Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
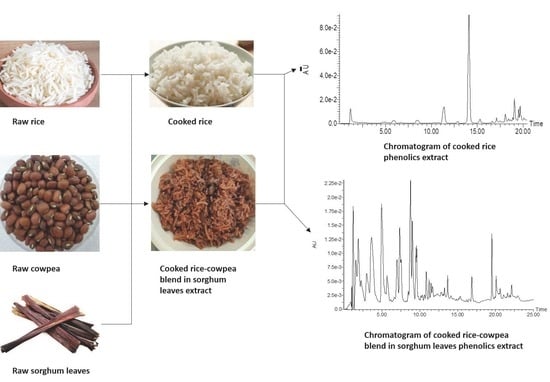
2.2. Source and Preparation of Samples
2.3. Extraction of Phenolic Compounds
2.4. Analysis of Constituent Phenolic Compounds
2.5. Total Phenolic Content
2.6. Antioxidant Activities
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition
3.2. Total Phenolic CONTENT and Antioxidant Activity
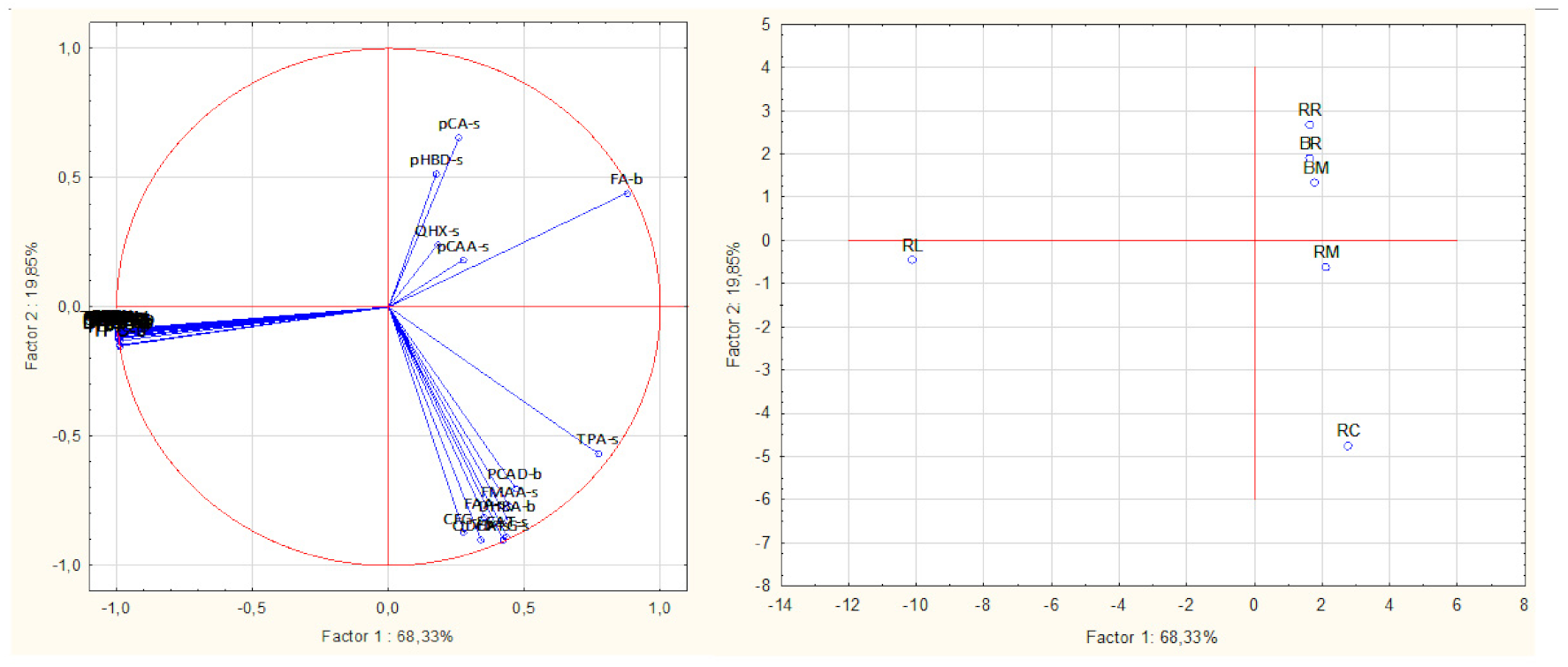
3.3. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Biannual report on global food markets. In Food Outlook; Ben-Belhassen, B., Abbassian, A., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 66–67. [Google Scholar]
- Somaratne, G.M.; Prasantha, B.D.R.; Dunuwila, G.R.; Chandrasekara, A.; Wijesinghe, D.G.N.G.; Gunasekara, D.C.S. Effect of polishing on glycemic index and antioxidant properties of red and white basmati rice. Food Chem. 2017, 237, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.D.; Subhasree, R.S.; Bhakyaraj, R.; Vidhyalakshmi, R. Brown rice-beyond the color reviving a lost health food—A review. Am.-Euras. J. Agron. 2009, 2, 67–72. [Google Scholar]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. Br. Med. J. 2012, 344, e1454. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Apea-Bah, F.B.; Minnaar, A.; Bester, M.J.; Duodu, K.G. Does a sorghum-cowpea composite porridge hold promise for contributing to alleviating oxidative stress? Food Chem. 2014, 157, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Giami, S.Y. Compositional and nutritional properties of selected newly developed lines of cowpea (Vigna unguiculata L. Walp). J. Food Compos. Anal. 2005, 18, 665–673. [Google Scholar] [CrossRef]
- Apea-Bah, F.B.; Serem, J.C.; Bester, M.J.; Duodu, K.G. Phenolic composition and antioxidant properties of Koose, a deep-fat fried cowpea cake. Food Chem. 2017, 237, 247–256. [Google Scholar] [CrossRef]
- Anyango, J.O.; de Kock, H.L.; Taylor, J.R.N. Impact of cowpea addition on the protein digestibility corrected amino acid score and other protein quality parameters of traditional African foods made from non-tannin and tannin sorghum. Food Chem. 2011, 124, 775–780. [Google Scholar] [CrossRef]
- Kayodé, A.P.P.; Bara, C.A.; Dalodé-Vieira, G.; Linnemann, A.R.; Nout, M.J.R. Extraction of antioxidant pigments from dye sorghum leaf sheaths. LWT-Food Sci. Technol. 2012, 46, 49–55. [Google Scholar] [CrossRef]
- Akogou, F.U.G.; Kayodé, A.P.P.; den Besten, H.M.W.; Linnemann, A.R. Extraction methods and food uses of a natural red colorant from dye sorghum. J. Sci. Food Agric. 2018, 98, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant activity of commercial wild rice and identification of flavonoid compounds in active fractions. J. Agric. Food Chem. 2009, 57, 7543–7551. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Apea-Bah, F.B.; Head, D.; Scales, R.; Bazylo, R.; Beta, T. Hydrothermal extraction, a promising method for concentrating phenolic antioxidants from red osier dogwood (Cornus stolonifer) leaves and stems. Heliyon 2020, 6, e05158. [Google Scholar] [CrossRef] [PubMed]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef]
- Hachibamba, T.; Dykes, L.; Awika, J.; Minnaar, A.; Duodu, K.G. Effect of simulated gastrointestinal digestion on phenolic composition and antioxidant capacity of cooked cowpea (Vigna unguiculata) varieties. Int. J. Food Sci. Technol. 2013, 48, 2638–2649. [Google Scholar] [CrossRef]
- Nderitu, A.M.; Dykes, L.; Awika, J.M.; Minnaar, A.; Duodu, K.G. Phenolic composition and inhibitory effect against oxidative DNA damage of cooked cowpeas as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2013, 141, 1763–1771. [Google Scholar] [CrossRef]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Xie, M.-X.; Li, S.; Jiang, M.; Wang, Y.-D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. Biochim. Biophys. Acta Gen. Subj. 2004, 1674, 205–214. [Google Scholar] [CrossRef]
- Tsai, P.-J.; She, C.-H. Significance of phenol-protein interactions in modifying the antioxidant capacity of peas. J. Agric. Food Chem. 2006, 54, 8491–8494. [Google Scholar] [CrossRef]
- Liu, E.-H.; Qi, L.-W.; Li, P. Structural relationship and binding mechanisms of five flavonoids with bovine serum albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure-affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Dykes, L.; Awika, J.M. Ultra performance liquid chromatography–tandem quadrupole mass spectrometry profiling of anthocyanins and flavonols in cowpea (Vigna unguiculata) of varying genotypes. J. Agric. Food Chem. 2012, 60, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Kayodé, A.P.P.; Nout, M.J.R.; Linnemann, A.R.; Hounhouigan, J.D.; Berghofer, E.; Siebenhandl-Ehn, S. Uncommonly high levels of 3-deoxyanthocyanidins and antioxidant capacity in the leaf sheaths of dye sorghum. J. Agric. Food Chem. 2011, 59, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 128, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein–flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.N.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| No. | λmax, nm | [M-H]− | MS/MS | Molecular Formula | Compound |
|---|---|---|---|---|---|
| 1 | 210, 279 | 451 | 289 (100), 451 (17) | C21H24O11 | Catechin 3-O-glucoside |
| 2 | 210, 280 | 137 | 137 (100), 101 (33), 109 (33) | C7H6O3 | Protocatechuic aldehyde |
| 3 | 210, 282 | 577 | 109 (100), 289 (68), 123 (63), 245 (44) | C15H14O6 | Procyanidin B |
| 4 | 284 | 121 | 121 (100) | C7H6O2 | p-hydroxybenzaldehyde |
| 5 | 210, 289 | 153 | 131 (100), 147 (28) | C7H6O4 | 2,4-dihydroxybenzoic acid |
| 6 | 210, 308 | 163 | 119 (100) | C9H8O3 | p-coumaric acid |
| 7 | 217, 322 | 193 | 134 (100), 178 (30) | C10H10O4 | Ferulic acid |
| 8 | 210, 319 | 355 | 209 (100), 191 (45), 147 (21) | C15H16O10 | p-coumaroyl aldaric acid |
| 9 | 210, 319 | 355 | 209 (100), 191 (45), 147 (21) | C15H16O10 | p-coumaroyl aldaric acid |
| 10 | 210, 326 | 385 | 191 (100), 209 (57), 147 (21) | C16H18O11 | Feruloylaldaric acid |
| 11 | 210, 326 | 385 | 191 (100), 209 (54), 147 (24) | C16H18O11 | Feruloylaldaric acid |
| 12 | 210, 326 | 385 | 191 (100), 209 (61), 147 (22) | C16H18O11 | Feruloylaldaric acid |
| 13 | 210, 327 | 399 | 191 (100), 173 (46), 147 (27) | C17H20O11 | Feruloyl methylaldaric acid |
| 14 | 210, 327 | 399 | 191 (100), 173 (46), 147 (27) | C17H20O11 | Feruloyl methylaldaric acid |
| 15 | 210, 327 | 399 | 191 (100), 173 (46), 147 (27) | C17H20O11 | Feruloyl methylaldaric acid |
| 16 | 210, 320 | 413 | 191 (100), 173 (56), 147 (21) | C22H22O8 | 1,3-coumaroyl-feruloyl-glycerol |
| 17 | 210, 279 | 435 | 435 (100), 137 (49), 299 (48), 161 (20), 135 (15), 273 (4) | C21H24O10 | (Epi)afzelechin O-glucoside |
| 18 | 211, 269, 370 | 625 | 625 (100), 300 (79), 301 (56) | C27H30O17 | Quercetin O-dihexoside |
| 19 | 212, 371 | 271 | 151 (100), 177 (30), 271 (12) | C15H12O5 | Naringenin |
| 20 | 210, 321 | 463 | 463 (100), 301 (83) | C21H20O12 | Quercetin O-hexoside |
| 21 | 212, 279, 487 | 269 | 269 (100) | C15H11O5+ | Luteolinidin |
| 22 | 210, 277 | 287 | 139 (100), 193 (39), 287 (17) | C15H12O6 | Eriodictyol |
| 23 | 210, 276, 320, 475 | 253 | 253 (100), 117 (30) | C15H11O4+ | Apigeninidin |
| 24 | 214, 335 | 285 | 285 (100), 133 (59) | C15H10O6 | Luteolin |
| 25 | 215, 267, 337 | 269 | 117 (100), 149 (27), 269 (13) | C15H10O5 | Apigenin |
| Sample | Raw Rice | Raw Cowpea | Raw Leaves | Raw Mix | Boiled Rice | Cooked Rice–Cowpea Blend | Total |
|---|---|---|---|---|---|---|---|
| Soluble free phenolics | |||||||
| p-hydroxybenzaldehyde | 22.1 ± 0.2 b | nd | nd | nd | 1.5 ± 0.0 a | nd | 23.6 ± 0.2 D |
| p-coumaric acid | 20.4 ± 0.6 a | nd | nd | nd | 22.4 ± 2.3 a | nd | 42.8 ± 2.1 F |
| p-coumaroyl aldaric acid | nd | 13.8 ± 1.2 b | nd | 5.5 ± 0.0 a | nd | 5.0 ± 0.1 a | 24.3 ± 1.4 D |
| p-coumaroyl aldaric acid | nd | nd | nd | nd | nd | 6.7 ± 0.1 a | 6.7 ± 0.1 B |
| Feruloyl methylaldaric acid | nd | nd | nd | 20.0 ± 2.0 a | nd | nd | 20.0 ± 2.0 D |
| Feruloylaldaric acid | nd | 19.6 ± 1.6 c | nd | 1.2 ± 0.0 a | nd | 10.0 ± 0.8 b | 30.8 ± 1.7 E |
| Feruloylaldaric acid | nd | 9.7 ± 0.9 a | nd | nd | nd | nd | 9.7 ± 0.9 B |
| Feruloyl methylaldaric acid | nd | 8.1 ± 0.4 a | nd | 2.0 ± 0.2 a | nd | 1.5 ± 0.1 a | 11.6 ± 0.4 B |
| Feruloyl methylaldaric acid | nd | 20.4 ± 0.7 b | nd | 21.8 ± 2.9 a | nd | 10.2 ± 0.3 a | 52.4 ± 0.4 G |
| Feruloyl methylaldaric acid | nd | 15.8 ± 0.8 a | nd | nd | nd | nd | 15.8 ± 0.8 C |
| 1,3-coumaroyl-feruloyl-glycerol | nd | 3.3 ± 0.1 a | nd | nd | nd | nd | 3.3 ± 0.1 A |
| Total | 42.5 ± 0.5 C | 90.7 ± 1.9 E | nd | 50.5 ± 1.2 D | 23.9 ± 2.0 A | 33.4 ± 0.4 B | |
| Insoluble bound phenolics | |||||||
| protocatechuic aldehyde | nd | 28.6 ± 0.5 c | nd | 13.4 ± 0.4 a | 17.5 ± 0.3 b | nd | 59.4 ± 0.5 B |
| p-hydroxybenzaldehyde | nd | nd | nd | nd | nd | nd | nd |
| 2,4-dihydroxy benzoic acid | nd | 17.2 ± 0.3 c | nd | 12.8 ± 0.8 b | 3.8 ± 0.1 a | nd | 33.8 ± 0.6 A |
| p-coumaric acid | 5.6 ± 0.4 a | 37.3 ± 0.2 c | 4208.1 ± 22.3 e | 50.7 ± 0.4 d | 24.3 ± 0.0 b | nd | 4326.1 ± 22.1 D |
| ferulic acid | 38.8 ± 1.3 b | 23.0 ± 0.1 a | nd | 40.0 ± 0.1 b | 39.8 ± 0.3 b | 39.5 ± 0.1 b | 181.2 ± 1.6 C |
| Total | 44.4 ± 1.7 B | 106.2 ± 1.0 D | 4208.1 ± 22.3 F | 116.9 ± 1.2 E | 85.5 ± 0.7 C | 39.5 ± 0.1 A | |
| Sample | Raw Rice | Raw Cowpea | Raw Leaves | Raw Mix | Boiled Rice | Cooked Rice–Cowpea Blend | Total |
|---|---|---|---|---|---|---|---|
| Soluble free flavonoids and anthocyanins | |||||||
| Catechin O-glucoside | nd | 328.9 ± 14.9 c | nd | 168.7 ± 6.2 b | nd | 57.3 ± 1.1 a | 554.9 ± 7.7 C |
| Procyanidin B1 | nd | 156.4 ± 0.9 c | nd | 70.2 ± 4.0 b | nd | 43.4 ± 0.9 a | 270.0 ± 4.1 B |
| (Epi)-afzelechin O-glucoside | nd | 255.9 ± 31.1 c | nd | 61.2 ± 5.5 a | nd | 105.9 ± 15.1 b | 422.9 ± 36.4 BC |
| Quercetin dihexoside | nd | 7.9 ± 0.2 c | nd | 3.8 ± 0.1 b | nd | 2.4 ± 0.2 a | 14.1 ± 0.4 A |
| Quercetin hexoside | nd | 16.4 ± 0.5 b | nd | nd | nd | 6.5 ± 0.3 a | 22.9 ± 0.5 A |
| Naringenin | nd | nd | 403.5 ± 4.1 b | 9.6 ± 0.2 a | nd | nd | 413.1 ± 4.1 BC |
| Luteolinidin | nd | nd | 3340.8 ± 308.0 b | 15.3 ± 0.1 a | nd | nd | 3356.1 ± 308.2 F |
| Eriodictyol | nd | nd | 2163.5 ± 171.9 c | 12.4 ± 0.3 b | nd | 5.4 ± 0.0 a | 2181.3 ± 171.6 E |
| Apigeninidin | nd | nd | 71,881.9 ± 351.6 c | 299.5 ± 2.7 b | nd | 3.4 ± 0.1 a | 72,184.8 ± 352.8 H |
| Luteolin | nd | nd | 1077.5 ± 36.2 b | 5.2 ± 0.2 a | nd | 1.5 ± 0.1 a | 1084.2 ± 36.1 D |
| Apigenin | nd | nd | 3630.3 ± 27.8 c | 17.7 ± 0.1 b | nd | 4.3 ± 0.4 a | 3652.3 ± 27.8 G |
| Total | nd | 765.5 ± 18.4 C | 82,497.6 ± 494.9 D | 663.6 ± 15.2 B | nd | 230.1 ± 5.2 A | |
| Sample | RAW RICE | Raw Cowpea | Raw Leaves | Raw Mix | Boiled Rice | Cooked Rice–Cowpea Blend |
|---|---|---|---|---|---|---|
| Soluble free phenolics | ||||||
| TPC | 0.2 ± 0.0 a | 5.6 ± 0.2 d | 75.5 ± 1.2 e | 2.4 ± 0.0 c | 0.2 ± 0.0 a | 1.8 ± 0.1 b |
| TEAC | 1.8 ± 0.0 a | 51.7 ± 1.2 d | 1950.0 ± 208.4 e | 31.3 ± 0.5 c | 2.3 ± 0.0 a | 26.6 ± 1.3 b |
| DPPH | 0.6 ± 0.0 a | 20.6 ± 1.3 d | 595.4 ± 138.2 e | 10.5 ± 0.5 c | 0.5 ± 0.0 a | 7.5 ± 0.3 b |
| ORAC | 11.1 ± 0.7 a | 624.0 ± 112.0 d | 36,530.2 ± 761.6 e | 456.0 ± 38.0 c | 11.2 ± 0.2 e | 103.7 ± 1.3 b |
| Total-free | 13.7 ± 0.8 a | 701.9 ± 114.3 d | 39,151.1 ± 771.4 e | 500.2 ± 39.8 c | 14.2 ± 0.5 a | 139.6 ± 2.1 b |
| Insoluble bound phenolics | ||||||
| TPC | 0.1 ± 0.0 a | 1.4 ± 0.3 c | 19.0 ± 1.0 d | 0.6 ± 0.1 b | 0.1 ± 0.0 a | 0.6 ± 0.0 b |
| TEAC | 1.9 ± 0.3 a | 18.0 ± 2.9 d | 307.7 ± 21.8 e | 6.5 ± 0.8 b | 1.6 ± 0.4 a | 9.8 ± 0.5 c |
| DPPH | 0.4 ± 0.0 a | 6.4 ± 0.9 c | 166.0 ± 16.2 d | 2.8 ± 0.7 b | 0.3 ± 0.0 a | 2.8 ± 0.1 b |
| ORAC | 80.6 ± 5.8 c | 180.7 ± 22.9 d | 3613.0 ± 76.8 e | 68.9 ± 5.7 b | 4.6 ± 0.3 a | 35.1 ± 0.7 b |
| Total-bond | 83 ± 5.8 c | 206.5 ± 23.1 d | 4105.7 ± 84.1 e | 78.8 ± 6.5 c | 6.6 ± 0.4 a | 48.3 ± 0.7 b |
| Overall total | 96.7 ± 5.9 b | 908.4 ± 115.8 e | 43,256.8 ± 780.6 f | 579.0 ± 40.4 d | 20.8 ± 0.6 a | 187.9 ± 2.4 c |
| Sample | Raw Rice | Raw Cowpea | Raw Leaves | Raw Mix | Boiled Rice | Cooked Rice–Cowpea Blend |
|---|---|---|---|---|---|---|
| Principal component 1 | 2.21788 | 6.39497 | 82.70456 | 3.72584 | 2.27364 | 2.68310 |
| Principal component 2 | 19.89433 | 63.53699 | 0.62503 | 1.11919 | 9.99322 | 4.83123 |
| Principal component 3 | 18.99977 | 3.14427 | 0.00002 | 2.28827 | 11.44832 | 64.11934 |
| Principal component 4 | 20.59325 | 8.62702 | 0.00091 | 49.92923 | 11.83996 | 9.00963 |
| Principal component 5 | 21.62809 | 1.63008 | 0.00280 | 26.27080 | 47.77819 | 2.69004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apea-Bah, F.B.; Li, X.; Beta, T. Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants. Foods 2021, 10, 2058. https://doi.org/10.3390/foods10092058
Apea-Bah FB, Li X, Beta T. Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants. Foods. 2021; 10(9):2058. https://doi.org/10.3390/foods10092058
Chicago/Turabian StyleApea-Bah, Franklin Brian, Xiang Li, and Trust Beta. 2021. "Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants" Foods 10, no. 9: 2058. https://doi.org/10.3390/foods10092058
APA StyleApea-Bah, F. B., Li, X., & Beta, T. (2021). Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants. Foods, 10(9), 2058. https://doi.org/10.3390/foods10092058