Anti-Inflammatory Action of Dietary Wild Olive (Acebuche) Oil in the Retina of Hypertensive Mice
Abstract
:1. Introduction
2. Materials and Methods
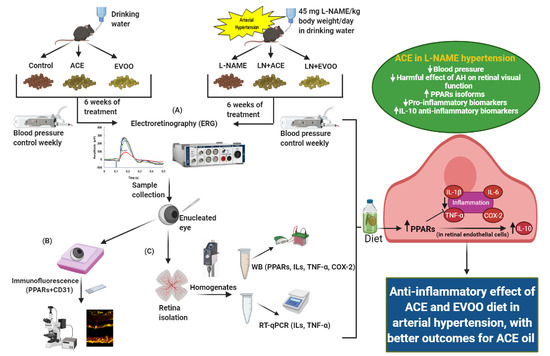
2.1. Study Design
2.2. Dietary Supplementation
2.3. Animal Characteristics
2.4. Electroretinography (ERG)
2.5. Sample Harvesting
2.6. Western Blotting Analyses
2.7. Real-Time PCR
2.8. Immunohistofluorescence
2.9. Statistical Analyses
3. Results
3.1. Validation of the Experimental Approach
3.2. Retinal Function Analyzed by Full-Field ERG
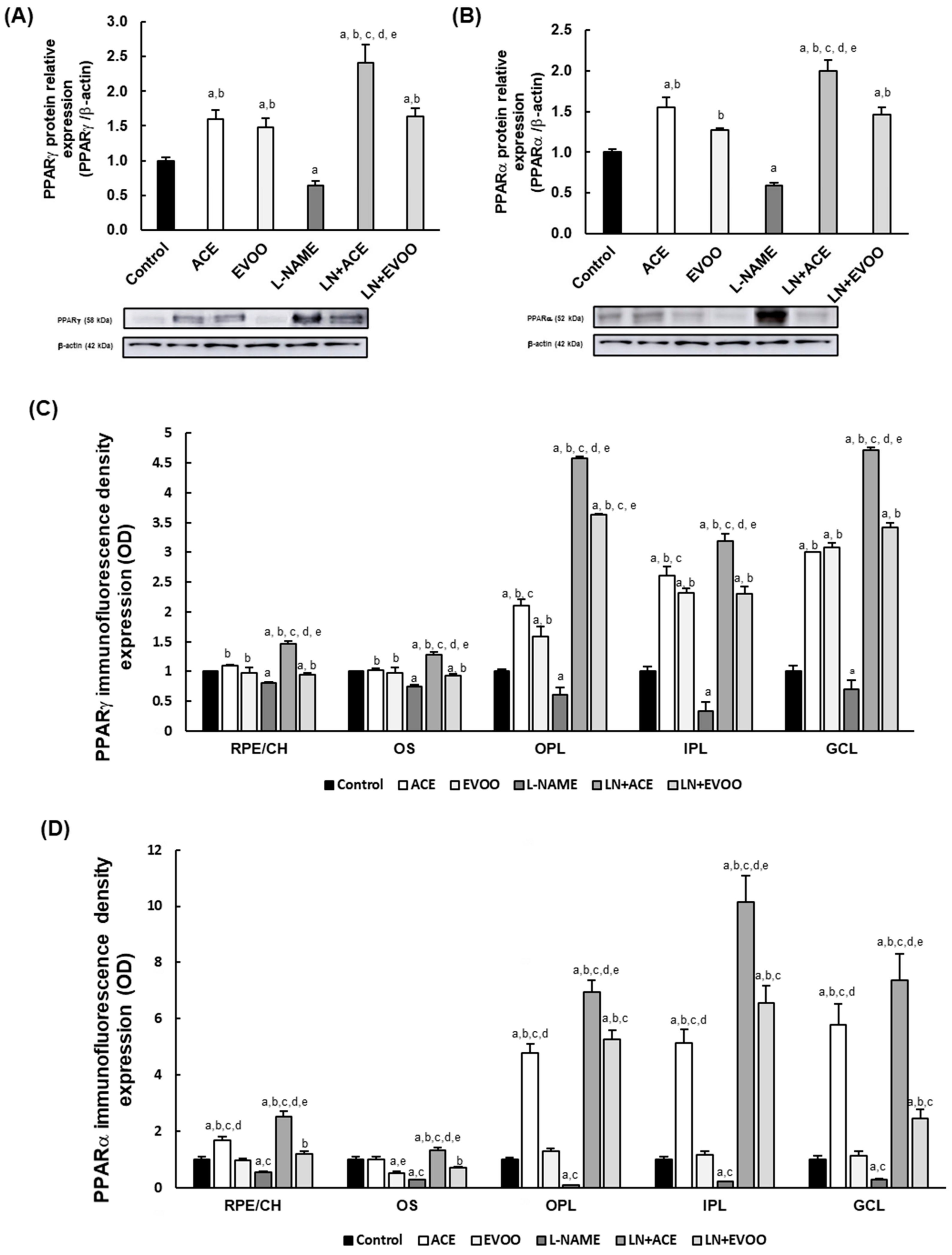
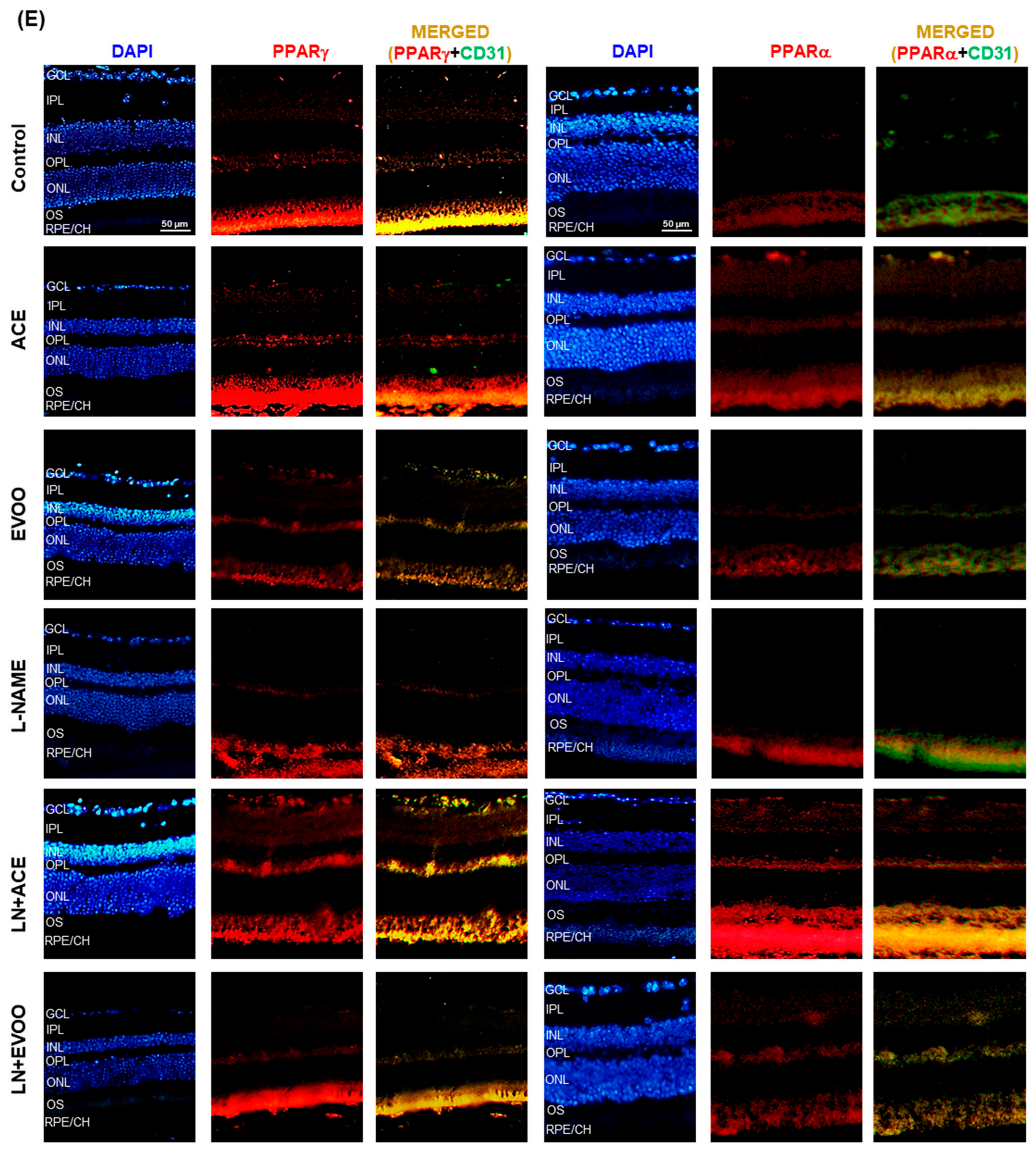
3.3. PPARs Expression in Retina Layers
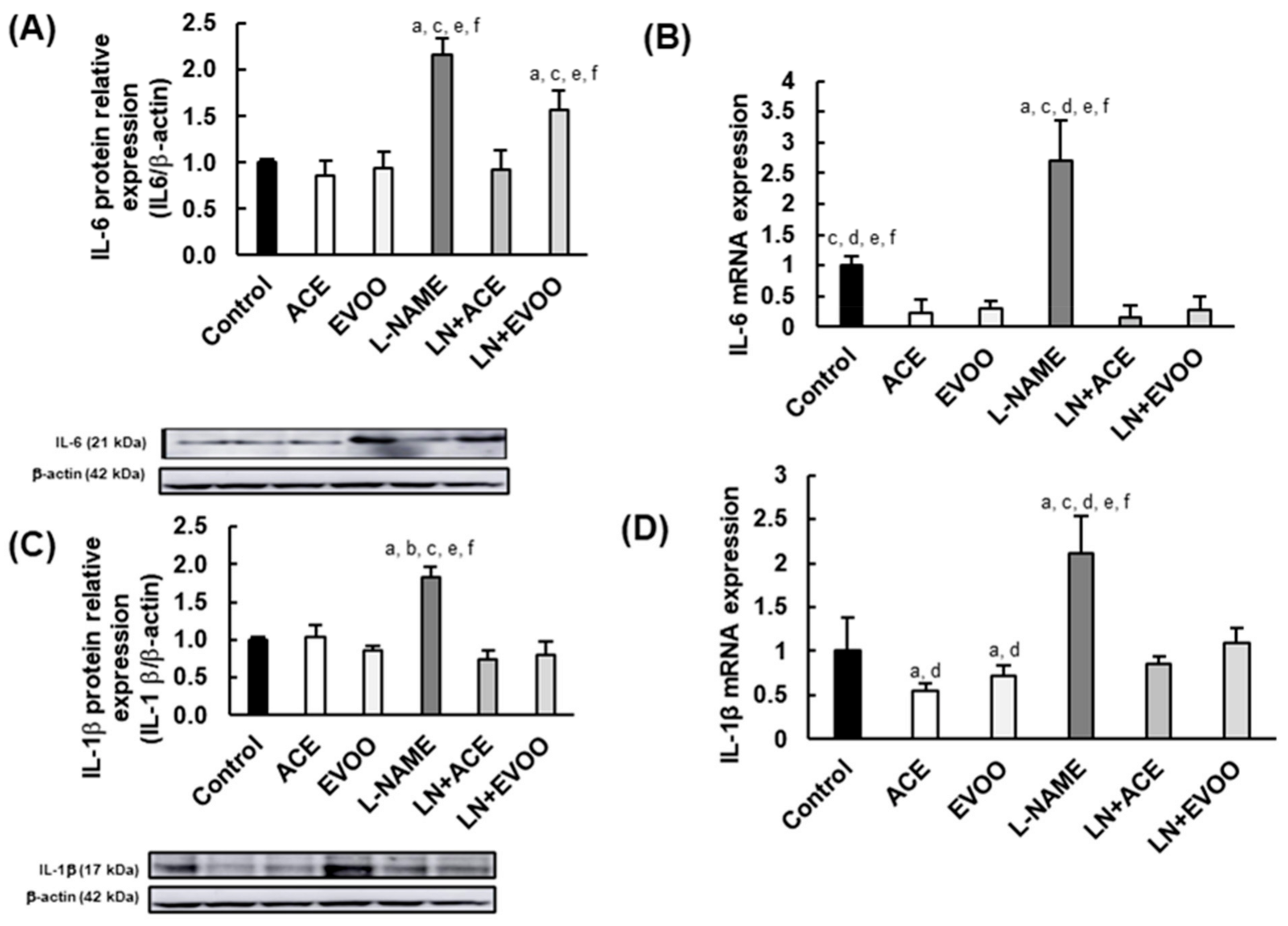
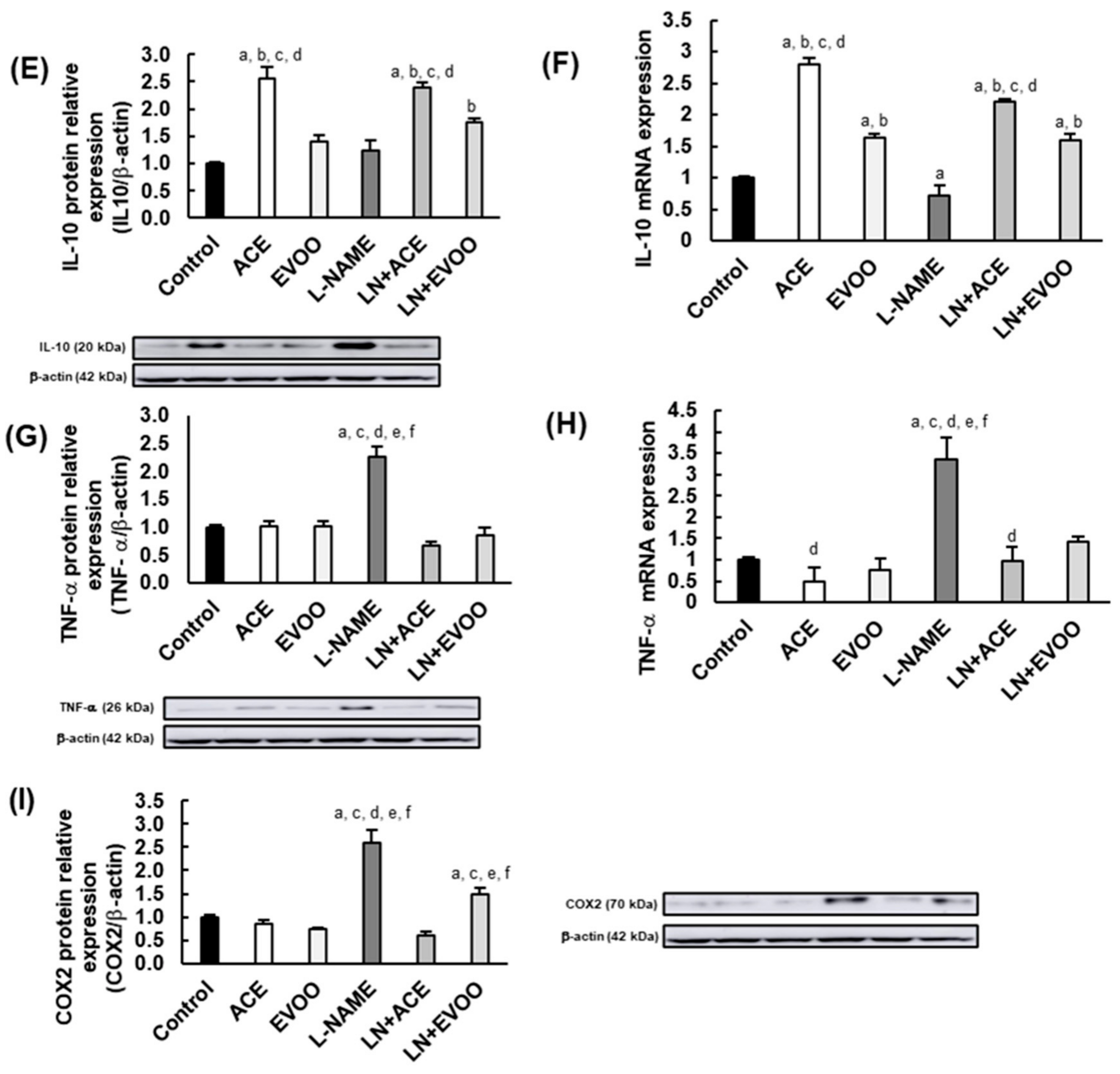
3.4. Inflammatory Biomarkers in the Retina
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef]
- Di Daniele, N.D.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A.D. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1018/2013 of 23 October 2013 amending Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods other than those referring to the reduction of disease risk and to children’s development and heal. Off. J. Eur. Union L 282 2013, 56, 43–45. Available online: http://eur-lex.europa.eu/pri/en/oj/dat/2003/l_285/l_28520031101en00330037.pdf (accessed on 26 July 2021).
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [Green Version]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Declerck, K.; Szarc vel Szic, K.; Palagani, A.; Heyninck, K.; Haegeman, G.; Morand, C.; Milenkovic, D.; Vanden Berghe, W. Epigenetic control of cardiovascular health by nutritional polyphenols involves multiple chromatin-modifying writer-reader-eraser proteins. Curr. Top. Med. Chem. 2015, 16, 788–806. [Google Scholar] [CrossRef]
- Szymańska, R.; Nowicka, B.; Kruk, J. Vitamin E—Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini-Rev. Med. Chem. 2017, 17, 1039–1052. [Google Scholar] [CrossRef]
- Pascual Fuster, V. Utilidad de los esteroles vegetales en el tratamiento de la hipercolesterolemia. Nutr. Hosp. 2017, 34, 62–67. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.d.C.A. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [Green Version]
- Genovese, A.; Mondola, F.; Paduano, A.; Sacchi, R. Biophenolic compounds influence the in-mouth perceived intensity of virgin olive oil flavours and off-flavours. Molecules 2020, 25, 1969. [Google Scholar] [CrossRef] [Green Version]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [Green Version]
- Maqueda, J.E. Estudio Analítico Comparado Entre el Aceite de Acebuchina y el Aceite de Oliva Virgen. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2005. [Google Scholar]
- Santana-Garrido, Á.; Reyes-Goya, C.; Pérez-Camino, M.C.; André, H.; Mate, A.; Vázquez, C.M.; Santana-garrido, Á.; Reyes-goya, C.; Carmen Pérez-Camino, M.; André, H.; et al. Retinoprotective effect of wild olive (Acebuche) oil- enriched diet against ocular oxidative stress induced by arterial hypertension. Antioxidants 2020, 9, 885. [Google Scholar] [CrossRef]
- Carnés Sánchez, J.; Iraola, V.M.; Sastre, J.; Florido, F.; Boluda, E.; Fernández-Caldas, L.; Carnes Sanchez, J.; Iraola, V.M.; Sastre, J.; Florido, F.; et al. Allergenicity and immunochemical characterization of six varieties of Olea europaea. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 313–318. [Google Scholar] [CrossRef]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M.; Mares, J.A.; Voland, R.P.; Sondel, S.A.; Millen, A.E.; LaRowe, T.; Moeller, S.M.; et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Cougnard-Grégoire, A.; Merle, B.M.J.; Rougier, J.F.K.M.B.; Delyfer, M.N.; Goff, M.L.; Samieri, C.; Dartigues, J.F.; Delcourt, C. Olive oil consumption and age-related macular degeneration: The alienor study. PLoS ONE 2016, 11, e0160240. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Rodríguez-Pérez, M.D.; Márquez-Estrada, L.; López-Villodres, J.A.; Reyes, J.J.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; De La Cruz, J.P. Neuroprotective Effect of Hydroxytyrosol in Experimental Diabetic Retinopathy: Relationship with Cardiovascular Biomarkers. J. Agric. Food Chem. 2018, 66, 637–644. [Google Scholar] [CrossRef]
- Benlarbi, M.; Jemai, H.; Hajri, K.; Mbarek, S.; Amri, E.; Jebbari, M.; Hammoun, I.; Baccouche, B.; Boudhrioua Mihoubi, N.; Zemmal, A.; et al. Neuroprotective effects of oleuropein on retina photoreceptors cells primary culture and olive leaf extract and oleuropein inhibitory effects on aldose reductase in a diabetic model: Meriones shawi. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Agita, A.; Thaha, M.; Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165. [Google Scholar]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Mehaffey, E.; Majid, D.S.A. Tumor necrosis factor-α, kidney function, and hypertension. Am. J. Physiol.-Ren. Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro Júnior, J.E.G.; Moraes, P.Z.; Rodriguez, M.D.; Simões, M.R.; Cibin, F.; Pinton, S.; Barbosa Junior, F.; Peçanha, F.M.; Vassallo, D.V.; Miguel, M.; et al. Cadmium exposure activates NADPH oxidase, renin–angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol. Lett. 2020, 333, 80–89. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Ouatu, A.; Rezus, C.; Ciocoiu, M.; Costea, C.F.; Floria, M. Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? Int. J. Hypertens. 2019, 2019, 3159283. [Google Scholar] [CrossRef] [PubMed]
- McMaster, W.G.; Kirabo, A.; Madhur, M.S.; Harrison, D.G. Inflammation, Immunity, and Hypertensive End-Organ Damage. Circ. Res. 2015, 116, 1022–1033. [Google Scholar] [CrossRef]
- Formanowicz, D.; Rybarczyk, A.; Radom, M.; Formanowicz, P. A role of inflammation and immunity in essential hypertension—modeled and analyzed using Petri nets. Int. J. Mol. Sci. 2020, 21, 3348. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2020, 100916. [Google Scholar] [CrossRef]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coban, E.; Nizam, I.; Topal, C.; Akar, Y. The Association of Low-Grade Systemic Inflammation with Hypertensive Retinopathy. Clin. Exp. Hypertens. 2011, 32, 528–531. [Google Scholar] [CrossRef]
- Silva, K.C.; Pinto, C.C.; Biswas, S.K.; De Faria, J.B.L.; De Faria, J.M.L. Hypertension increases retinal inflammation in experimental diabetes: A possible mechanism for aggravation of diabetic retinopathy by hypertension. Curr. Eye Res. 2007, 32, 533–541. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Santana-Garrido, Á.; Reyes-Goya, C.; André, H.; Aramburu, Ó.; Mate, A.; Vázquez, C.M. Sunitinib-induced oxidative imbalance and retinotoxic effects in rats. Life Sci. 2020, 257, 118072. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- He, W.-S.; Zhu, H.; Chen, Z.-Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Hernáez, A.; Farràs, M.; Fitó, M. Olive oil phenolic compounds and high-density lipoprotein function. Curr. Opin. Lipidol. 2016, 27, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.M.; Rothwell, P.M. Blood pressure and the brain: The neurology of hypertension. Pract. Neurol. 2020, 20, 100–111. [Google Scholar] [CrossRef]
- Sicard, P.; Acar, N.; Grégoire, S.; Lauzier, B.; Bron, A.M.; Creuzot-Garcher, C.; Bretillon, L.; Vergely, C.; Rochette, L. Influence of rosuvastatin on the NAD(P)H oxidase activity in the retina and electroretinographic response of spontaneously hypertensive rats. Br. J. Pharmacol. 2007, 151, 979–986. [Google Scholar] [CrossRef] [Green Version]
- Negretto, A.D.; Rosa, A.A.M.; Nakashima, A.A.; Ortega, K.C.; Mion Júnior, D.; Oyamada, M.K.; Nakashima, Y. Avaliação da retinopatia hipertensiva através do potencial oscilatório do eletrorretinograma. Arq. Bras. Oftalmol. 2008, 71, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-garrido, Á.; Reyes-goya, C.; Fernández-bobadilla, C.; Blanca, A.J.; Mate, A.; Vázquez, C.M. NADPH oxidase—Induced oxidative stress in the eyes of hypertensive rats. Mol. Vis. 2021, 28, 161–178. [Google Scholar]
- Akay, F.; Gundogan, F.C.; Yolcu, U.; Toyran, S.; Uzun, S. Choroidal thickness in systemic arterial hypertension. Eur. J. Ophthalmol. 2015, 26, 152–157. [Google Scholar] [CrossRef]
- Lim, H.B.; Lee, M.W.; Park, J.H.; Kim, K.; Jo, Y.J.; Kim, J.Y. Changes in Ganglion Cell–Inner Plexiform Layer Thickness and Retinal Microvasculature in Hypertension: An Optical Coherence Tomography Angiography Study. Am. J. Ophthalmol. 2019, 199, 167–176. [Google Scholar] [CrossRef]
- Machida, S.; Kondo, M.; Jamison, J.A.; Khan, N.W.; Kononen, L.T.; Sugawara, T.; Bush, R.A.; Sieving, P.A. P23H rhodopsin transgenic rat: Correlation of retinal function with histopathology. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3200–3209. [Google Scholar]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Doroshenko, E.R.; Drohomyrecky, P.C.; Gower, A.; Whetstone, H.; Cahill, L.S.; Ganguly, M.; Spring, S.; Yi, T.J.; Sled, J.G.; Dunn, S.E. Peroxisome Proliferator-Activated Receptor-δ Deficiency in Microglia Results in Exacerbated Axonal Injury and Tissue Loss in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 371. [Google Scholar] [CrossRef]
- Shen, L.Q.; Child, A.; Weber, G.M.; Folkman, J.; Aiello, L.P. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Chen, Q.; Jiang, N.; Liang, X.; Li, J.; Zong, R.; Huang, C.; Qiu, Y.; Ma, J.X.; Liu, Z. PPARα-dependent effects of palmitoylethanolamide against retinal neovascularization and fibrosis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef] [Green Version]
- Joharapurkar, A.; Patel, V.; Kshirsagar, S.; Patel, M.S.; Savsani, H.; Jain, M. Effect of dual PPAR-α/γ agonist saroglitazar on diabetic retinopathy and oxygen-induced retinopathy. Eur. J. Pharmacol. 2021, 899, 174032. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X.; et al. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlinowski, J.; Jozkowicz, A. PPAR Gamma and Angiogenesis: Endothelial Cells Perspective. J. Diabetes Res. 2016, 2016, 8492353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farràs, M.; Valls, R.M.; Fernández-Castillejo, S.; Giralt, M.; Solà, R.; Subirana, I.; Motilva, M.J.; Konstantinidou, V.; Covas, M.I.; Fitó, M. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J. Nutr. Biochem. 2013, 24, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-De-Moraes, I.M.; Gonçalves-De-Albuquerque, C.F.; Kurz, A.R.M.; De Jesus Oliveira, F.M.; Pereira de Abreu, V.H.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 oleic acid, the main compound of olive oil, mitigates inflammation during experimental sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef] [Green Version]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Omega-3 Reduces Pathological Retinal Angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Rossino, M.G.; Casini, G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.L.; Chin, K.Y. The biological activities of oleocanthal from a molecular perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef] [Green Version]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Bao, J.; Yan, W.; Xu, K.; Chen, M.; Chen, Z.; Ran, J.; Xiong, Y.; Wu, L. Oleanolic Acid Decreases IL-1 β -Induced Activation of Fibroblast-Like Synoviocytes via the SIRT3-NF- κ B Axis in Osteoarthritis. Oxid. Med. Cell. Longev. 2020, 2020, 7517219. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic acid exerts a neuroprotective effect against microglial cell activation by modulating cytokine release and antioxidant defense systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Yan, D.Y.; Wu, C.Y.; Xuan, J.W.; Jin, C.Q.; Hu, X.L.; Bao, G.D.; Bian, Y.J.; Hu, Z.C.; Shen, Z.H.; et al. Maslinic acid prevents IL-1β-induced inflammatory response in osteoarthritis via PI3K/AKT/NF-κB pathways. J. Cell. Physiol. 2021, 236, 1939–1949. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.; Park, E.K.; Bae, J.S. Maslinic acid ameliorates inflammation via the downregulation of NF-κB and STAT-1. Antioxidants 2020, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Ferreira, D.; Esperandim, V.R.; Toldo, M.P.A.; Kuehn, C.C.; Do Prado Júnior, J.C.; Cunha, W.R.; Silva, M.L.A.e.; Albuquerque, S.d. In vivo activity of ursolic and oleanolic acids during the acute phase of Trypanosoma cruzi infection. Exp. Parasitol. 2013, 134, 455–459. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Giner, R.M. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J. Nat. Prod. 2013, 76, 1113–1120. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily use of extra virgin olive oil with high oleocanthal concentration reduced body weight, waist circumference, alanine transaminase, inflammatory cytokines and hepatic steatosis in subjects with the metabolic syndrome: A 2-month intervention study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]


| Primary Antibody | Origin | Dilution | Secondary Antibody | Dilution | Reference |
|---|---|---|---|---|---|
| Anti-PPARγ | Mouse monoclonal | 1:2000 | Goat Anti-Mouse | 1:4000 | SCB |
| Anti-PPARα | Mouse monoclonal | 1:2000 | Goat Anti-Mouse | 1:4000 | SCB |
| Anti-IL-6 | Mouse monoclonal | 1:1000 | Goat Anti-Rabbit | 1:2000 | SCB |
| Anti-IL-1β | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-IL-10 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-TNF-α | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-COX2 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-β-Actin | Mouse monoclonal | 1:20,000 | Goat Anti-Mouse | 1:30,000 | SCB |
| Gene | Forward Primer (5′→ 3′) | Reverse Primer (5′→3′) |
|---|---|---|
| IL-6 | CTCTGCAAGAGACTTCCATCC | TTCTGCAAGTGCATCATCGT |
| IL-1β | CCGTGGACCTTCCAGGATGA | GGGAAGGTCACACACCAGCA |
| IL-10 | CTGGACAACATACTGCTAACCG | GGGCATCACTTCTACCAGGTAA |
| TNF-α | CCACGCTCTTCTGTCTACTG | ACTTGGTGGTTTGCTACGAC |
| GAPDH | GCCAAAAGGGTCATCATCTCCGC | GGATGACCTTGCCCACAGCCTTG |
| Primary Antibody | Origin | Dilution | Reference |
|---|---|---|---|
| Anti-PPARγ | Mouse monoclonal | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| Anti-PPARα | Mouse monoclonal | 1:200 | Santa Cruz Biotechnology |
| Anti-CD31 | Rabbit monoclonal | 1:200 | Rockland Immunochemicals, Limerick, PA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Garrido, Á.; Reyes-Goya, C.; Milla-Navarro, S.; de la Villa, P.; André, H.; Vázquez, C.M.; Mate, A. Anti-Inflammatory Action of Dietary Wild Olive (Acebuche) Oil in the Retina of Hypertensive Mice. Foods 2021, 10, 1993. https://doi.org/10.3390/foods10091993
Santana-Garrido Á, Reyes-Goya C, Milla-Navarro S, de la Villa P, André H, Vázquez CM, Mate A. Anti-Inflammatory Action of Dietary Wild Olive (Acebuche) Oil in the Retina of Hypertensive Mice. Foods. 2021; 10(9):1993. https://doi.org/10.3390/foods10091993
Chicago/Turabian StyleSantana-Garrido, Álvaro, Claudia Reyes-Goya, Santiago Milla-Navarro, Pedro de la Villa, Helder André, Carmen M. Vázquez, and Alfonso Mate. 2021. "Anti-Inflammatory Action of Dietary Wild Olive (Acebuche) Oil in the Retina of Hypertensive Mice" Foods 10, no. 9: 1993. https://doi.org/10.3390/foods10091993