, C. Blood proteins as functional ingredients. Sustain. Meat Prod. Process. 2019, 85–101. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Valenzuela, C.; Brito, A.; Weinborn, V.; Flores, S.; Arredondo, M. The effect of proteins from animal source foods on heme iron bioavailability in humans. Food Chem. 2016, 196, 733–738. [Google Scholar] [CrossRef]
- Pares, D.; Toldra, M.; Saguer, E.; Carretero, C. Scale-up of the process to obtain functional ingredients based in plasma protein concentrates from porcine blood. Meat Sci. 2014, 96, 304–310. [Google Scholar] [CrossRef]
- Komeroski, M.R.; Homem, R.V.; Schmidt, H.; Rockett, F.C.; Lira, L.; Farias, D.; Kist, T.L.; Doneda, D.; Rios, A.; Oliveira, V.R. Effect of whey protein and mixed flours on the quality parameters of gluten-free breads. Int. J. Gastron. Food Sci. 2021, 24, 100361. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT—Food Sci. Technol. 2021, 149, 111904. [Google Scholar] [CrossRef]
- Torres, I.C.; Amigo, J.M.; Knudsen, J.C.; Tolkach, A.; Mikkelsen, B.; Ipsen, R. Rheology and microstructure of low-fat yoghurt produced with whey protein microparticles as fat replacer. Int. Dairy J. 2018, 81, 62–71. [Google Scholar] [CrossRef]
- Sharma, L.G.; Pandey, L.M. Thermomechanical process induces unfolding and fibrillation of bovine serum albumin. Food Hydrocoll. 2020, 112, 106294. [Google Scholar]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-casein: Isolation, properties and functionality. A review. Int. Dairy J. 2016, 66, 115–125. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Li, C.; Dai, T.; Chen, J.; Li, X.; Li, T.; Liu, C.; Mcclements, D.J. Protein-polyphenol functional ingredients: The foaming properties of lactoferrin are enhanced by forming complexes with procyanidin. Food Chem. 2021, 339, 128145. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, e1–e301. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Guo, X.N.; Gao, F.; Zhu, K.X. Effect of fresh egg white addition on the quality characteristics and protein aggregation of oat noodles. Food Chem. 2020, 330, 127319. [Google Scholar] [CrossRef]
- Sang, S.; Xu, D.; Ma, Y.; Jin, Y.; Wu, F.; Xu, X. Effect of egg yolk on the properties of wheat dough and bread. Food Biosci. 2020, 37, 100689. [Google Scholar] [CrossRef]
- Li, J.; Zhai, J.; Gu, L.; Su, Y.; Gong, L.; Yang, Y.; Chang, C. Hen egg yolk in food industry—A review of emerging functional modifications and applications. Trends Food Sci. Technol. 2021, 115, 12–21. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Ye, X.; Liu, D.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019, 274, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Z.; Sun, G.; Gao, Y.; Xu, J. Ferritin, a novel vehicle for iron supplementation and food nutritional factors encapsulation. Trends Food Sci. 2015, 44, 189–200. [Google Scholar] [CrossRef]
- Montero, P.; Ávalos, A.; Pérez-Mateos, M. Characterization of polyphenoloxidase of prawns (Penaeus japonicus). Alternatives to inhibition: Additives and high-pressure treatment. Food Chem. 2001, 75, 317–324. [Google Scholar] [CrossRef]
- Nozawa, H.; Mamegoshi, S.I.; Seki, N. Partial purification and characterization of six transglutaminases from ordinary muscles of various fishes and marine invertebrates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 313–317. [Google Scholar] [CrossRef]
- Yang, N.; Fan, X.; Yu, W.; Huang, Y.; Yu, C.; Konno, K.; Dong, X. Effects of microbial transglutaminase on gel formation of frozen-stored longtail southern cod (patagonotothen ramsayi) mince. LWT—Food Sci. Technol. 2020, 128, 109444. [Google Scholar] [CrossRef]
- Ahmed, Z.; Donkor, O.; Street, W.A.; Vasiljevic, T. Calpains- and cathepsins-induced myofibrillar changes in post-mortem fish: Impact on structural softening and release of bioactive peptides. Trends Food Sci. 2015, 45, 130–146. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Wei, Z.; Zhang, H.; Dong, M.; Huang, M.; Han, M.; Xu, X.; Zhou, G. Modification of myofibrillar protein via glycation: Physicochemical characterization, rheological behavior and solubility property. Food Hydrocoll. 2020, 105, 105852. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Liu, Q.; Chen, Q.; Kong, B. Filamentous myosin in low-ionic strength meat protein processing media: Assembly mechanism, impact on protein functionality, and inhibition strategies. Trends Food Sci. 2021, 112, 25–35. [Google Scholar] [CrossRef]
- Zheng, H.; Han, M.; Yun, B.; Xu, X.; Zhou, G. Combination of high pressure and heat on the gelation of chicken myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2019, 52, 122–130. [Google Scholar] [CrossRef]
- Motoki, M.; Seguro, K. Transglutaminase and its use for food processing. Trends Food Sci. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of chicken myoflbrillar protein induced by transglutaminase crosslinking. J. Food Eng. 2011, 107, 226–233. [Google Scholar] [CrossRef]
- Lee, H.C.; Jang, H.S.; Kang, I.; Chin, K.B. Effect of red bean protein isolate and salt levels on pork myofibrillar protein gels mediated by microbial transglutaminase. LWT—Food Sci. Technol. 2017, 76, 95–100. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of myofibrillar/pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocoll. 2012, 27, 394–400. [Google Scholar] [CrossRef]
- Lee, H.C.; Kang, I.; Chin, K.B. Effect of mungbean [Vigna radiata (L.) Wilczek] protein isolates on the microbial transglutaminase-mediated porcine myofibrillar protein gels at various salt concentrations. Meat Sci. 2015, 101, 112. [Google Scholar] [CrossRef]
- Ramirez-Suarez, J.C.; Addo, K.; Xiong, Y.L. Gelation of mixed myofibrillar/wheat gluten proteins treated with microbial transglutaminase. Food Res. Int. 2005, 38, 1143–1149. [Google Scholar] [CrossRef]
- Niu, H.; Xia, X.; Wang, C.; Kong, B.; Liu, Q. Thermal stability and gel quality of myofibrillar protein as affected by soy protein isolates subjected to an acidic pH and mild heating. Food Chem. 2018, 242, 188–195. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, L.; Li, R.; Zheng, B.; Rea, M.C.; Miao, S. Effect of plant protein mixtures on the microstructure and rheological properties of myofibrillar protein gel derived from red sea bream (pagrosomus major). Food Hydrocoll. 2019, 96, 537–545. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Myoprotein–phytophenol interaction: Implications for muscle food structure-forming properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2801–2824. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Glucose oxidase promotes gallic acid-myofibrillar protein interaction and thermal gelation. Food Chem. 2019, 293, 529–536. [Google Scholar] [CrossRef]
- Jia, N.; Wang, L.; Shao, J.; Liu, D.; Kong, B. Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 2017, 127, 45–50. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y.L. Dual role (anti- and pro-oxidant) of gallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. J. Agric. Food Chem. 2016, 64, 3054–3061. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.A.; Carne, A.; Mcconnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Wismer-Pedersen, J. Use of haemoglobin in foods—A review. Meat Sci. 1988, 24, 31–45. [Google Scholar] [CrossRef]
- Salvador, P.; Toldra, M.; Pares, D.; Carretero, C.; Saguer, E. Color stabilization of porcine hemoglobin during spray-drying and powder storage by combining chelating and reducing agents. Meat Sci. 2009, 83, 328–333. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Kong, B.; Liu, Q.; Chang, Z.; Yang, H.; Jiang, Y. Application of glycosylated nitrosohemoglobin in meat batters: Color formation and antimicrobial activity. Adv. Mater. Res. 2012, 1915, 554–556. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Kong, B.; Liu, Q.; Yang, H.; Zhao, Y.; Jiang, Y. Effect of glycosylated nitrosohemoglobin on quality of cooked meat batters during chill storage. Adv. J. Food Sci. Technol. 2013, 5, 19–23. [Google Scholar] [CrossRef]
- Pereira, A.D.; Gomide, L.A.M.; Cecon, P.R.; Fontes, E.A.F.; Fontes, P.R.; Ramos, E.M.; Vidigal, J.G. Evaluation of mortadella formulated with carbon monoxide-treated porcine blood. Meat Sci. 2014, 97, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.R.; Gomide, L.A.M.; Fontes, E.A.F.; Ramos, E.M.; Ramos, A.L.S. Composition and color stability of carbon monoxide treated dried porcine blood. Meat Sci. 2010, 85, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhou, Y.; Lu, Y.; Lv, Y.; Chi, Y.; He, Q. Effect of Tea Polyphenols on the Oxidation and Color Stability of Porcine Hemoglobin. Food Chem. 2019, 84, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Wang, R.; Nakai, S. Preparation of Colorless Globin from Bovine Hemoglobin using Sodium Alginate. Food Sci. 1990, 55, 577–578. [Google Scholar] [CrossRef]
- Yang, J.; Lin, C. Functional properties of porcine blood globin decolourized by different methods. Int. J. Food Sci. Technol. 1998, 33, 419–427. [Google Scholar] [CrossRef]
- Silva, J.G.; Morais, H.A.; Silvestre, M.P.C. Comparative study of the functional properties of bovine globin isolates and sodium caseinate. Food Res. Int. 2003, 36, 73–80. [Google Scholar] [CrossRef]
- Shi, J.; Roos, A.; Schouten, O.; Zheng, C.; Vink, C.; Vonk, B.; Kliphuis, A.; Schaap, A.; Edens, L. Properties of hemoglobin decolorized with a histidine-specific protease. J. Food Sci. 2015, 80, 1202–1208. [Google Scholar] [CrossRef]
- Silva, J.G.; Morais, H.A.; Oliveira, A.L.; Silvestre, M.P.C. Addition effects of bovine blood globin and sodium caseinate on the quality characteristics of raw and cooked ham pté. Meat Sci. 2003, 63, 177–184. [Google Scholar] [CrossRef]
- Chen, H.; Xu, S.; Wang, Z. Interaction between flaxseed gum and meat protein. J. Food Eng. 2007, 80, 1051–1059. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G.; Biliaderis, C.G. Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. J. Agric. Food Chem. 1994, 42, 1891–1895. [Google Scholar] [CrossRef]
- Trius, A.; Sebranek, J.G.; Lanier, T. Carrageenans and their use in meat products. Crit. Rev. Food Sci. Nutr. 1996, 36, 69–85. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, X.; Li, P.; Xu, B.; Chen, C. Water holding capacity of sodium-reduced chicken breast myofibrillar protein gel as affected by combined CaCl2 and high-pressure processing. Int. J. Food Sci. Technol. 2020, 55, 601–609. [Google Scholar] [CrossRef]
- Goemaere, O.; Glorieux, S.; Govaert, M.; Steen, L.; Fraeye, I. Phosphate elimination in emulsified meat products: Impact of protein-based ingredients on quality characteristics. Foods 2021, 10, 882. [Google Scholar] [CrossRef]
- Baugreet, S.; Gomez, C.; Auty, M.A.E.; Kerry, J.P.; Hamill, R.M.; Brodkorb, A. In vitro digestion of protein-enriched restructured beef steaks with pea protein isolate, rice protein and lentil flour following sous vide processing. Innov. Food Sci. Emerg. Technol. 2019, 54, 153–161. [Google Scholar] [CrossRef]
- Chandan, R.C. Milk composition, physical and processing characteristics. Manuf. Yogurt Fermented Milks 2006, 17–40. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT—Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Tran Le, T.; Sabatino, P.; Heyman, B.; Kasinos, M.; Dinh, H.H.; Dewettinck, K.; Martins, J.; Van Der Meeren, P. Improved heat stability by whey protein–surfactant interaction. Food Hydrocoll. 2011, 25, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Thongngam, M.; McClements, D.J. Isothermal titration calorimetry study of the interactions between chitosan and a bile salt (sodium taurocholate). Food Hydrocoll. 2005, 19, 813–819. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Guo, H.; Ding, Q.; Liu, R.; Ren, F. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Wu, X.; Huang, X.; Wang, F.; Liu, S. Unfolding and refolding of bovine serum albumin induced by cetylpyridinium bromide. Biophys. J. 2005, 88, 3518–3524. [Google Scholar] [CrossRef] [Green Version]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [Green Version]
- Woodman, O.L.; Meeker, W.F.; Boujaoude, M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharmacol. 2005, 46, 302–309. [Google Scholar] [CrossRef]
- Xiao, J.; Suzuki, M.; Jiang, X.; Chen, X.; Yamamoto, K.; Ren, F.; Xu, M. Influence of B-ring hydroxylation on interactions of flavonols with bovine serum albumin. J. Agric. Food Chem. 2008, 56, 2350–2356. [Google Scholar] [CrossRef]
- Smith, C.; Halliwell, B.; Aruoma, O.I. Protection by albumin against the pro-oxidant actions of phenolic dietary components. Food Chem. Toxicol. 1992, 30, 483–489. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kim, S.; Lee, N.; Kwon, M.; Yang, I.; Kim, M.J.; Cheong, S.G.; Kwon, D.; Lee, J.Y.; Oh, H.B.; et al. Amantadine-resistant influenza A viruses isolated in South Korea from 2003 to 2009. Antivir. Res. 2009, 84, 199–202. [Google Scholar] [CrossRef]
- Holt, C. Structure and stability of bovine casein micelles. Adv. Protein Chem. 1992, 43, 63–151. [Google Scholar]
- Kumar, S.; Clarke, A.R.; Hooper, M.L.; Horne, D.S.; Law, A.J.; Leaver, J.; Springbett, A.; Stevenson, E.; Simons, J.P. Milk composition and lactation of beta-casein-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 6138–6142. [Google Scholar] [CrossRef] [Green Version]
- Udabage, P.; Mckinnon, I.A.N.; Augustin, M.A. Mineral and casein equilibria in milk: Effects of added salts and calcium-chelating agents. J. Dairy Res. 2000, 67, 361–370. [Google Scholar] [CrossRef]
- Tsuchita, H.; Suzuki, T.; Kuwata, T. The effect of casein phosphopeptides on calcium absorption from calcium-fortified milk in growing rats. Br. J. Nutr. 2001, 85, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucey, J.A.; Fox, P.F. Importance of calcium and phosphate in cheese manufacture: A review. J. Dairy Sci. 1993, 76, 1714–1724. [Google Scholar] [CrossRef]
- Lara-Castellanos, M.J.; Azuara, E.; Jimenez-Fernandez, V.M.; Luna-Solano, G.; Jimenez, M. Effect of casein replacement by modified casein on physicochemical, textural, sensorial properties and microbiological stability of fresh cheese. Int. Dairy J. 2021, 112, 104864. [Google Scholar] [CrossRef]
- Gamiz-Hernandez, A.P.; Angelova, I.N.; Send, R.; Sundholm, D.; Kaila, V.R.I. Protein-induced color shift of carotenoids in β-crustacyanin. Angew. Chem. Int. Ed. 2015, 54, 11564–11566. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.; Leibman, A. Lactoferrin and transferrin: A comparative study. Biochim. Biophys. Acta Protein Struct. 1972, 257, 314–323. [Google Scholar] [CrossRef]
- Cunningham, F.E.; Proctor, V.A.; Goetsch, S.J. Egg-white lysozyme as a food preservative: An overview. World Poult. Sci. J. 1991, 47, 141–163. [Google Scholar] [CrossRef]
- Fleming, A.; Allison, V.D. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1922, 93, 306–317. [Google Scholar]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef]
- Nakamura, S.; Kato, A.; Kobayashi, K. New antimicrobial characteristics of lysozyme-dextran conjugate. J. Agric. Food Chem. 1991, 39, 647–650. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, Q.; Geng, F.; Ma, M. Characterization of the interaction between hen egg white lysozyme and ovalbumin. Food Biosci. 2020, 36, 100674. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, L.; Xia, M.; Huang, X.; Isobe, K.; Handa, A.; Cai, Z. Role of lysozyme on liquid egg white foaming properties: Interface behavior, physicochemical characteristics and protein structure. Food Hydrocoll. 2021, 120, 106876. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part B 2021, 109, 1045–1058. [Google Scholar] [CrossRef]
- Pranata, M.P.; González-Buesa, J.; Chopra, S.; Kim, K.; Pietri, Y.; Ng, P.K.W.; Matuana, L.M.; Almenar, E. Egg white protein film production through extrusion and calendering processes and its suitability for food packaging applications. Food Bioprocess Technol. 2019, 12, 714–727. [Google Scholar] [CrossRef]
- Huang, X.; Luo, X.; Liu, L.; Dong, K.; Yang, R.; Lin, C.; Song, H.; Li, S.; Huang, Q. Formation mechanism of egg white protein/κ-carrageenan composite film and its application to oil packaging. Food Hydrocoll. 2020, 105, 105780. [Google Scholar] [CrossRef]
- Tiimob, B.J.; Rangari, V.K.; Mwinyelle, G.; Abdela, W.; Evans, P.G.; Abbott, N.; Samuel, T.; Jeelani, S. Tough aliphatic-aromatic copolyester and chicken egg white flexible biopolymer blend with bacteriostatic effects. Food Packag. Shelf Life 2018, 15, 9–16. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Simpson, B.K. Application of high hydrostatic pressure to control enzyme related fresh seafood texture deterioration. Food Res. Int. 1996, 29, 569–575. [Google Scholar] [CrossRef]
- Busconi, L.; Folco, E.J.; Martone, C.B.; Sanchez, J.J. Postmortem changes in cytoskeletal elements of fish muscle1. J. Food Biochem. 1989, 13, 443–451. [Google Scholar] [CrossRef]
- Hernández-Herrero, M.M.; Duflos, G.; Malle, P.; Bouquelet, S. Collagenase activity and protein hydrolysis as related to spoilage of iced cod (Gadus morhua). Food Res. Int. 2003, 36, 141–147. [Google Scholar] [CrossRef]
- Harper, E. Collagenases. Annu. Rev. Biochem. 1980, 49, 1063–1078. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Benjakul, S. Prevention of melanosis in crustaceans by plant polyphenols: A review. Trends Food Sci. 2019, 85, 1–9. [Google Scholar] [CrossRef]
- Calvo, M.M.; Tzamourani, A.; Martínez-Alvarez, O. Halophytes as a potential source of melanosis-inhibiting compounds.mechanism of inhibition of a characterized polyphenol extract of purslane (Portulaca oleracea). Food Chem. 2021, 355, 129649. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritin: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Orino, K.; Lehman, L.; Tsuji, Y.; Ayaki, H.; Torti, S.V.; Torti, F.M. Ferritin and the response to oxidative stress. Biochem. J. 2001, 357, 241–247. [Google Scholar] [CrossRef]
- Moroz, C.; Chetrit, A.; Kahn, M.; Modan, B. FBL blood test as a predictive marker of breast cancer in high risk women. Med. Oncol. 1997, 14, 39–42. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.; Ma, G.; Wang, A.; He, W.; Wang, P.; Liu, Y.; Liu, J.; Sun, R. Gene expression of ferritin in tissue of the pacific white shrimp, Litopenaeus vannamei after exposure to pH stress. Aquaculture 2008, 275, 356–360. [Google Scholar] [CrossRef]
- Maiti, B.; Khushiramani, R.; Tyagi, A.; Karunasagar, I.; Karunasagar, I. Recombinant ferritin protein protects penaeus monodon infected by pathogenic Vibrio harveyi. Dis. Aquat. Org. 2010, 88, 99–105. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Chang, X.; Xie, X.; Liang, Y.; Wang, K.; Zheng, W.; Liu, H. A cqferritin protein inhibits white spot syndrome virus infection via regulating iron ions in red claw crayfish cherax quadricarinatus. Dev. Comp. Immunol. 2018, 82, 104–112. [Google Scholar] [CrossRef]
- Lemonnier, H.; Wabete, N.; Pham, D.; Lignot, J.H.; Barri, K.; Mermoud, I.; Royer, F.; Boulo, V.; Laugier, T. Iron deposits turn blue shrimp gills to orange. Aquaculture 2021, 540, 736697. [Google Scholar] [CrossRef]
- Cianci, M.; Rizkallah, P.J.; Olczak, A.; Raftery, J.; Chayen, N.E.; Zagalsky, P.F.; Helliwell, J.R. The molecular basis of the coloration mechanism in lobster shell: β-crustacyanin at 3.2-resolution. Proc. Natl. Acad. Sci. USA 2002, 99, 9795–9800. [Google Scholar] [CrossRef] [Green Version]
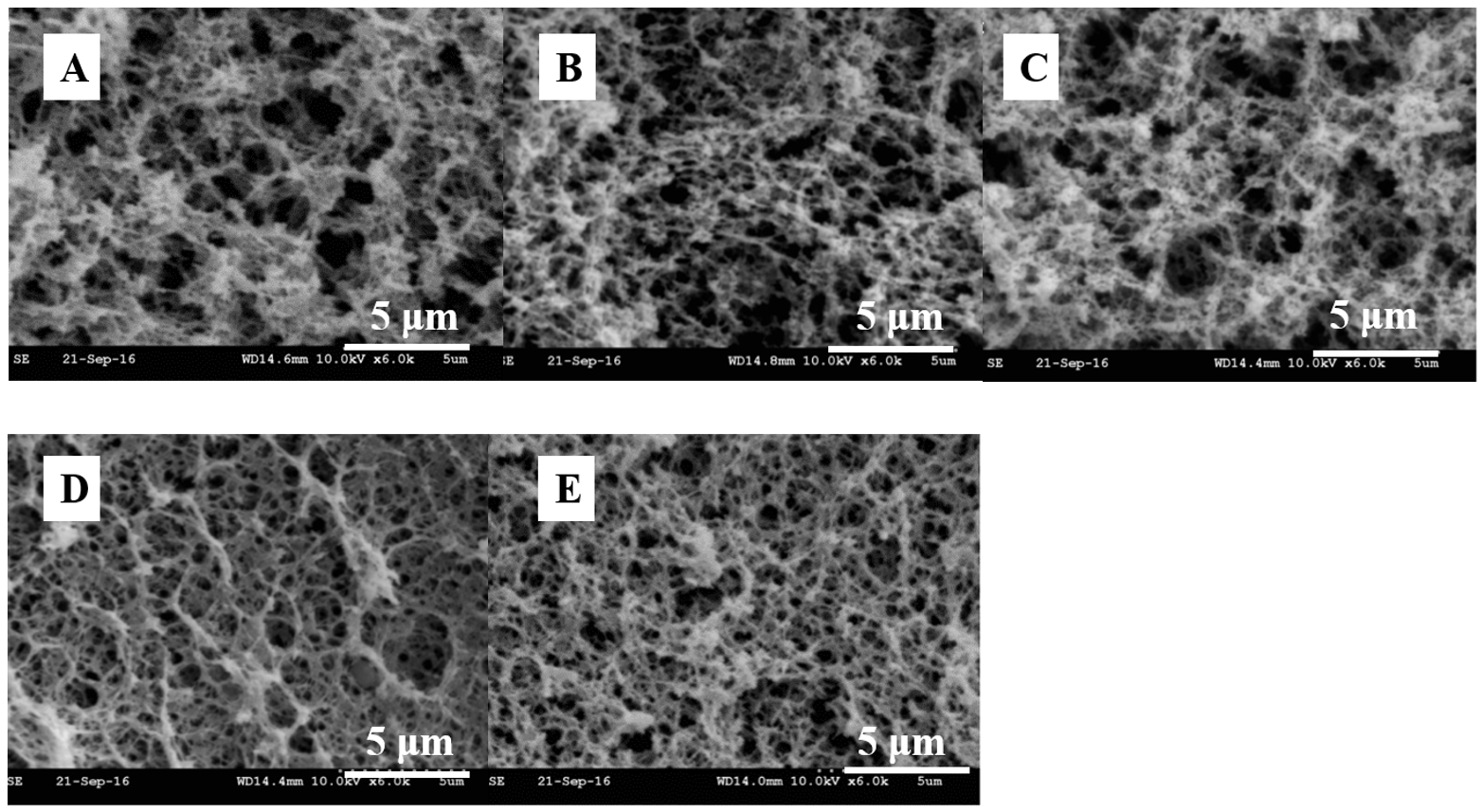

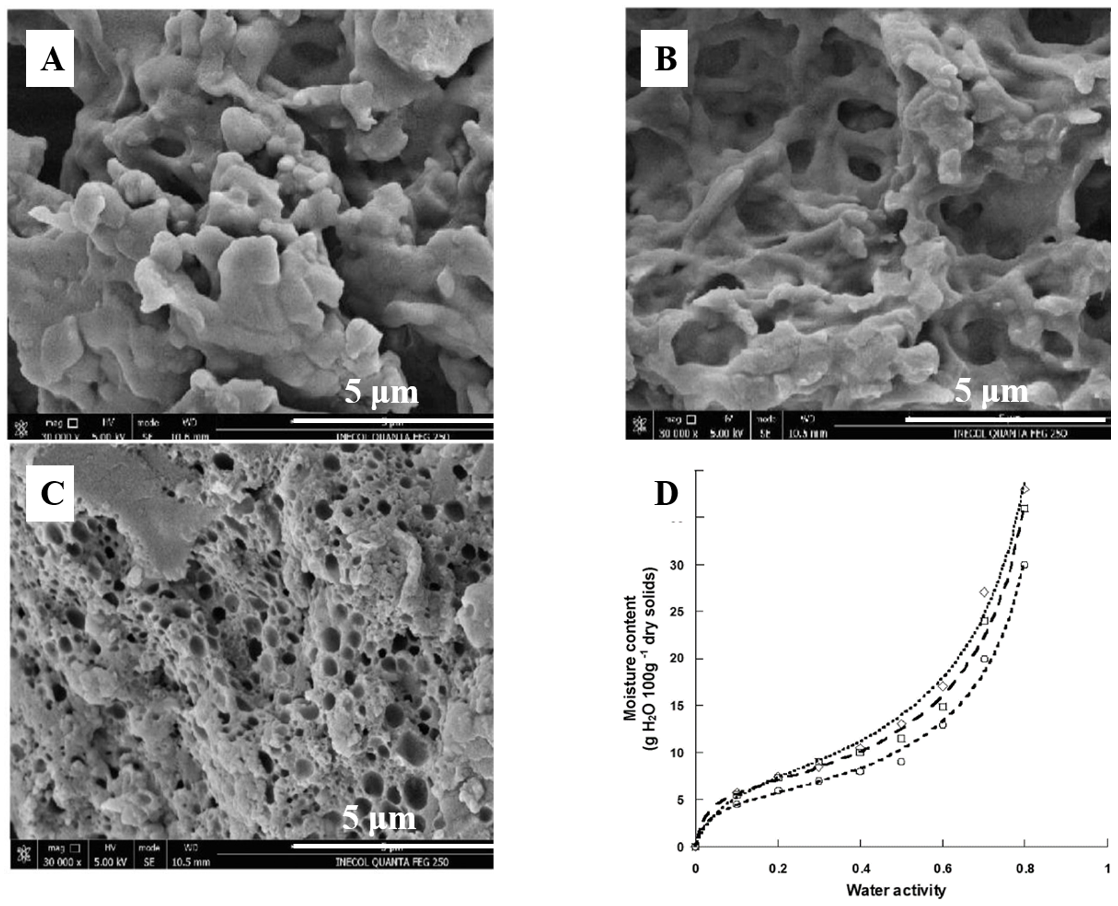

| Food Sources | Main Proteins | Relationships with Product Quality | References |
|---|---|---|---|
| Meat | Myofibrillar protein | Form heat-induced gel Related to the water retention, chewiness and juiciness of meat products. | [13,14] |
| Sarcoplasmic protein | Related to the color of meat products. Influence gel properties, such as water holding capacity. Indirectly affects meat tenderness. | [15,16] | |
| Connective tissue | Affect the texture, tenderness and water holding capacity of meat products. | [17] | |
| Hemoglobin | Soluble, foaming, emulsifying, colorant, and nutritional supplement. | [18,19] | |
| Plasma protein | Foaming, solubility, emulsifying, gelling properties, and fat binder. | [18,20] | |
| Milk | Whey protein | Emulsification, foaming, gelling, water binding properties. Used as thickener, stabilizers, and fat substitutes in yogurt. | [21,22,23] |
| Bovine serum albumin | Physiological model protein, foaming, emulsifying, gelling properties. | [24] | |
| Casein | Solubility, emulsifying, gelling, foaming, stability. Can be used to make food films. | [25,26,27] | |
| Lactoferrin | Biological activities: iron metabolism regulation, immune regulation, antibacterial, antitumor, anti-inflammatory. Can be used as iron solubilizer. | [28,29] | |
| Egg | Egg white proteins | Gelling, water holding capacity, foaming and emulsifying properties. Can be used as thickener to give the product good taste and texture. Enhance the nutritional level. Can be used as food packaging film. | [30,31] |
| Egg yolk proteins | Gelling, emulsification, film formation. Can be used as gelata, adhesive or color former to improve the nutritional value, flavor and taste of the product. | [32,33] | |
| Lysozyme | Antibacterial activity. Extend the shelf life of food. | [34] | |
| Fishery products | Collagen | Used as emulsifier, foaming agent, stabilizer and nutrients in the product. | [35,36] |
| Ferritin | Can be used as iron supplement. | [37] | |
| Polyphenol oxidase | Cause melanosis of crustaceans. | [38] | |
| Transglutaminase | Improve functional properties of proteins, such as gelation. | [39,40] | |
| Cathepsins | Promoting degradation of myofibrillar protein. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Xu, C.; Gan, J.; Jiang, Z.; Wang, Y.; Cao, X. Roles of Proteins/Enzymes from Animal Sources in Food Quality and Function. Foods 2021, 10, 1988. https://doi.org/10.3390/foods10091988
Lv C, Xu C, Gan J, Jiang Z, Wang Y, Cao X. Roles of Proteins/Enzymes from Animal Sources in Food Quality and Function. Foods. 2021; 10(9):1988. https://doi.org/10.3390/foods10091988
Chicago/Turabian StyleLv, Chenyan, Chen Xu, Jing Gan, Zhenghui Jiang, Yumeng Wang, and Xueli Cao. 2021. "Roles of Proteins/Enzymes from Animal Sources in Food Quality and Function" Foods 10, no. 9: 1988. https://doi.org/10.3390/foods10091988
APA StyleLv, C., Xu, C., Gan, J., Jiang, Z., Wang, Y., & Cao, X. (2021). Roles of Proteins/Enzymes from Animal Sources in Food Quality and Function. Foods, 10(9), 1988. https://doi.org/10.3390/foods10091988





