Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Purple Sweet Potato Starch (SPS)
2.3. Preparation of Sweet Potato Peel (SPP) Powder
2.4. Preparation of SPS and SPS/SPP Films
2.5. Color Change of CA with pH
2.6. Characterization of Films
2.6.1. Thickness
2.6.2. Water Content, Water Solubility, and Swelling Degree
2.6.3. Water Vapor Permeability (WVP)
2.6.4. Colorimetric Analysis
2.6.5. Mechanical Properties
2.6.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.6.7. Scanning Electron Microscopy (SEM)
2.6.8. Thermogravimetric Analysis (TGA)
2.7. Feasibility of Anthocyanin Migration
2.8. Direct Food Contact
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Film
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.2. Surface Morphology
3.1.3. Thickness
3.1.4. Water Content (WC)
3.1.5. Water Solubility
3.1.6. Swelling Degree
3.1.7. Water Vapor Permeability (WVP)
3.2. Mechanical Properties of Films
3.3. Thermal Analysis
3.4. Color Analysis
3.4.1. Color Parameters of Prepared Films
3.4.2. Color Change of CA Solution
3.4.3. Color Change of Anthocyanin Incorporated SPS and SPS/SPP Films at pH 1–12
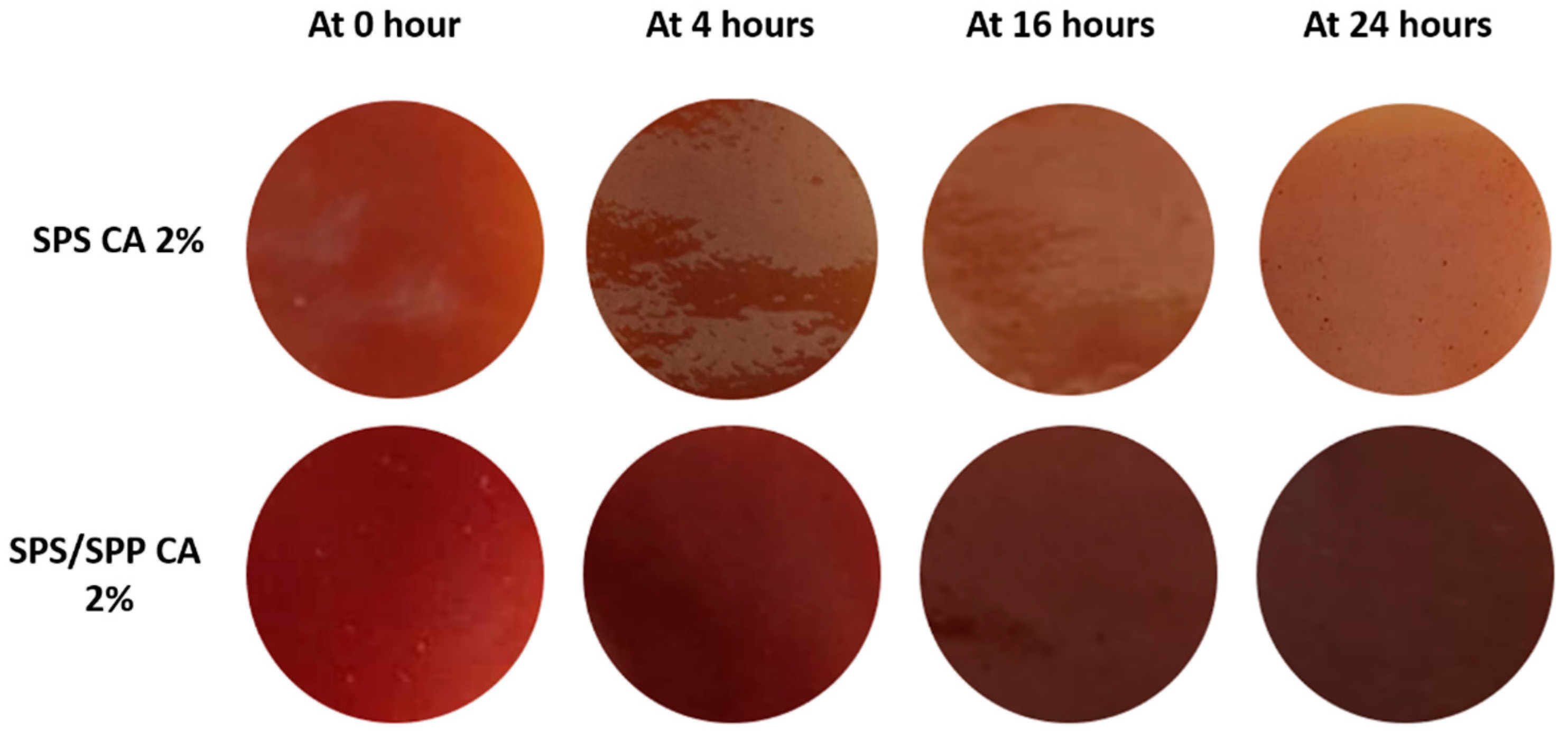
3.4.4. Direct Food Contact
3.5. Anthocyanin Migration Feasibility Test
Migration from Film to Food Simulants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango kernel starch-gum composite films: Physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Majid, N.A.; Tawakkal, I.S.M.A.; Basha, R.K.; Nordin, N.; Shapi’i, R.A. Tapioca starch films reinforced with microcrystalline cellulose for potential food packaging application. Food Sci. Technol. 2019, 39, 605–612. [Google Scholar] [CrossRef]
- Chivrac, F.; Angellier-Coussy, H.; Guillard, V.; Pollet, E.; Avérous, L. How does water diffuse in starch/montmorillonite nano-biocomposite materials? Carbohydr. Polym. 2010, 82, 128–135. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Abdullah, S.N.; Halim, M.R.A.; Shari, E.S.; Ismail, N.A.; Yusoff, M.M. Growth and yield performance of five purple sweet potato (ipomoea batatas) accessions on colluvium soil. Pertanika J. Trop. Agric. Sci. 2018, 41, 975–986. [Google Scholar]
- Mohd Hanim, A.B.; Chin, N.L.; Yusof, Y.A. Physico-chemical and flowability characteristics of a new variety of Malaysian sweet potato, VitAto Flour. Int. Food Res. J. 2014, 21, 2099–2107. [Google Scholar]
- Soison, B.; Jangchud, K.; Jangchud, A.; Harnsilawat, T.; Piyachomkwan, K. Characterization of starch in relation to flesh colors of sweet potato varieties. Int. Food Res. J. 2015, 22, 2302–2308. [Google Scholar]
- Lai, Y.C.; Wang, S.Y.; Gao, H.Y.; Nguyen, K.M.; Nguyen, C.H.; Shih, M.C.; Lin, K.H. Physicochemical properties of starches and expression and activity of starch biosynthesis-related genes in sweet potatoes. Food Chem. 2016, 199, 556–564. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of Sweet Potato Starch-Based Nanocomposite Films Activated With Thyme Essential Oil on the Shelf-Life of Baby Spinach Leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef]
- Rydz, J.; Musioł, M.; Zawislak-Węgrzyńska, B.; Sikorska, W. Chapter 14—Present and Future of Biodegradable Polymers for Food Packaging Applications. In Handbook of Food Bioengineering—Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 20, pp. 431–467. [Google Scholar] [CrossRef]
- Shukor, U.A.A.; Nordin, N.; Tawakkal, I.S.M.A.; Talib, R.A.; Othman, S.H. Chapter 9—Utilization of jackfruit peel waste for the production of biodegradable and active antimicrobial packaging films. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Saba, N., Jawaid, M., Thariq, M., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 171–192. [Google Scholar] [CrossRef]
- Othman, S.H.; Tarmiti, N.A.N.; Shapi’i, R.A.; Zahiruddin, S.M.M.; Tawakkal, I.S.M.A.; Basha, R.K. Starch/banana pseudostem biocomposite films for potential food packaging applications. BioResources 2020, 15, 3984–3998. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Johar, N.; Ahmad, I. Starch biocomposite film reinforced by multiscale rice husk fiber. Compos. Sci. Technol. 2017, 151, 147–155. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Wang, Q. Sustainable packaging biocomposites from polylactic acid and wheat straw: Enhanced physical performance by solid state shear milling process. Compos. Sci. Technol. 2018, 158, 34–42. [Google Scholar] [CrossRef]
- Cruz-Gálvez, A.M.; Castro-Rosas, J.; Rodríguez-Marín, M.L.; Cadena-Ramírez, A.; Tellez-Jurado, A.; Tovar-Jiménez, X.; Gómez-Aldapa, C.A. Antimicrobial activity and physicochemical characterization of a potato starch-based film containing acetonic and methanolic extracts of Hibiscus sabdariffa for use in sausage. LWT 2018, 93, 300–305. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A. Effect of the incorporation of antimicrobial/antioxidant proteins on the properties of potato starch films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Zahiruddin, S.M.M.; Othman, S.H.; Tawakkal, I.S.M.A.; Talib, R.A. Mechanical and thermal properties of tapioca starch films plasticized with glycerol and sorbitol. Food Res. 2019, 3, 157–163. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Laroque, D.A.; de Andrade, L.M.; Carciofi, B.A.M.; Tenório, J.A.S.; de Andrade, C.J. Production of active cassava starch films; effect of adding a biosurfactant or synthetic surfactant. React. Funct. Polym. 2019, 144, 104368. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- de Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- da Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends. Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bhandari, B.; Yang, C. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M. Fabrication and characterization of alizarin colorimetric indicator based on cellulose-chitosan to monitor the freshness of minced beef. Sens. Actuators B Chem. 2019, 285, 519–528. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.T.; da Rosa Zavareze, E. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Cheng, C.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Shaari, N.; Shamsudin, R.; Nor, M.Z.M.; Hashim, N. Quality Attributes of Malaysia Purple-Fleshed Sweet Potato at Different Peel Condition. Agronomy 2021, 11, 872. [Google Scholar] [CrossRef]
- Silva, H.R.D.; Assis, D.D.C.D.; Prada, A.L.; Silva, J.O.C.; Sousa, M.B.D.; Ferreira, A.M.; Amado, J.R.R.; Carvalho, H.D.O.; Santos, A.V.T.D.L.T.D.; Carvalho, J.C.T. Obtaining and characterization of anthocyanins from Euterpe oleracea (açaí) dry extract for nutraceutical and food preparations. Rev. Bras. Farmacogn. 2019, 29, 677–685. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard test methods for tensile properties of thin plastic sheeting, method D882-10. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2010; Volume 87, pp. 3–5. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Compositional Analysis by Thermogravimetry (E1131-08). In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015; Volume 8, p. 6. [Google Scholar]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Sanches-Silva, A.; Motta, J.F.G.; Andrade, M.; de Araújo Neves, I.; Teófilo, R.F.; de Carvalho, M.G.; de Melo, N.R. Combined use of essential oils applied to protein base active food packaging: Study in vitro and in a food simulant. Eur. Polym. J. 2017, 93, 75–86. [Google Scholar] [CrossRef]
- Baner, A.; Bieber, W.; Figge, K.; Franz, R.; Piringer, O. Alternative Fatty Food Simulants for Migration Testing of Polymeric Food Contact Materialsd. Food Addit. Contam. 1992, 9, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Migration of antimicrobial agents from starch-based films into a food simulant. LWT Food Sci. Technol. 2013, 50, 432–438. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava/sugar palm fiber reinforced cassava starch hybrid composites: Physical, thermal and structural properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef]
- Luchese, C.L.; Frick, J.M.; Patzer, V.L.; Spada, J.C.; Tessaro, I.C. Synthesis and characterization of biofilms using native and modified pinhão starch. Food Hydrocoll. 2015, 45, 203–210. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Xue Mei, L.; Mohammadi Nafchi, A.; Ghasemipour, F.; Mat Easa, A.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A study of controlled uptake and release of anthocyanins by oxidized starch microgels. J. Agric. Food Chem. 2013, 61, 5880–5887. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Water vapor permeability and solubility of films from hydrolyzed whey protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Haas Costa, T.M.; de Oliveira Rios, A.; Hickmann Flôres, S. Valorization of food-grade industrial waste in the obtaining active biodegradable films for packaging. Ind. Crop. Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. The structural characteristics of starches and their functional properties. CyTA J. Food. 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control. 2021, 126, 108039. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Versino, F.; García, M.A. Cassava (Manihot esculenta) starch films reinforced with natural fibrous filler. Ind. Crop. Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of Chitosan–Starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Thermal, mechanical, and physical properties of seaweed/sugar palm fibre reinforced thermoplastic sugar palm Starch/Agar hybrid composites. Int. J. Biol. Macromol. 2017, 97, 606–615. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Anthocyanin Pigments—Bioactivity and Coloring Properties. J. Food Sci. 2004, 69, C419–C425. [Google Scholar] [CrossRef]
- Ananga, A.; Georgiev, V.; Ochieng, J.; Phills, B.; Tsolov, V. Production of Anthocyanins in Grape Cell Cultures: A Potential Source of Raw Material for Pharmaceutical, Food, and Cosmetic Industries. In The Mediterranean Genetic Code—Grapevine and Olive; IntechOpen: London, UK, 2013; pp. 247–287. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012047. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.K.; Costa, D.; Yamashita, F.; Gerschenson, L.N.; Grossmann, M.V. Mixture design for evaluation of potassium sorbate and xanthan gum effect on properties of tapioca starch films obtained by extrusion. Mater. Sci. Eng. C 2010, 30, 196–202. [Google Scholar] [CrossRef]
- Bastos, D.C.; Santos, A.E.F.; da Silva, M.L.V.J.; Simão, R.A. Hydrophobic corn starch thermoplastic films produced by plasma treatment. Ultramicroscopy 2009, 109, 1089–1093. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Del Nobile, M.A.; Panizza, A.; Corbo, M.R.; Nicolais, L. A general approach to describe the antimicrobial agent release from highly swellable films intended for food packaging applications. J. Control. Release 2003, 90, 97–107. [Google Scholar] [CrossRef]
- Bordignon, C.L., Jr.; Francescatto, V.; Nienow, A.A.; Calvete, E.; Reginatto, F.H. Influência do pH da solução extrativa no teor de antocianinas em frutos de morango. Ciência Tecnol. Aliment. 2009, 29, 183–188. [Google Scholar] [CrossRef][Green Version]








| Sample | Thickness (mm) | Water Content (%) | Water Solubility (%) | Swelling (%) | WVP (10−11 g/Pa h m) |
|---|---|---|---|---|---|
| SPS CA 0% | 0.103 ± 0.001 g | 8.14 ± 0.34 a | 15.4 ± 0.19 c | 6.61 ± 0.55 h | 1.81 ± 0.12 ab |
| SPS CA 1% | 0.138 ± 0.004 f | 7.90 ± 0.67 a | 45.23 ± 10.29 b | 98.34 ± 0.23 e | 1.34 ± 0.35 ab |
| SPS CA 1.5% | 0.155 ± 0.005 e | 8.20 ± 0.47 a | 65.29 ± 0.81 a | 91.87 ± 0.19 f | 0.68 ± 0.08 b |
| SPS CA 2% | 0.205 ± 0.003 c | 8.37 ± 1.17 a | 60.72 ± 5.14 a | 148.07 ± 2.73 b | 0.76 ± 0.58 b |
| SPS/SPP CA 0% | 0.161 ± 0.002 d | 7.50 ±1.01 a | 22.83 ± 1.63 c | 70.81 ± 1.15 g | 2.36 ± 0.79 a |
| SPS/SPP CA 1% | 0.209 ± 0.006 c | 8.43 ± 0.82 a | 57.26 ± 2.88 ab | 128.68 ± 0.96 c | 2.45 ± 0.40 a |
| SPS/SPP CA 1.5% | 0.219 ± 0.004 b | 7.44 ± 0.74 a | 57.60 ± 5.93 ab | 102.94 ± 0.96 d | 2.51 ± 0.90 a |
| SPS/SPP CA 2% | 0.238 ± 0.006 a | 6.77 ± 0.16 a | 60.58 ± 7.24 a | 223.32 ± 2.38 a | 2.37 ± 0.85 a |
| Samples | DTG Peak Temperature (°C) | Weight Loss (%) |
|---|---|---|
| SPS CA 0% | 356.83 | 56.34 |
| SPS CA 1% | 350.54 | 43.45 |
| SPS CA 1.5% | 355.95 | 29.79 |
| SPS CA 2% | 354.71 | 31.81 |
| SPS/SPP CA 0% | 335.90 | 40.34 |
| SPS/SPP CA 1% | 332.20 | 30.04 |
| SPS/SPP CA 1.5% | 335.16 | 28.73 |
| SPS/SPP CA 2% | 344.13 | 26.27 |
| Sample | L | a | b | ΔE | WI |
|---|---|---|---|---|---|
| SPS | 91.95 ± 0.06 a | −0.92 ± 0.09 f | 6.19 ± 0.11 g | 10.01 ± 0.08 g | 89.80 ± 0.07 a |
| SPS CA 1% | 75.32 ± 0.83 b | 8.68 ± 0.44 e | 13.17 ± 0.49 e | 29.09 ± 1.03 f | 70.71 ± 1.03 b |
| SPS CA 1.5% | 63.87 ± 1.30 c | 16.55 ± 0.65 d | 14.33 ± 0.41 d | 42.03 ± 1.47 e | 57.75 ± 1.47 c |
| SPS CA 2% | 55.03 ± 0.45 e | 20.61 ± 0.66 c | 18.21 ± 0.64 c | 52.50 ± 0.77 d | 47.28 ± 0.76 d |
| SPS/SPP | 58.33 ± 0.87 d | 16.93 ± 0.61 d | 41.28 ± 0.45 a | 60.97 ± 0.51 c | 38.94 ± 0.51 e |
| SPS/SPP CA 1% | 37.56 ± 0.26 f | 22.88 ± 0.35 a | 19.09 ± 0.49 b | 68.93 ± 0.25 b | 30.81 ± 0.25 f |
| SPS/SPP CA 1.5% | 33.47 ± 0.42 g | 21.53 ± 0.58 b | 13.20 ± 1.03 e | 70.88 ± 0.39 a | 28.83 ± 0.38 g |
| SPS/SPP CA 2% | 32.12 ± 0.33 h | 20.89 ± 0.90 bc | 11.67 ± 0.92 f | 71.68 ± 0.50 a | 28.02 ± 0.49 g |
| pH Value | L | a | b | ΔE |
|---|---|---|---|---|
| pH 1 | 55.98 ± 0.02 j | 75.69 ± 0.04 a | −14.19 ± 0.07 i | 88.58 ± 0.02 a |
| pH 2 | 62.91 ± 0.02 h | 65.22 ± 0.08 c | −10.56 ± 0.10 h | 75.61 ± 0.07 c |
| pH 3 | 56.69 ± 0.96 i | 73.81 ± 0.82 b | −10.5 ± 0.57 h | 86.08 ± 0.29 b |
| pH 4 | 62.95 ± 0.03 h | 52.9 ± 0.20 d | −17.31 ± 0.05 j | 66.70 ± 0.19 d |
| pH 5 | 69.65 ± 0.03 g | 26.07 ± 0.11 e | −13.89 ± 0.07 i | 42.21 ± 0.10 g |
| pH 6 | 73.52 ± 0.06 e | 12.93 ± 0.08 f | −7.78 ± 0.07 g | 30.36 ± 0.10 i |
| pH 7 | 74.29 ± 0.01 d | −2.29 ± 0.04 g | 3.71 ± 0.03 e | 26.09 ± 0.01 k |
| pH 8 | 74.35 ± 0.02 d | −9.36 ± 0.07 k | 2.08 ± 0.10 f | 27.43 ± 0.02 j |
| pH 9 | 70.18 ± 0.03 f | −21.73 ± 0.03 l | 9.61 ± 0.10 d | 38.23 ± 0.04 h |
| pH 10 | 78.24 ± 0.01 b | −8.72 ± 0.05 j | 40.98 ± 0.06 c | 47.34 ± 0.06 f |
| pH 11 | 77.00 ± 0.03 c | −4.65 ± 0.03 h | 54.74 ± 0.06 b | 59.68 ± 0.06 e |
| pH 12 | 84.32 ± 0.18 a | −5.87 ± 0.06 i | 57.32 ± 0.15 a | 59.85 ± 0.18 e |
| pH Value | Images after the Color Change | |||||
|---|---|---|---|---|---|---|
| SPS CA 1% | SPS CA 1.5% | SPS CA 2% | SPS/SPP CA 1% | SPS/SPP CA 1.5% | SPS/SPP CA 2% | |
| pH 1 |  |  |  |  |  |  |
| pH 2 |  |  |  |  |  |  |
| pH 3 |  |  |  |  |  |  |
| pH 4 |  |  |  |  |  |  |
| pH 5 |  |  |  |  |  |  |
| pH 6 |  |  |  |  |  |  |
| pH 7 |  |  |  |  |  |  |
| pH 8 |  |  |  |  |  |  |
| pH 9 |  |  |  |  |  |  |
| pH 10 |  |  |  |  |  |  |
| pH 11 |  |  |  |  |  |  |
| pH 12 |  |  |  |  |  |  |
| SPS CA1% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 62.28 ± 0.67 ab | 18 ± 0.54 a | 10.08 ± 0.44 f | 32.12 ± 0.40 c |
| pH 2 | 62.01 ± 1.77 ab | 13.31 ± 0.24 b | 10.67 ± 0.63 def | 30.24 ± 1.26 d |
| pH 3 | 59.26 ± 3.34 cd | 12.06 ± 1.10 c | 10.31 ± 1.57 ef | 32.14 ± 2.33 c |
| pH 4 | 61.14 ± 1.64 bc | 9.93 ± 0.39 d | 10.42 ± 0.70 ef | 29.68 ± 1.15 d |
| pH 5 | 54.50 ± 1.86 e | 7.28 ± 0.98 e | 10.38 ± 2.07 ef | 32.24 ± 1.86 b |
| pH 6 | 64.1 ± 1.27 a | 6.94 ± 0.40 e | 12.24 ± 0.75 cd | 26.80 ± 0.77 e |
| pH 7 | 64.56 ± 0.35 a | 6.11 ± 0.07 f | 13.13 ± 0.20 c | 26.56 ± 0.24 e |
| pH 8 | 62.01 ± 2.07 ab | 9.44 ± 0.28 d | 12.86 ± 0.33 c | 29.57 ± 1.67 d |
| pH 9 | 58.76 ± 0.80 cd | 2.83 ± 0.08 g | 12 ± 0.42 cde | 30.97 ± 0.60 cd |
| pH 10 | 59.03 ± 0.61 cd | 0.9 ± 0.07 i | 13.69 ± 0.33 c | 31.23 ± 0.49 cd |
| pH 11 | 58.17 ± 1.27 d | 1.89 ± 0.14 h | 25.24 ± 1.82 b | 37.95 ± 0.28 a |
| pH 12 | 60.96 ± 0.84 bc | 3.24 ± 0.07 g | 30.6 ± 0.52 a | 39.57 ± 0.16 a |
| SPS CA 1.5% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 50.32 ± 1.46 c | 24.21 ± 0.95 a | 11.63 ± 0.74 cd | 45.53 ± 0.70 de |
| pH 2 | 60.83 ± 1.36 a | 16.49 ± 0.86 b | 14.10 ± 0.61 a | 33.77 ± 1.70 h |
| pH 3 | 57.90 ± 0.51 a | 14.19 ± 0.14 c | 13.41 ± 0.17 ab | 34.97 ± 0.35 gh |
| pH 4 | 52.24 ± 0.63 bc | 14.03 ± 0.17 c | 12.29 ± 0.27 bcd | 39.56 ± 0.43 f |
| pH 5 | 53.83 ± 1.93 b | 8.37 ± 0.18 d | 12.42 ± 0.34 bcd | 36.55 ± 1.65 g |
| pH 6 | 42.07 ± 0.84 ef | 8.09 ± 0.10 d | 9.50 ± 0.25 f | 47.09 ± 0.76 cd |
| pH 7 | 40.48 ± 1.82 f | 8.53 ± 0.23 d | 11.17 ± 0.72 de | 49.00 ± 1.62 c |
| pH 8 | 46.65 ± 2.93 d | 8.74 ± 0.31 d | 12.80 ± 1.73 abc | 43.53 ± 2.25 e |
| pH 9 | 45.68 ± 4.09 d | 3.78 ± 0.14 e | 10.22 ± 1.31 ef | 43.21 ± 3.70 e |
| pH 10 | 43.70 ± 0.90 de | −0.59 ± 0.75 f | 12.85 ± 1.09 abc | 45.59 ± 0.59 de |
| pH 11 | 32.17 ± 0.42 h | −0.10 ± 0.46 f | 12.44 ± 0.09 bcd | 56.74 ± 0.43 a |
| pH 12 | 36.41 ± 1.33 g | −0.12 ± 0.19 f | 13.84 ± 0.70 a | 52.89 ± 1.11 b |
| SPS CA 2% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 50.14 ± 1.37 bc | 21.98 ± 0.39 b | 11.37 ± 0.41 d | 43.32 ± 0.99 bcd |
| pH 2 | 49.62 ± 1.03 bc | 24.87 ± 0.84 a | 11.16 ± 0.55 de | 45.27 ± 0.80 b |
| pH 3 | 51.28 ± 0.52 ab | 15.87 ± 0.40 c | 10.08 ± 0.36 ef | 39.60 ± 0.43 g |
| pH 4 | 48.85 ± 0.44 c | 15.92 ± 0.47 c | 11.25 ± 0.40 de | 41.85 ± 0.32 def |
| pH 5 | 45 ± 0.78 d | 11.26 ± 0.27 d | 9.07 ± 0.36 f | 44.13 ± 0.69 bc |
| pH 6 | 52.62 ± 0.48 a | 8.88 ± 0.14 e | 12.70 ± 0.24 c | 36.10 ± 0.44 h |
| pH 7 | 48.66 ± 0.92 c | 8.37 ± 0.07 e | 12.12 ± 0.15 cd | 39.88 ± 0.91 fg |
| pH 8 | 48.36 ± 0.62 c | 8.25 ± 0.33 e | 11.71 ± 0.24 cd | 40.15 ± 0.64 efg |
| pH 9 | 36.75 ± 1.33 e | 3.41 ± 0.59 f | 6.83 ± 1.17 g | 51.17 ± 1.26 a |
| pH 10 | 44.33 ± 2.05 d | −0.26 ± 0.10 h | 12.08 ± 0.41 cd | 43.44 ± 2.05 bcd |
| pH 11 | 37.19 ± 2.33 e | 3.05 ± 0.20 fg | 22.00 ± 1.79 b | 50.56 ± 2.34 a |
| pH 12 | 45.50 ± 1.59 d | 2.61 ± 0.12 g | 24.43 ± 0.86 a | 42.17 ± 1.55 cde |
| SPS/SPS CA 1% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 29.75 ± 0.50 c | 46.75 ± 0.56 a | 43.56 ± 1.65 bc | 85.26 ± 0.86 d |
| pH 2 | 20.87 ± 0.79 e | 35.05 ± 1.52 d | 44.9 ± 0.97 b | 86.89 ± 0.52 cd |
| pH 3 | 32.31 ± 1.44 b | 43.64 ± 1.64 b | 37.72 ± 0.49 d | 79.03 ± 0.38 f |
| pH 4 | 38.64 ± 1.11 a | 35.79 ± 1.02 cd | 21.46 ± 0.59 h | 63.72 ± 0.35 i |
| pH 5 | 33.98 ± 0.21 b | 29.02 ± 0.93 e | 26.85 ± 0.66 g | 65.96 ± 0.80 h |
| pH 6 | 28.73 ± 0.67 c | 29.86 ± 0.24 e | 34.31 ± 0.62 e | 73.63 ± 0.91 g |
| pH 7 | 33.47 ± 3.08 b | 23.86 ± 1.2 f | 31.43 ± 2.05 f | 66.24 ± 3.88 h |
| pH 8 | 25.92 ± 1.48 d | 33.88 ± 1.92 d | 42.71 ± 1.95 bc | 81.48 ± 0.65 e |
| pH 9 | 19.92 ± 0.71 e | 37.49 ± 2.41 c | 43.95 ± 0.16 b | 88.17 ± 0.46 bc |
| pH 10 | 11.84 ± 1.80 g | 6.21 ± 2.10 g | 41.21 ± 2.30 c | 85.92 ± 0.67 d |
| pH 11 | 14.27 ± 1.41 f | 3.2 ± 0.12 h | 57.42 ± 2.54 a | 92.37 ± 0.44 a |
| pH 12 | 8.44 ± 1.37 h | 2.02 ± 0.28 h | 42.36 ± 2.22 bc | 89.26 ± 0.21 b |
| SPS/SPS CA 1.5% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 30.02 ± 0.73 d | 46.65 ± 0.79 a | 47.70 ± 0.10 bc | 87.12 ± 0.04 b |
| pH 2 | 22.79 ± 0.46 g | 42.90 ± 0.25 b | 53.39 ± 0.47 a | 93.28 ± 0.53 a |
| pH 3 | 26.24 ± 0.46 f | 41.78 ± 0.14 c | 46.35 ± 0.86 c | 86.57 ± 0.84 b |
| pH 4 | 30.34 ± 0.26 cd | 37.6 ± 0.18 d | 34.93 ± 0.16 e | 76.08 ± 0.15 cd |
| pH 5 | 32.58 ± 0.61 b | 28.08 ± 0.33 f | 29.27 ± 0.08 f | 67.66 ± 0.31 e |
| pH 6 | 28.1 ± 1.26 e | 30.75 ± 0.29 e | 35.87 ± 0.23 e | 75.20 ± 0.98 d |
| pH 7 | 35.10 ± 0.42 a | 20.66 ± 0.19 h | 30.28 ± 0.16 f | 63.30 ± 0.22 f |
| pH 8 | 31.57 ± 1.77 bc | 26.2 ± 1.35 g | 28.64 ± 4.36 f | 67.54 ± 3.76 e |
| pH 9 | 31.62 ± 0.54 bc | 17.38 ± 0.33 i | 21.91 ± 1.25 h | 62.11 ± 0.81 f |
| pH 10 | 19.84 ± 0.14 h | −2 ± 0.04 l | 38.69 ± 0.17 d | 77.47 ± 0.09 c |
| pH 11 | 16.39 ± 0.82 i | 2.17 ± 0.03 k | 48.90 ± 0.65 b | 85.67 ± 0.41 b |
| pH 12 | 30.81 ± 0.83 cd | 5.49 ± 0.53 j | 24.49 ± 0.38 g | 61.64 ± 0.83 f |
| SPS/SPS CA 2% | ||||
| pH Value | L | a | b | ΔE |
| pH 1 | 28.56 ± 0.25 b | 45.79 ± 0.30 a | 48.54 ± 0.10 a | 88.10 ± 0.09 a |
| pH 2 | 31.45 ± 0.86 a | 46.51 ± 0.18 a | 46.03 ± 1.50 b | 85.23 ± 1.31 b |
| pH 3 | 29.23 ± 1.08 b | 42.17 ± 1.10 b | 42.97 ± 0.22 d | 82.93 ± 0.27 c |
| pH 4 | 26.17 ± 0.19 c | 38.02 ± 0.08 c | 41.96 ± 0.03 d | 82.67 ± 0.10 cd |
| pH 5 | 29.17 ± 1.15 b | 28.34 ± 0.46 e | 30.52 ± 0.23 g | 71.07 ± 0.86 f |
| pH 6 | 26.23 ± 0.30 c | 28.77 ± 0.56 e | 37.06 ± 0.65 f | 76.47 ± 0.23 e |
| pH 7 | 22.34 ± 1.40 d | 33.32 ± 1.76 d | 37.17 ± 0.22 f | 81.42 ± 1.73 d |
| pH 8 | 20.97 ± 0.72 e | 37.64 ± 2.79 c | 46.53 ± 0.48 b | 88.69 ± 1.33 a |
| pH 9 | 20.81 ± 0.59 e | 39.26 ± 0.16 c | 46.26 ± 0.12 b | 89.35 ± 0.36 a |
| pH 10 | 17.63 ± 0.84 f | −4.64 ± 0.07 h | 44.22 ± 0.65 c | 82.26 ± 0.43 cd |
| pH 11 | 14.42 ± 0.35 g | 3.48 ± 0.21 g | 38.92 ± 0.80 e | 82.39 ± 0.59 cd |
| pH 12 | 26.49 ± 0.79 c | 12.23 ± 0.77 f | 26.26 ± 0.71 h | 67.14 ± 0.50 g |
| At Hour | pH of Chicken | Chicken in Contact with | ||||
|---|---|---|---|---|---|---|
| No Film | SPS CA 0% | SPS CA 2% | SPS/SPP CA 0% | SPS/SPP CA 2% | ||
| 0 | pH 5 |  |  |  |  |  |
| 4 | pH 6 |  |  |  |  |  |
| 16 | pH 7 |  |  |  |  |  |
| 24 | pH 8 |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. https://doi.org/10.3390/foods10092005
Sohany M, Tawakkal ISMA, Ariffin SH, Shah NNAK, Yusof YA. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods. 2021; 10(9):2005. https://doi.org/10.3390/foods10092005
Chicago/Turabian StyleSohany, Mouluda, Intan Syafinaz Mohamed Amin Tawakkal, Siti Hajar Ariffin, Nor Nadiah Abdul Karim Shah, and Yus Aniza Yusof. 2021. "Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films" Foods 10, no. 9: 2005. https://doi.org/10.3390/foods10092005
APA StyleSohany, M., Tawakkal, I. S. M. A., Ariffin, S. H., Shah, N. N. A. K., & Yusof, Y. A. (2021). Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods, 10(9), 2005. https://doi.org/10.3390/foods10092005






