Microcapsules Consisting of Whey Proteins-Coated Droplets of Lipids Embedded in Wall Matrices of Spray-Dried Microcapsules Consisting Mainly of Non-Fat Milk Solids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microencapsulation by Spray Drying
2.3. Analyses
2.3.1. Particle Size Distribution
2.3.2. Surface Excess
2.3.3. Protein and Fat Content
2.3.4. Composition of Proteins Adsorbed at the O/W Interface
2.3.5. Core Retention
2.3.6. Microencapsulation Efficiency (MEE)
2.3.7. Oxidative Stability
2.3.8. Scanning Electron Microscopy (SEM)
2.3.9. Statistical Analysis
3. Results
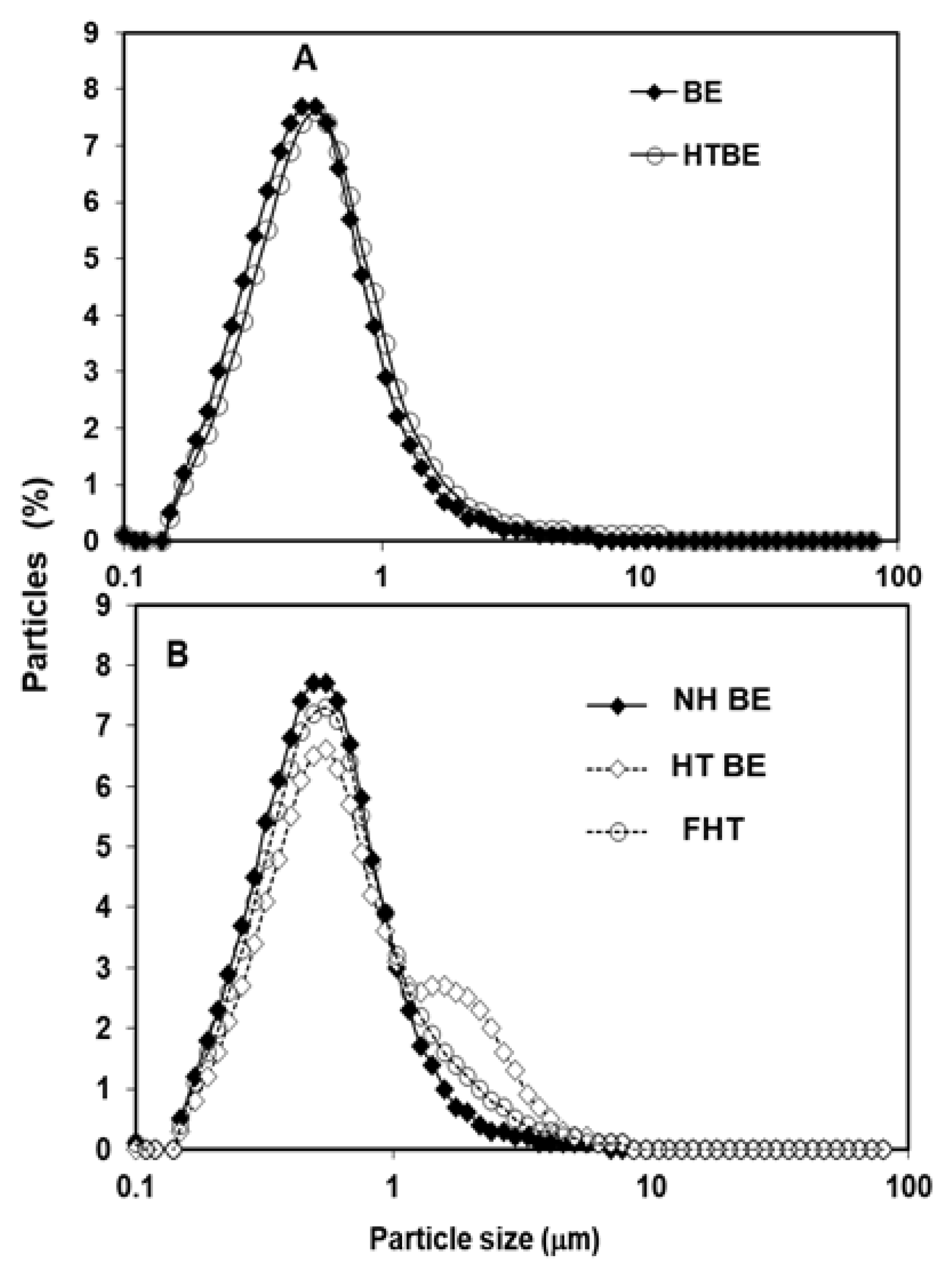
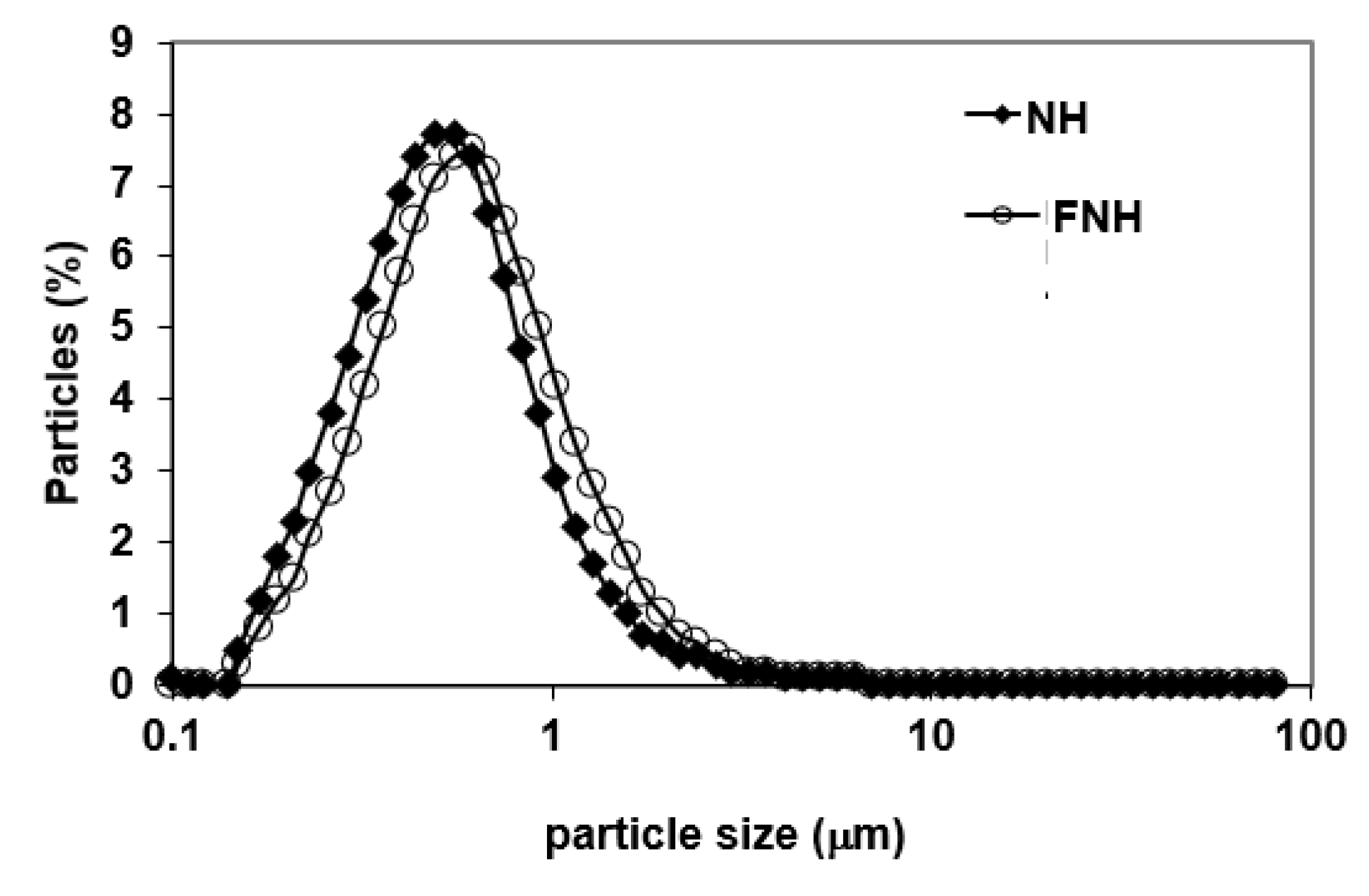
3.1. Particle Size Distribution of CIWEs
3.2. Surface Excess and Protein-Specific-Load
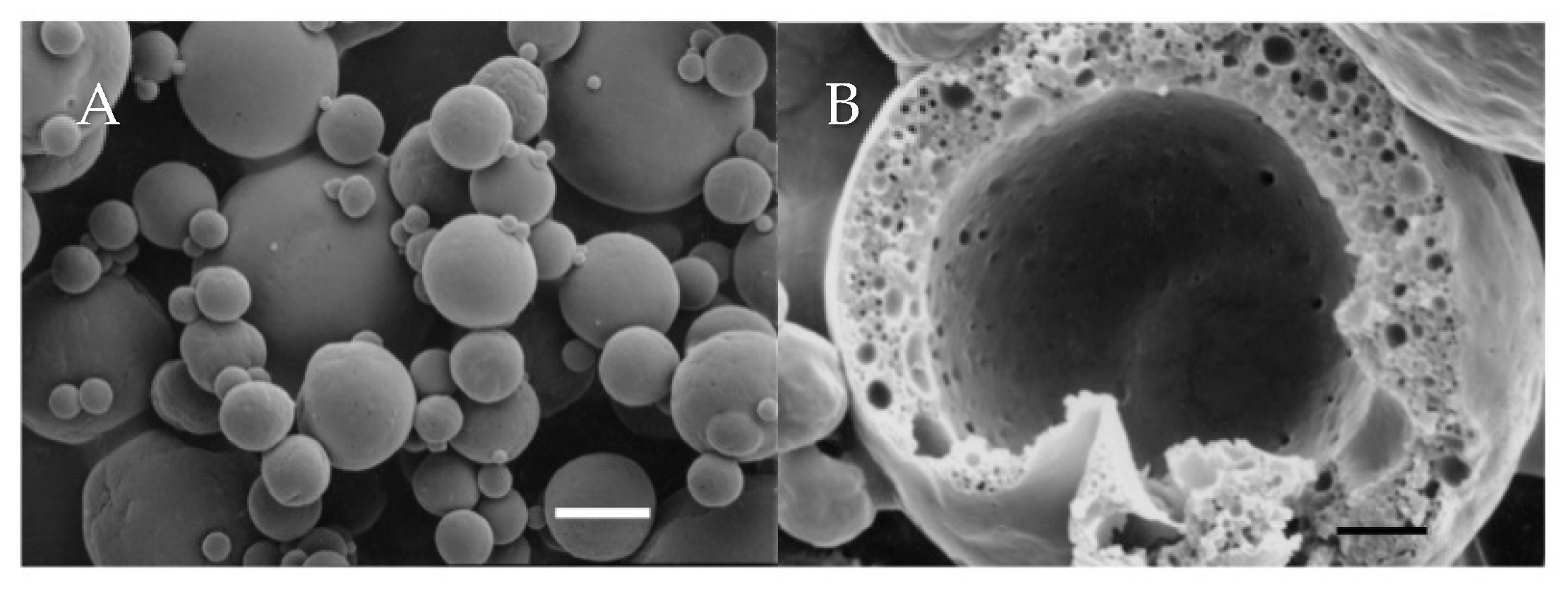
3.3. Microstructure, Core Retention and Microencapsulation Efficiency
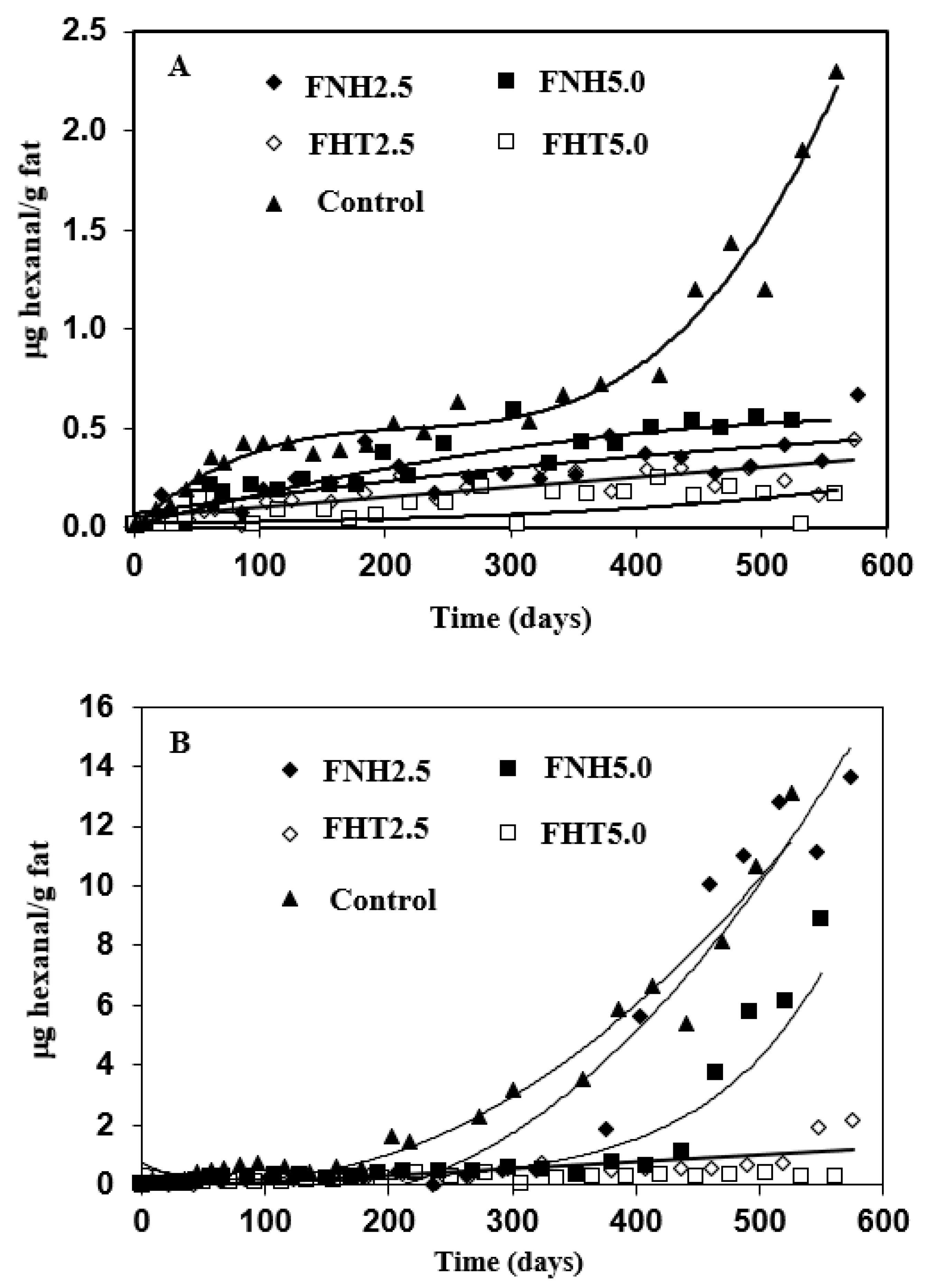
3.4. Oxidative Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gharibzahedi, S.M.T.; George, S.; Greiner, R.; Estevinho, B.N.; Frutos Fernández, M.J.; McClements, D.J.; Roohinejad, S. New Trends in the Microencapsulation of Functional Fatty Acid-Rich Oils Using Transglutaminase Catalyzed Crosslinking. Compr. Rev. Food Sci. Food Saf. 2018, 17, 274–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The New Paradigm for Lipid Oxidation and Insights to Microencapsulation of Omega-3 Fatty Acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and Oxidative Stability of PUFA-Rich Oil Microencapsulated by Spray Drying Using Pea Protein and Pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Hermida, L.G.; Gallardo, G. Chapter 14-Food Applications of Microencapsulated Omega-3 Oils. In Microencapsulation and Microspheres for Food Applications; Sagis, L.M.C., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 271–299. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chen, Q.; Shi, J. Microencapsulation of food ingredients for functional foods. In Functional Food Ingredients and Nutraceuticals; Shi, J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 267–318. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Sanguansri, L.; Augustin, M.A. Microencapsulation and Delivery of Omega-3 Fatty Acids. Functional Food Ingredients and Nutraceuticals: Processing Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 13, pp. 373–407. [Google Scholar]
- Sahin-Yesilcubuk, N.; Akoh, C.C. Encapsulation technologies for lipids. In Food Lipids; Akoh, C.C., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 453–476. [Google Scholar]
- Vega, C.; Roos, Y.H. Invited review: Spray-dried dairy and dairy-like-emulsions compositional considerations. J. Dairy Sci. 2006, 89, 383–401. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.O. Nano-Microencapsulation Technology and Applications in Fortified and Functional Foods. Functional Food Ingredients and Nutraceuticals: Processing Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 13, pp. 319–371. [Google Scholar]
- Estevinho, B.N.; Rocha, F. Chapter 7-Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 191–222. [Google Scholar] [CrossRef]
- Sagis, L.M. Microencapsulation and Microspheres for Food Applications; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Milad, F.; Francesco, D.; Julian, M.D. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Chang, C.; Nickerson, M.T. Encapsulation of omega 3-6-9 fatty acids-rich oils using protein-based emulsions with spray drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mat. Sci. Eng. C-Mater. 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. In Microencapsulation in the Food Industry; Academic Press: San Diego, CA, USA, 2014; pp. 211–226. [Google Scholar] [CrossRef]
- Can Karaca, A.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Tang, C.-H. Microencapsulation properties of protein isolates from three selected Phaseolus legumes in comparison with soy protein isolate. LWT-Food Sci. Technol. 2014, 55, 74–82. [Google Scholar] [CrossRef]
- Zhang, J.; Rosenberg, Y.; Rosenberg, M. Microencapsulation properties of wall systems consisting of WHPI and carbohydrates. Aims Agric. Food 2018, 3, 66–84. [Google Scholar] [CrossRef]
- Rosenberg, M.; Rosenberg, Y.; Frenkel, L. Microencapsulation of model oil in wall matrices consisting of SPI and maltodextrins. Aims Agric. Food 2016, 1, 33–51. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Ind. Crop. Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Ting, S.; Jiang, J.; Liu, Y. Correlating emulsion properties to microencapsulation efficacy and nutrients retention in mixed proteins system. Food Res. Int. 2019, 115, 44–53. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Fäldt, P.; Bergenståhl, B. Spray-dried whey protein/lactose/soybean oil emulsions. 1. Surface composition and particle structure. Food Hydrocoll. 1996, 10, 421–429. [Google Scholar] [CrossRef]
- Fäldt, P.; Bergenståhl, B. Spray-dried whey protein/lactose/soybean oil emulsions. 2. Redispersability, wettability and particle structure. Food Hydrocoll. 1996, 10, 431–439. [Google Scholar] [CrossRef]
- Moreau, D.L.; Rosenberg, M. Microstructure and Fat Extractability in Microcapsules Based on Whey Proteins or Mixtures of Whey Proteins and Lactose. Food Struct. 1993, 12, 457–468. [Google Scholar]
- Rosenberg, M.; Young, S.L.; Brooker, B.E.; Colombo, V.E. Whey Proteins as Microencapsulating Agents-Microencapsulation of Anhydrous Milkfat-Structure Evaluation. Food Struct. 1993, 12, 31–41. [Google Scholar]
- Sheu, T.Y.; Rosenberg, M. Microencapsulation by Spray Drying Ethyl Caprylate in Whey Protein and Carbohydrate Wall Systems. J. Food Sci. 1995, 60, 98–103. [Google Scholar] [CrossRef]
- Sheu, T.Y.; Rosenberg, M. Microstructure of Microcapsules Consisting of Whey Proteins and Carbohydrates. J. Food Sci. 1998, 63, 491–494. [Google Scholar] [CrossRef]
- Moreau, D.L.; Rosenberg, M. Oxidative Stability of Anhydrous Milkfat Microencapsulated in Whey Proteins. J. Food Sci. 1996, 61, 39–43. [Google Scholar] [CrossRef]
- Moreau, D.L.; Rosenberg, M. Porosity of Whey Protein-Based Microcapsules Containing Anhydrous Milkfat Measured by Gas Displacement Pycnometry. J. Food Sci. 1998, 63, 819–823. [Google Scholar] [CrossRef]
- Moreau, D.L.; Rosenberg, M. Porosity of Microcapsules with Wall Systems Consisting of Whey Proteins and Lactose Measured by Gas Displacement Pycnometry. J. Food Sci. 1999, 64, 405–409. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, H.; Taylor, M.W. Composition and Structure of Fat Globule Surface Layers in Recombined Milk. J. Food Sci. 1996, 61, 28–32. [Google Scholar] [CrossRef]
- Walstra, P.W.J.; Geurts, T.J. Homogenization. In Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 279–296. [Google Scholar]
- Sharma, S.K.; Dalgleish, D.G. Interactions between milk serum proteins and synthetic fat globule membrane during heating of homogenized whole milk. J. Agric. Food Chem. 1993, 41, 1407–1412. [Google Scholar] [CrossRef]
- Rosenberg, M.; Rosenberg, Y.; Zhang, J. Microencapsulation of a Model Oil in Wall System Consisting of Wheat Proteins Isolate (WHPI) and Lactose. Appl. Sci. 2018, 8, 1944. [Google Scholar] [CrossRef] [Green Version]
- Vega, C.; Douglas Goff, H.; Roos, Y.H. Casein molecular assembly affects the properties of milk fat emulsions encapsulated in lactose or trehalose matrices. Int. Dairy J. 2007, 17, 683–695. [Google Scholar] [CrossRef]
- Hooi, R.; Barbano, D.M.; Bradley, R.L.; Budde, D.; Bulthaus, M.; Chettiar, M.; Lynch, J.; Reddy, R.; Arnold, E.A. Chemical and Physical Methods. In Standard Methods for the Examination of Dairy Products; Arnold, E.A., Ed.; American Public Health Association: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Euston, S.E.; Singh, H.; Munro, P.A.; Dalgleish, D.G. Competitive Adsorption between Sodium Caseinate and Oil-Soluble and Water-Soluble Surfactants in Oil-in-Water Emulsions. J. Food Sci. 1995, 60, 1124–1131. [Google Scholar] [CrossRef]
- Bak, K.H.; Richards, M.P. Hexanal as a Predictor of Development of Oxidation Flavor in Cured and Uncured Deli Meat Products as Affected by Natural Antioxidants. Foods 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Flores, R.; Ye, A.; Singh, H. Interactions of Whey Proteins during Heat Treatment of Oil-in-Water Emulsions Formed with Whey Protein Isolate and Hydroxylated Lecithin. J. Agric. Food Chem. 2005, 53, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Malaki Nik, A.; Wright, A.J.; Corredig, M. Interfacial design of protein-stabilized emulsions for optimal delivery of nutrients. Food Funct. 2010, 1, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Properties of Emulsions Stabilized with Milk Proteins: Overview of Some Recent Developments. J. Dairy Sci. 1997, 80, 2607–2619. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Adsorption of protein and the stability of emulsions. Trends Food Sci. Technol. 1997, 8, 1–6. [Google Scholar] [CrossRef]
- Liang, Y.; Matia-Merino, L.; Gillies, G.; Patel, H.; Ye, A.; Golding, M. The heat stability of milk protein-stabilized oil-in-water emulsions: A review. Curr. Opin. Colloid 2017, 28, 63–73. [Google Scholar] [CrossRef]
- Rosenberg, M.; Lee, S.L. Microstructure of Whey Protein/Anhydrous Milkfat Emulsions. Food Struct. 1993, 12, 14. [Google Scholar]
- Dalgleish, D.G. Food emulsions—their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- Damodaran, S.; Anand, K. Sulfhydryl−Disulfide Interchange-Induced Interparticle Protein Polymerization in Whey Protein-Stabilized Emulsions and Its Relation to Emulsion Stability. J. Agric. Food Chem. 1997, 45, 3813–3820. [Google Scholar] [CrossRef]
- Sliwinski, E.L.; Roubos, P.J.; Zoet, F.D.; van Boekel, M.A.J.S.; Wouters, J.T.M. Effects of heat on physicochemical properties of whey protein-stabilised emulsions. Colloids Surf. B Biointerfaces 2003, 31, 231–242. [Google Scholar] [CrossRef]
- Liang, Y.; Patel, H.; Matia-Merino, L.; Ye, A.; Golding, M. Structure and stability of heat-treated concentrated dairy-protein-stabilised oil-in-water emulsions: A stability map characterisation approach. Food Hydrocoll. 2013, 33, 297–308. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Surel, C.; Foucquier, J.; Perrot, N.; Mackie, A.; Garnier, C.; Riaublanc, A.; Anton, M. Composition and structure of interface impacts texture of O/W emulsions. Food Hydrocoll. 2014, 34, 3–9. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Baron, C.P.; Let, M.B.; Brüggemann, D.A.; Pedersen, L.R.L.; Jacobsen, C. Homogenization Conditions Affect the Oxidative Stability of Fish Oil Enriched Milk Emulsions: Oxidation Linked to Changes in Protein Composition at the Oil−Water Interface. J. Agric. Food Chem. 2007, 55, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Matsumura, Y. Proteins at liquid interfaces: Role of the molten globule state. Colloids Surf. B Biointerfaces 1994, 3, 1–17. [Google Scholar] [CrossRef]
- Young, S.L.; Sarda, X.; Rosenberg, M. Microencapsulating Properties of Whey Proteins. 2. Combination of Whey Proteins with Carbohydrates. J. Dairy Sci. 1993, 76, 2878–2885. [Google Scholar] [CrossRef]
- Young, S.L.; Sarda, X.; Rosenberg, M. Microencapsulating Properties of Whey Proteins. 1. Microencapsulation of Anhydrous Milk Fat. J. Dairy Sci. 1993, 76, 2868–2877. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Drusch, S.; Berg, S. Extractable oil in microcapsules prepared by spray-drying: Localisation, determination and impact on oxidative stability. Food Chem. 2008, 109, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Cai, L.; Fang, L. Characteristics on the oxidation stability of infant formula powder with different ingredients during storage. Food Sci. Nutr. 2020, 8, 6392–6400. [Google Scholar] [CrossRef]
- Azarbad, M.H.; Jeleń, H. Determination of Hexanal—An Indicator of Lipid Oxidation by Static Headspace Gas Chromatography (SHS-GC) in Fat-Rich Food Matrices. Food Anal. Methods 2015, 8, 1727–1733. [Google Scholar] [CrossRef]
- Cluskey, S.M.; Connolly, J.F.; Devery, R.; O’Brien, B.; Kelly, J.; Harrington, D.; Stanton, C. Lipid and Cholesterol Oxidation in Whole Milk Powder during Processing and Storage. J. Food Sci. 1997, 62, 331–337. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Dobarganes, C.; Márquez-Ruiz, G. Heterogeneous Aspects of Lipid Oxidation in Dried Microencapsulated Oils. J. Agric. Food Chem. 2006, 54, 1722–1729. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Variables affecting lipid oxidation in dried microencapsulated oils. Grasas Aceites 2003, 54, 304–314. [Google Scholar] [CrossRef] [Green Version]

| BE Type | d3,2 (μm) | Surface Excess (mg/m2) | ||
|---|---|---|---|---|
| BE | FE | BE | FE | |
| NH1.0 | 0.43 ALa | 0.54 AKa | 1.39 CDLb | 3.75 AKb |
| NH1.5 | 0.40 BLa | 0.43 BKa | 1.64 BCLb | 3.64 BCKa |
| NH2.0 | 0.39 BKa | 0.39 CKb | 1.77 BCLb | 3.26 BCKa |
| NH2.5 | 0.37 CLa | 0.40 CKa | 1.98 BLb | 2.91 CKb |
| NH5.0 | 0.36 CKb | 0.36 DKb | 2.90 ALc | 3.35 BCKc |
| HT1.0 | 0.44 BLa | 0.50 AKb | 2.24 BLa | 4.05 BKa |
| HT1.5 | 0.39 CDLa | 0.42 BKa | 2.45 BLa | 3.05 CDKb |
| HT2.0 | 0.40 CKa | 0.41 BKa | 2.32 BLa | 2.80 DKb |
| HT2.5 | 0.38 DLa | 0.41 BKa | 2.40 BLa | 4.28 BKa |
| HT5.0 | 0.47 Aka | 0.45 BLa | 4.73 ALa | 6.57 AKb |
| BE Type | α-Lac (mg/g Fat) | β-Lg (mg/g Fat) | CNs (mg/g Fat) | WP/TP (%) | ||
|---|---|---|---|---|---|---|
| BE | FE | BE | FE | |||
| NH1.0 | 1.79 Ca | 1.06 BCb | 11.16 Cb | 21.11 Bb | 43.53 Aa | 33.8 |
| NH1.5 | 2.40 Ba | 1.70 CDa | 14.35 Cb | 24.65 Ba | 28.18 Ba | 48.3 |
| NH2.0 | 2.95 Ba | 1.56 Cb | 20.71 Bb | 25.17 Bb | 17.71 Ca | 60.1 |
| NH2.5 | 3.44 Ba | 1.79 Ba | 21.66 Bb | 36.22 Ab | 14.85 Da | 71.9 |
| NH5.0 | 6.39 Aa | 4.46 Ab | 27.75 Ac | 36.23 Ab | 13.54 Ea | 75.0 |
| HT1.0 | 1.93 Ba | 1.89 Ca | 15.27 Da | 27.68 Da | 35.60 Ab | 45.4 |
| HT1.5 | 2.11 Bb | 1.86 Ca | 19.33 Ca | 27.62 Da | 14.95 Bb | 66.3 |
| HT2.0 | 1.98 Bb | 1.98 Ba | 24.50 BCa | 38.77 Ca | 14.66 Bb | 73.5 |
| HT2.5 | 2.06 Bb | 1.88 Ca | 28.05 Ba | 46.98 Ba | 12.54 Cb | 79.6 |
| HT5.0 | 5.79 Ab | 10.70 Aa | 39.79 Aa | 61.49 Aa | 7.60 Db | 90.5 |
| FE Type | WPI (%) | CR (%) | MEE (%) |
|---|---|---|---|
| NH1.0 | 1.0 | 94.9 Bb | 88.1 Ba |
| NH1.5 | 1.5 | 93.8 Ba | 87.4 Ca |
| NH2.0 | 2.0 | 97.6 Aa | 86.6 Da |
| NH2.5 | 2.5 | 94.4 Ba | 88.4 Bb |
| NH5.0 | 5.0 | 97.3 Aa | 90.8 Ab |
| FHT1.0 | 1.0 | 96.7 Aa | 77.9 Db |
| FHT1.5 | 1.5 | 93.6 BCa | 78.9 Db |
| FHT2.0 | 2.0 | 93.0 CDb | 86.9 Ca |
| FHT2.5 | 2.5 | 90.3 Db | 91.3 Ba |
| FHT5.0 | 5.0 | 96.0 Aa | 93.3 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Rosenberg, Y.; Rosenberg, M. Microcapsules Consisting of Whey Proteins-Coated Droplets of Lipids Embedded in Wall Matrices of Spray-Dried Microcapsules Consisting Mainly of Non-Fat Milk Solids. Foods 2021, 10, 2105. https://doi.org/10.3390/foods10092105
Wang M, Rosenberg Y, Rosenberg M. Microcapsules Consisting of Whey Proteins-Coated Droplets of Lipids Embedded in Wall Matrices of Spray-Dried Microcapsules Consisting Mainly of Non-Fat Milk Solids. Foods. 2021; 10(9):2105. https://doi.org/10.3390/foods10092105
Chicago/Turabian StyleWang, Minghua, Yael Rosenberg, and Moshe Rosenberg. 2021. "Microcapsules Consisting of Whey Proteins-Coated Droplets of Lipids Embedded in Wall Matrices of Spray-Dried Microcapsules Consisting Mainly of Non-Fat Milk Solids" Foods 10, no. 9: 2105. https://doi.org/10.3390/foods10092105





