Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet
Abstract
:1. Introduction
2. Type of Resistant Starch and Its Content in Mediterranean Food
3. Resistant Starch in Human Nutritional Intervention Studies: GI and Impact on Inflammation and Gut Microbiome
3.1. Resistant Starch and Enhancement of Glycemic Control
3.2. Resistant Starch, Gut Microbiome and Inflammation
3.3. RS, Blood Lipid Profile and Cytokines Levels
4. Potential Mechanisms of Resistant Starch in Prevention of Colon Cancer
5. Food Claims Regarding Resistant Starch
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Juyal, R.; Hossain, M.M.; Singh, A. Non-communicable diseases viewed as “collateral damage” of our decisions: Fixing accountabilities and finding sloutions in primary care settings. J. Fam. Med. Prim. Care 2020, 9, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Gortmaker, S.L.; Swinburn, B.A.; Levy, D.; Carter, R.; Mabry, P.L.; Finegood, D.; Huang, T.; Moodie, M. Changing the future of obesity: Science, policy, and action. Lancet 2011, 378, 838–847. [Google Scholar] [CrossRef] [Green Version]
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.; Depres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Hörmann-Wallner, M.; Krause, R.; Alfaro, B.; Jilani, H.; Laureati, M.; Almi, V.L.; Sandell, M.; Sandvik, P.; Zeinstra, G.Z.; Methven, L. Intake of Fibre-Associated Foods and Texture Preferences in Relation to Weight Status Among 9-12 Years Old Children in 6 European Countries. Front Nutr. 2021, 8, 633807. [Google Scholar] [CrossRef]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to microRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Longeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Woo, K.; Seib, P. Cross-linked resistant starch: Preparation and properties 1. Cereal Chem. 2002, 79, 819–825. [Google Scholar] [CrossRef]
- Hasjim, J.; Lee, S.-O.; Hendrich, S.; Setiawan, S.; Setiawan, A.; Ai, Y.; Jane, J.L. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010, 87, 257–262. [Google Scholar] [CrossRef]
- Ibrahim, O.M. Effects of processing and additives on starch physicochemical and digestibility properties. Carbohydr. Polym. Technol. Appl. 2021, 2, 100039. [Google Scholar]
- Brighenti, F.; Casiraghi, M.C.; Baggio, C. Resistant starch in the Italian diet. Br. J. Nutr. 1998, 80, 333–341. [Google Scholar]
- Platel, K.; Shurpalekar, K.S. Resistant starch content of Indian foods. Plant. Foods Hum. Nutr. 1994, 45, 91–95. [Google Scholar] [CrossRef]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant Starch Content in Foods Commonly Consumed in the United States: A Narrative Review. J. Acad. Nutr. Diet 2020, 120, 230–244. [Google Scholar] [CrossRef]
- Fabbri, A.D.T.; Schacht, R.W.; Crosby, G.A. Evaluation of Resistant Starch Content of Cooked Black Beans, Pinto Beans and Chickpeas. NFS J. 2016, 3, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Narwojsz, A.; Borowska, E.J.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. Effect of Different Methods of Thermal Treatment on Starch and Bioactive Compounds of Potato. Plant Foods Hum. Nutr. 2020, 75, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiturong, P.; Laosirisathian, N.; Sirithunyalug, B.; Eitssayeam, S.; Sirilun, S.; Chaiyana, W.; Sirithunyalug, J. Physicochemical and prebiotic properties of resistant starch from Musa sapientum Linn., ABB group, cv. Kluai Namwa Luang. Heliyon 2020, 6, e05789. [Google Scholar] [CrossRef]
- Okumus, B.N.; Tacer-Caba, Z.; Kahraman, K.; Nilufer-Erdil, D. Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chem. 2018, 240, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Polesi, L.F.; Sarmento, S.B.S. Structural and physicochemical characterization of resistant starch prepared using hydrolysis and heat treatments of chickpea starch. Starch-Starke 2011, 63, 226–235. [Google Scholar] [CrossRef]
- Dingting, Z.; Zhen, M.; Xinzhong, H. Isolated Pea Resistant Starch Substrates with Different Structural Features Modulate the Production of Short-Chain Fatty Acids and Metabolism of Microbiota in Anaerobic Fermentation In Vitro. J. Agric. Food Chem. 2021, 69, 5392–5404. [Google Scholar]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef]
- Guo, J.; Liu, L.; Lian, X.; Li, L.; Wu, H. The properties of different cultivars of Jinhai sweet potato starches in China. Int. J. Biol. Macromol. 2014, 67, 1–6. [Google Scholar] [CrossRef]
- Barreto, F.F.V.; Bello-Pérez, L.A. Chemical, Structural, Technological Properties and Applications of Andean Tuber Starches: A Review. Food Rev. 2021, 1–16. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.T.; Poh, G.Y.Y.; Kelvin, K.; Goh, T.; Wee, M.S.W.; Henry, C.J. Comparison of physicochemical properties of jackfruit seed starch with potato and rice starches. Int. J. Food Prop. 2021, 24, 364–379. [Google Scholar] [CrossRef]
- Eerlingen, R.C.; Crombez, M.; Delcour, J.A. Enzyme-Resistant starch. I. Quantitative and qualitative influence of incubation-time and temperature of autoclaved starch on resistant starch formation. Cereal Chem. 1993, 70, 339–344. [Google Scholar]
- Shamai, K.; Shimoni, E.; Bianco-Peled, H. Small-angle X-ray scattering of resistant starch type III. Biomacromolecules 2004, 5, 219–223. [Google Scholar] [CrossRef]
- Mutungi, C.; Onyango, C.; Doert, T.; Paasch, S.; Thiele, S.; Machill, S.; Jaros, D.; Rohm, H. Long- and short-range structural changes of recrystallised cassava starch subjected to in vitro digestion. Food Hydrocoll. 2011, 25, 477–485. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Molecular-Organization in Starches—A C-13 Cp Mas Nmr-Study. J. Am. Chem. Soc. 1985, 107, 7040–7044. [Google Scholar] [CrossRef]
- Thèrien, H. Study of hydration of cross-linked high amylose starch by solid-state 13C NMR spectroscopy. Carbohydr. Res. 2007, 342, 1525–1529. [Google Scholar] [CrossRef]
- Thompson, D.B. Strategies for the manufacture of resistant starch. Trends Food Sci. Technol. 2000, 11, 245–253. [Google Scholar] [CrossRef]
- Katoh, E.; Murata, K.; Fujita, N. 13C CP/MAS NMR Can Discriminate Genetic Backgrounds of Rice Starch. ACS Omega 2020, 5, 24592–24600. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernandez-Hernandez, O.; Rodrıguez-Sanchez, S.; Sanz, M.L.; Martınez-Castro, I. Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Mahadevamma, S.; Prashanth, K.H.; Tharanathan, R. Resistant starch derived from processed legumes–Purification and structural characterization. Carbohydr. Polym. 2003, 54, 215–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Zhang, Y.; Chen, K.; Chang, H.; Ma, C.; Jiang, S.; Huo, D.; Liu, W.; Jha, R.; et al. Synergistic Effects of the Jackfruit Seed Sourced Resistant Starch and Bifidobacterium pseudolongum subsp. globosum on Suppression of Hyperlipidemia in Mice. Foods. 2021, 10, 1431. [Google Scholar] [CrossRef]
- Johansson, E.V.; Nilsson, A.C.; Östman, E.M.; Björck, I.M.E. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr. J. 2013, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.C.; Johansson-Boll, E.V.; Bjorck, I.M. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernelbased product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 2017, 61, 1600129. [Google Scholar] [CrossRef] [PubMed]
- Ayua, E.O.; Nkhata, S.G.; Namaumbo, S.J.; Kamau, E.H.; Ngoma, T.N.; Aduol, K.O. Polyphenolic inhibition of enterocytic starch digestion enzymes and glucose transporters for managing type 2 diabetes may be reduced in food systems. Heliyon 2021, 12, e06245. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.; Sánchez-Zapata, E.; Pérez-Álvarez, J. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Burton, P.; Lightowler, H. The impact of freezing and toasting on the glycaemic response of white bread. Eur. J. Clin. Nutr. 2008, 62, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003, 83, 395–402. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values. Diabet. Care 2008, 3, 2281–2283. [Google Scholar] [CrossRef] [Green Version]
- Salmeron, J.; Monson, J.E.; Stampfer, M.S.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary Fiber, Glycemic Load and Risk of Non-Insulin Dependent Diabetes Mellitus on Woman. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary Fiber, Glycemic Response and Diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physico-Chemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Bertoft, E.; Blennow, A. Structure of Potato Starch. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Elsevier: Copenaghen, Denmark, 2008; pp. 83–98. [Google Scholar]
- Pérez, S.; Bertoft, E. The Molecular Structures of Starch Components and Their Contribution to the Architecture of Starch Granules: A Comprehensive Review. Starch 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Lynch, D.R.; Liu, Q.; Tarn, T.R.; Bizimungu, B.; Chen, Q.; Harris, P.; Chik, C.L.; Skjodt, N.M. Glycemic Index—A Review and Implications for the Potato Industry. Am. J. Potato Res. 2007, 84, 179–190. [Google Scholar] [CrossRef]
- Fernandes, G.; Velangi, A.; Wolever, T.M.S. Glycemic Index of Potatoes Commonly Consumed in North America. J. Acad. Nutr. Diet. 2005, 105, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Dodevska, M.S.; Sobajic, S.S.; Djordjevic, P.B.; Dimitrijevic-Sreckovic, V.S.; Spasojevic-Kalimanovska, V.S.; Djordjevic, B.I. Effects of total fibre or resistant starch-rich diets within lifestyle intervention in obese prediabetic adults. Eur. J. Nutr. 2016, 55, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, C.; Re, A.; Pisconti, S.; Trombetti, M.; Perri, M.; Colosimo, M.; D’Amato, G.; Gallelli, L.; Cannataro, R.; Molinario, C.; et al. Dysbiosis in intestinal microbiome linked to fecal blood determined by direct hybridization. Biotech 2020, 10, 358. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, Y.Y.; Hoffmann, C.; Bittinger, K.; Chen, Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Asp, N.G.; van Amelsvoort, J.M.M.; Hautvast, J.G.A.J. Nutritional implications of resistant starch. Nutr. Res. Rev. 1996, 9, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and non-starch polysaccharides. Physiol Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Hu, Y.; Brown, I.L.; Young, G.P. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. J. Nutr. Metab. 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpaia, N.; Rudensky, A.Y. Microbial metabolites control gut inflammatory responses. Proc. Natl. Acad. Sci. USA 2014, 111, 2058–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F.; Phillips, G.J. Diet, Genes, and Microbes: Complexities of Colon Cancer Prevention. Toxicol Pathol. 2014, 42, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, 23, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Bush, J.R.; Alfa, M.J. Increasing levels of Parasutterella in the gut microbiome correlate with improving low-density lipoprotein levels in healthy adults consuming resistant potato starch during a randomised trial. BMC Nutr. 2020, 11, 72. [Google Scholar]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018, 4, 260. [Google Scholar] [CrossRef] [Green Version]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems 2021, 6, e00115–e00121. [Google Scholar]
- Luo, R.; Li, X.; Jiang, R.; Gao, X.; Lu, Z.; Hua, W. Serum Concentrations of Resistin and Adiponectin and Their Relation-ship to Insulin Resistance in Subjects with Impaired Glucose Tolerance. J. Int. Med. Res. 2012, 40, 621–630. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Lee, A.; Weidauer, L.A.; Wey, H.E.; Beare, T.M.; Specker, B.L.; Dey, M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double-blind controlled crossover intervention. Mol. Nutr. Food Res. 2014, 58, 1365–1369. [Google Scholar] [CrossRef]
- Park, O.J.; Ekang, N.; Chang, M.J.; Kim, W.K. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J. Nutr. Sci. Vitaminol. 2004, 50, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Bai, H.; Yu, Q.; Yan, J.; Zhao, L.; Wang, S.; Zhaoping, L.; Wang, Q.; Chen, L. High-resistant starch, low-protein flour intervention on patients with early type 2 diabetic nephropathy: A randomized trial. J. Ren Nutr. 2019, 29, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.C.; Björck, I.M.E.; Nilsson, A.C. Impact of rye-based evening meals on cognitive functions, mood and cardiometabolic risk factors: A randomized controlled study in healthy middle-aged subjects. Nutr. J. 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tan, L.; Kong, L. Impact of dietary intake of resistant starch on obesity and associated metabolic profiles in human: A systematic review of the literature. Crit. Rev. Food 2021, 61, 889–905. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory function of buckwheat-resistant starch supplementation on lipid profile and gut microbiota in mice fed with a high-fFat diet. J. Food. Sci 2019, 84, 2674–2681. [Google Scholar] [CrossRef] [PubMed]
- Gargari, B.P.; Namazi, N.; Khalili, M.; Sarmadi, B.; Jafarabadi, M.A.; Dehghan, P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement. Ther. Med. 2015, 23, 810–815. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Stintzi, A. Resistant starch, microbiome, and precision modulation. Gut Microbes. 2021, 13, 1926842. [Google Scholar] [CrossRef]
- Esgalhado, M.; Kemp, J.A.; Azevedo, R.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018, 9, 6508–6516. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Peterson, C.M.; Beyl, R.A.; Marlatt, K.L.; Martin, C.K.; Aryana, K.J.; Marco, M.L.; Martin, R.J.; Keenan, M.J.; Ravussin, E. Effect of 12 wk of resistant starch supplementation on cardiometabolic risk factors in adults with prediabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 492–501. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Gholami, A.; Hariri, M. Effect of resistant starch type 2 on inflammatory mediators: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 56, 102597. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.B.; Navarro, S.L.; Hullar, M.A.J.; Lampem, J.W. Diet and Gut Microbes Act Coordinately to Enhance Programmed Cell Death and Reduce Colorectal Cancer Risk. Dig. Dis. Sci. 2020, 65, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.; Martin, R.J. Role of Resistant Starch in Improving Gut Health, Adiposity, and Insulin Resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Humphreys, K.J.; Conlon, M.A.; Young, G.P.; Topping, D.L.; Hu, Y.; Winter, J.M.; Bird, A.R.; Cobiac, L.; Kennedy, N.A.; Michael, M.Z.; et al. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: A randomized trial. Cancer Prev. Res. 2014, 7, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Bingham, S.A.; Cummings, J.H. Starch intake and colorectal cancer risk: An international comparison. Br. J. Cancer 1994, 69, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.A.; Coxhead, J.M.; Mathers, J.C. Anti-cancer effects of butyrate: Use of micro-array technology to investigate mechanisms. Proc. Nutr. Soc. 2003, 62, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, J.C.; Smith, H.; Carter, S. Dose-response effects of raw potato starch on small-intestinal escape, large-bowel fermentation and gut transit time in the rat. Br. J. Nutr. 1997, 78, 1015–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.P.; Cherut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Nutr. Soc. 2003, 62, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, K.; Shirazi-Beechey, S.P. Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol. 2006, 25, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toden, S.; Bird, A.R.; Topping, D.L.; Conlon, M.A. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol. Ther. 2006, 5, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Conlon, M.A.; Kerr, C.A.; McSweeney, C.S.; Dunne, R.A.; Shaw, J.M.; Kang, S.; Bird, A.R.; Morell, M.K.; Lockett, T.J.; Molloy, P.L.; et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a Western diet. J. Nutr. 2012, 142, 832–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordonaro, M. Crosstalk between Wnt signaling and RNA processing in colorectal cancer. J. Cancer 2013, 4, 96–103. [Google Scholar] [CrossRef]
- Bordonaro, M.; Mariadason, J.M.; Aslam, F.; Heerdt, B.G.; Augenlicht, L.H. Butyrate-induced apoptotic cascade in colonic carcinoma cells: Modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A but not curcumin. Cell Growth Differ. 1999, 10, 713–720. [Google Scholar]
- Lazarova, D.L.; Bordonaro, M.; Carbone, R.; Sartorelli, A.C. Linear relationship between Wnt activity levels and apoptosis in colorectal carcinoma cells exposed to butyrate. Int. J. Cancer 2004, 110, 523–531. [Google Scholar] [CrossRef]
- Del Cornò, M.; Donninelli, G.; Conti, L.; Gessani, S. Linking Diet to Colorectal Cancer: The Emerging Role of MicroRNA in the Communication between Plant and Animal Kingdoms. Front. Microbiol. 2017, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Bauer-Marinovic, M.; Florian, S.; Müller-Schmehl, K.; Glatt, H.; Jacobasch, G. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis 2006, 27, 1849–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of Intestinal Microbial-Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients. 2019, 11, 1026. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Schumacher, U.; Ronaasen, V.; Coates, M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut 1995, 36, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarr, S.E.; Ridlon, J.M.; Hylemon, P.B. Diet, anaerobic bacterial metabolism, and colon cancer: A review of the literature. J. Clin. Gastroenterol. 2005, 39, 98–109. [Google Scholar] [PubMed]
- Jacobasch, G.; Dongowski, G.; Schmiedl, D.; Muller-Schmehl, K. Hydrothermal treatment of Novelose 330 results in high yield of resitant starch type 3 with beneficial prebiotic properties and decreased secondary bile acid formation in rats. Br. J. Nutr. 2006, 95, 1063–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Scientific opinion on the substantiation of health claims related to resistant starch and reduction of post-prandial glycaemic responses (ID 681), “digestive health benefits” (ID 682) and “favours a normal colon metabolism” (ID 783) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2024.
- Food and Drug Administration, Department of Health and Human Services. High-Amylose Starch and Diabetes. Docket Number FDA2015-Q-2352; Food and Drug Administration: College Park, MD, USA, 2015.



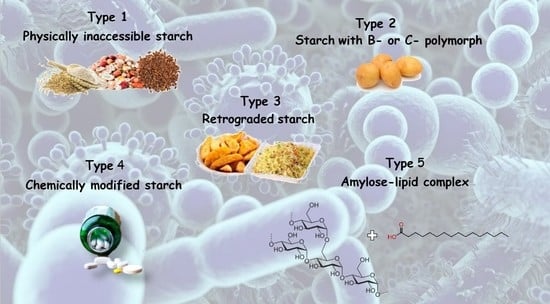
| Classification | Description | Example |
|---|---|---|
| RS1 | Physically inaccessible starch | Whole grains |
| RS2 | Starch with B- or C-polymorph | Uncooked potato, high-amylose maize starch |
| RS3 | Retrograded starch | Cooked and cooled potato starch |
| RS4 | Chemically modified starch | Cross-linked starch in thickeners |
| RS5 | Amylose–lipid complex | Palmitic acid-amylose complex |
| Sample | TT | TS (%) | RDS (%) | SDS (%) | RS (%) | HI | pGI | Reference |
|---|---|---|---|---|---|---|---|---|
| Bean flour | RAW | 43.14 ± 0.14 c | 82.11 ± 0.43 b | 10.25 ± 0.45 i | 7.64 ± 0.58 f | 87.71 ± 0.44 b | 87.86 ± 0.60 a | |
| ANN | 43.06 ± 0.33 c | 77.93 ± 0.68 d | 11.11 ± 0.54 h | 10.96 ± 0.19 c | 78.11 ± 0.59 e | 82.59 ± 0.49 d | [17,18,19] | |
| HMT | 43.65 ± 0.31 c | 59.63 ± 0.65 g | 25.11 ± 0.96 c | 15.26 ± 0.43 a | 64.67 ± 0.27 j | 75.21 ± 0.11 e | ||
| Broad bean flour | RAW | 43.43 ± 0.57 c | 80.26 ± 0.22 c | 11.14 ± 0.44 h | 8.60 ± 0.32 e | 84.66 ± 0.42 c | 86.18 ± 0.58 b | |
| ANN | 42.26 ± 0.67 d | 73.75 ± 0.33 e | 16.61 ± 0.55 e | 9.64 ± 0.26 d | 76.42 ± 0.03 f | 81.66 ± 0.04 d | [17,18,19] | |
| HMT | 42.11 ± 0.76 d | 60.52 ± 0.68 g | 26.19 ± 0.76 c | 13.29 ± 0.43 b | 68.42 ± 0.80 g | 77.27 ± 0.08 e | ||
| Chickpea flour | RAW | 45.32 ± 0.29 b | 85.26 ± 0.77 a | 9.05 ± 0.76 j | 5.69 ± 0.46 g | 90.15 ± 0.39 a | 89.20 ± 0.37 a | |
| ANN | 44.95 ± 0.87 b | 77.10 ± 0.19 d | 14.26 ± 0.20 f | 8.64 ± 0.09 e | 76.16 ± 0.85 f | 81.52 ± 0.27 d | [22] | |
| HMT | 44.99 ± 0.55 b | 61.10 ± 0.37 g | 27.11 ± 0.89 b | 11.79 ± 0.78 b | 67.21 ± 0.16 h | 76.60 ± 0.49 e | ||
| Lentil flour | RAW | 47.25 ± 0.11 a | 80.06 ± 0.34 c | 12.68 ± 0.65 g | 7.26 ± 0.61 f | 82.16 ± 0.49 d | 84.81 ± 0.83 c | |
| ANN | 47.61 ± 0.98 a | 70.60 ± 0.44 f | 19.26 ± 0.39 d | 10.14 ± 0.65 c | 75.33 ± 0.55 f | 81.06 ± 0.65 d | [20] | |
| HMT | 47.65 ± 0.54 a | 59.60 ± 0.97 g | 30.14 ± 0.65 a | 10.26 ± 0.17 c | 66.36 ± 0.47 i | 76.14 ± 0.69 e | ||
| Pea | H1 | 59.9 ± 1.78 c | 3.7 ± 0.12 | [23] | ||||
| H2 | 3.2 ± 0.11 | |||||||
| Wheat | H1 | 69.8 ± 1.10 d | 1.9 ± 0.21 | [14,15,16] | ||||
| H2 | 1.8 ± 0.14 | |||||||
| Rice | H1 | 81.4 ± 1.10 f | 1.4 ± 0.16 | [28] | ||||
| H2 | 1.2 ± 0.08 | |||||||
| Barley | H1 | 65.6 ± 0.76 c | 2.8 ± 0.23 | [39,40] | ||||
| H2 | 2.6 ± 0.09 | |||||||
| Potato | H1 | 85.51 ± 1.64 g | 1.8 ± 0.15 | [18,28] | ||||
| H2 | 1.7 ± 0.08 |
| Sample | TS (%) | Thermal Treatment | RS (%) | Reference |
|---|---|---|---|---|
| Bean | 38.34 + 0.7 | Boiled | 4.96 + 0.9 a (12.9) * | |
| Cooked | 8.45 + 1.1 b (22.0) * | [17,19] | ||
| Reheated | 8.24 + 0.3 b (21.5) * | |||
| Chickpea | 41.36 + 1.0 | Boiled | 4.35 + 0.4 a (10.5) * | |
| Cooked | 5.48 + 0.2 b (13.2) * | [17,19] | ||
| Reheated | 5.58 + 0.1 b (13.5) * | |||
| Lentil | 46.72 + 2.1 | Boiled | 7.56 + 0.6 a (16.2) * | |
| Cooked | 8.60 + 0.3 bc (18.4) * | [20] | ||
| Reheated | 7.62 + 0.3 ab (16.3) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cione, E.; Fazio, A.; Curcio, R.; Tucci, P.; Lauria, G.; Cappello, A.R.R.; Dolce, V. Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods 2021, 10, 2062. https://doi.org/10.3390/foods10092062
Cione E, Fazio A, Curcio R, Tucci P, Lauria G, Cappello ARR, Dolce V. Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods. 2021; 10(9):2062. https://doi.org/10.3390/foods10092062
Chicago/Turabian StyleCione, Erika, Alessia Fazio, Rosita Curcio, Paola Tucci, Graziantonio Lauria, Anna Rita Rita Cappello, and Vincenza Dolce. 2021. "Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet" Foods 10, no. 9: 2062. https://doi.org/10.3390/foods10092062
APA StyleCione, E., Fazio, A., Curcio, R., Tucci, P., Lauria, G., Cappello, A. R. R., & Dolce, V. (2021). Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods, 10(9), 2062. https://doi.org/10.3390/foods10092062