Abstract
Lacticaseibacillus zeae strains, isolated from raw milk and fermented dairy products, are closely related to the Lacticaseibacillus species that has beneficial probiotic properties. However, it is difficult to distinguish those using conventional methods. In this study, a unique gene was revealed to differentiate L. zeae from other strains of the Lacticaseibacillus species and other species by pan-genome analysis, and a real-time PCR method was developed to rapidly and accurately detect the unique gene. The genome analysis of 141 genomes yielded an 17,978 pan-genome. Among them, 18 accessory genes were specifically present in five genomes of L. zeae. The glycosyltransferase family 8 was identified as a unique gene present only in L. zeae and not in 136 other genomes. A primer designed from the unique gene accurately distinguished L. zeae in pure and mixed DNA and successfully constructed the criterion for the quantified standard curve in real-time PCR. The real-time PCR method was applied to 61 strains containing other Lacticaseibacillus species and distinguished L. zeae with 100% accuracy. Also, the real-time PCR method was proven to be superior to the 16S rRNA gene method in the identification of L. zeae.
1. Introduction
Lactobacillus genus has been reclassified, as such species previously belonging to Lactobacillus casei group now are allotted to Lacticaseibacillus genus [1,2]. The genus Lacticaseibacillus consists of 26 species (L. absianus, L. baoqingensis, L. brantae, L. camelliae, L. casei, L. chiayiensis, L. daqingensis, L. hegangensis, L. hulanensis, L. jixianensis, L. manihotivorans, L. mingshuiensis, L. nasuensis, L. pantheris, L. paracasei, L. porcinae, L. rhamnosus, L. saniviri, L. sharpeae, L. songhuajiangensis, L. suibinensis, L. suilingensis, L. thailandensis, L. yichunensis, L. zeae, and L. zhaodongensis), and Lacticaseibacillus zeae is one of the members of the Lacticaseibacillus genus, along with L. casei, L. paracasei, L. rhamnosus, and L. chiayiensis. However, the taxonomic position of L. zeae has long been debated. In 2008, the use of the name L. zeae rejected for contravening Rules 51b (1) and (2) of the International Code of Nomenclature of Bacteria [3], and only the three species L. casei, L. paracasei, and L. rhamnosus were included in the Lacticaseibacillus species [4]. However, the name L. zeae has since been reported to be legitimate and was validly published [5]. L. zeae has also been justified as a designation for an independent species based on the results of phenotypic characterization and whole-genome sequence-based analysis [5]. With the recent revival of the name L. zeae, therefore, an accurate method is needed to detect this species.
Traditionally, lactic acid bacteria have been identified by biochemical analysis, but classical identification tools cannot distinguish among some species with similar phenotypes [6]. Therefore, molecular methodologies such as amplified ribosomal DNA restriction analysis (ARDRA), randomly amplified polymorphic DNA (RAPD), and repetitive sequence-based PCR have been used to identify lactic acid bacteria [7,8,9]. Among these methodologies, polymerase chain reaction (PCR) is more cost-effective and faster than other molecular tools for the identification of lactic acid bacteria [10]. The main standard marker for differentiation of lactic acid bacteria is the 16S rRNA gene, but it is difficult to discriminate among closely related species such as Lacticaseibacillus species using this marker [11,12,13]. In particular, this gene has a high sequence similarity between L. zeae and other Lacticaseibacillus species of 98.7–99.9%, so it cannot be used to accurately distinguish species in the group [14]. Therefore, an alternative novel target gene is needed as a marker for the identification of L. zeae.
Although it is possible to identify and differentiate lactic acid bacteria by whole genome sequencing, it is time-consuming and costly compared to molecular methodologies [15]. Recently, some researchers have developed a PCR method that can efficiently differentiate closely related bacterial species based on the whole genome analysis [12,13]. However, the development of the PCR method to distinguish L. zeae from other closely related species using a marker obtained based on the pangenome has rarely been reported. This study revealed a unique gene of L. zeae that can be used to accurately distinguish it from other Lactobacillus-related species based on pan-genome analysis, and a real-time PCR method was developed that can detect this unique gene by a designed primer.
2. Materials and Methods
2.1. Pan-Genome Analysis
A total of 141 genome sequences representing nine lactic acid bacterial species were downloaded from the National Center for Biotechnology Information (NCBI) (Table 1). To overcome the limitation that 141 genomes contained only 5 out 26 species in the genus Lacticaseibacillus, nine species isolated from raw milk, the main habitat of L. zeae, were included in the genome analysis [16]. The pan-genome was analyzed by a pan-genome workflow using the Anvi’o program version 6.0 [17]. The genome sequences were arranged based on the distribution of orthologous gene clusters using the Markov Cluster Algorithm (MCL). Pan-genome profiles of the Lactobacillus-related species genome sequences were generated using the bacterial pan-genome analysis pipeline (BPGA) as described in the manual provided by developers [18]. The protein files of 141 genome sequences obtained from NCBI served as the input file for the BPGA analysis. Protein homologs were then clustered by USEARCH with 50% sequence similarity as a cut-off, which is the default setting value. The pan- and core-genome phylogeny analyses were constructed using 20 random orthologous protein clusters [19]. Each orthologous cluster is aligned with a cluster of orthologous groups (COG) database (http://www.ncbi.nlm.nih.gov/COG/ accessed on 4 December 2020) to assign categories to representative protein sequences. Since some proteins in lactobacilli genomes can fit more than one COG classification, and some proteins have no COG assigned, COG analysis restricted the analysis to known protein types. The unique genes of L. zeae were discovered by analyzing the accessory-genome, the set of proteins present in some, but not all genomes, and L. zeae specific primer was developed by selecting a gene suitable for primer design among them.

Table 1.
Genome features used in the analysis.
The unique gene for L. zeae was compared with other strains through BLASTP search against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, and sequence alignment was performed using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/ accessed on 31 August 2021).
2.2. Bacterial Strains and DNA Extraction
The bacterial strains used in this study are listed in Table 2. All reference strains were collected from the Korean Culture Center of Microorganisms (KCCM, Seoul, Korea), the NITE Biological Resource Center (NBRC, Chiba, Japan), the Korean Collection for Type Cultures (KCTC, Daejeon, Korea), the Korean Agricultural Culture Collection (KACC, Jeonju, Korea), and the Microorganism and Gen Bank (MGB, Gwangju, Korea).

Table 2.
List of bacterial strains used in this study.
The isolated strain was isolated from raw milk. Raw milk sample was obtained from the ranch in Korea. The udder was washed prior to collecting raw milk and then directly placed into sterile tubes. After collection, raw milk was maintained at 4 °C during transfer to the laboratory. For isolation of L. zeae, the serially diluted samples were spread on lactobacilli MRS agar (Difco, Becton & Dickinson, Sparks, MD, USA) plate and incubated at 37 °C for 48 h under anaerobic conditions. The different colonies were selected and identified by 16S rRNA gene sequencing. An isolate suspected of L. zeae was selected and designated as the Laboratory Isolate (LI) 220.
All reference strains and isolate were cultured anaerobically in lactobacilli MRS broth (Difco) at 37 °C for 48 h. For extraction of genomic DNA, 1 mL of cultured cells was pelleted by centrifugation at 13,600× g for 10 min and suspended in 200 µL of lysis buffer. According to the manufacturer’s instruction, genomic DNA was extracted using a DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany). DNA concentration and purity of reference strains were measured using a MaestroNano® spectrophotometer (Maestrogen, Las Vegas, NV, USA).
2.3. Real-Time PCR Conditions
The specificity of the designed primer was confirmed using pure and mixed DNA of nine species, including species mainly found in raw milk and closely related to L. zeae. For the preparation of mixed DNA, DNA was extracted from the cells of nine species and 20 ng of each mixed to provide a template for PCR amplification. The standard curve for quantification was generated by L. zeae KACC 11461 serially diluted from 103 to 109 CFU/mL [20]. PCR was performed with CFX96 Deep Well Real-time System (Bio-Rad, Hercules, CA, USA) with a 20 µL reaction mixture containing 20 ng of DNA, 500 nM of primer pair, and 10 µL of 2X Thunderbird SYBR® qPCR Mix (Toyobo, Osaka, Japan). The amplification consisted of an initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 5 s and 60 °C for 30 s. The amplicon was then heated to a temperature from 65 °C to 95 °C, with increments of 0.5 °C, to generate a melting curve.
2.4. Evaluation and Application of Real-Time PCR
The real-time PCR developed in this study was evaluated using 61 bacterial strains (Table 2). The real-time PCR was conducted according to the conditions described in the previous Section 2.3. The strain amplified by real-time PCR was verified by 16S rRNA gene sequencing with 27F/1492R primers. The amplicons were purified using the QIAquick PCR purification kit (Qiagen), and purified amplicons were sequenced. The 16S rRNA gene sequences were then confirmed by BLAST searches.
Our method was applied to spiked food sample. The milk sample was purchased from a local market in Korea and was confirmed to be free of L. zeae. A spiked milk sample was prepared according to a previous study [21,22]. Briefly, 25 mL milk sample was inoculated with L. zeae at a concentration of 103 to 109 CFU/mL. Genomic DNA was extracted inoculated sample according to the method described above, and real-time PCR was performed.
3. Results and Discussion
3.1. Pan-Genome Analysis
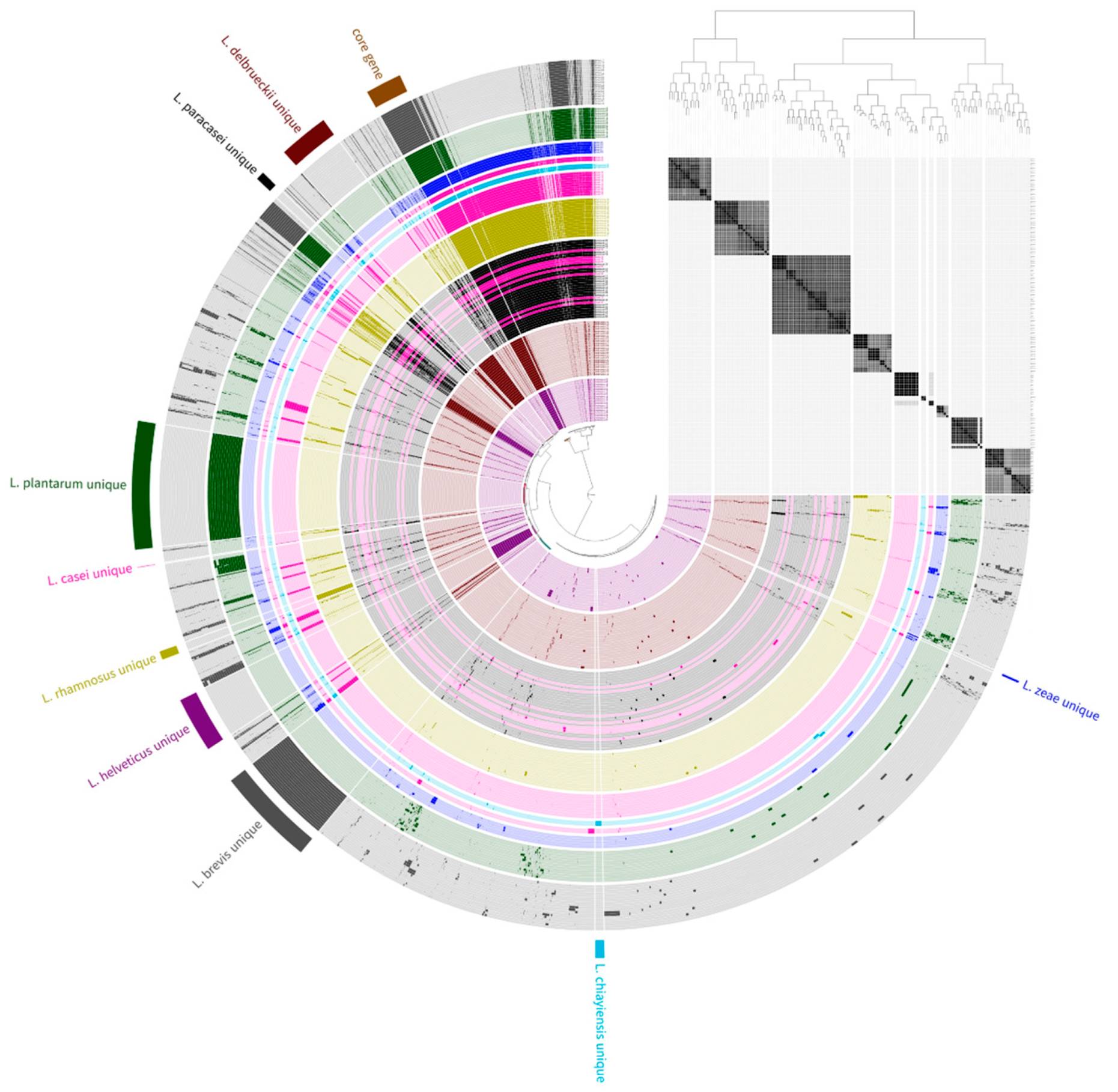
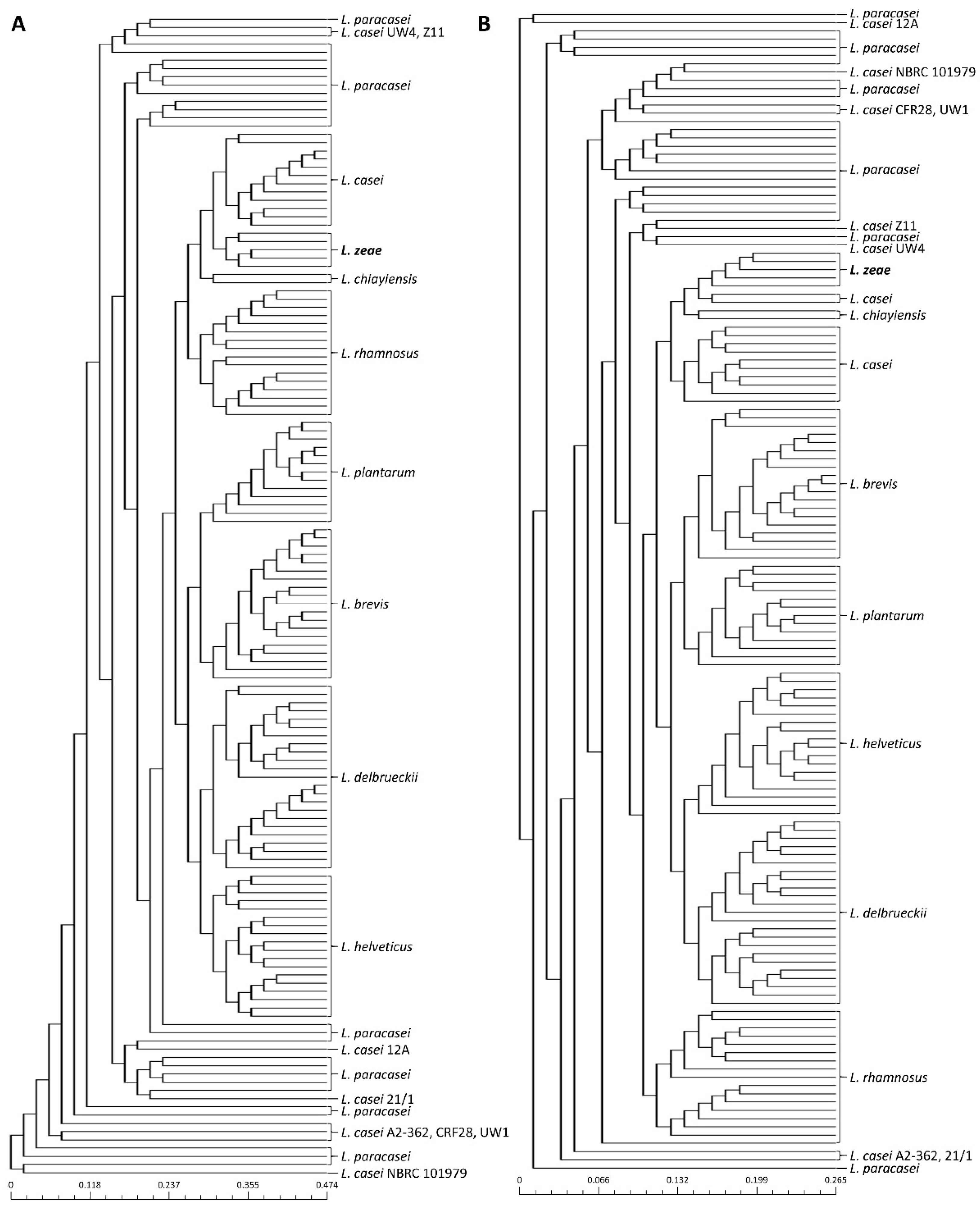
Previous studies have reported that public databases contain misnamed genomes for phenotypically closely related species [23,24,25]. Therefore, phylogenetic analysis based on pan-genome was performed to avoid causing incorrect results due to the misclassification of genome sequences used in this study prior to the genome analysis of L. zeae. The phylogenetic tree based on the pan-genome was mostly clustered by species, resulting in two major clusters (Figure 1). The first cluster included L. helveticus and L. delbrueckii, and the second included Lacticaseibacillus species, L. plantarum, and L. brevis. However, eight genomes of L. casei were clustered with L. paracasei instead of L. casei. Consistent with the results of a previous study [25], we suggested that L. casei 12A, 12/1, A2-362, UW4, Z11, NBRC 101979, UW1, and CRF28 should be moved out and placed into the L. paracasei. Similar results were also confirmed in the phylogenetic analysis based on binary pan-matrix and concatenated core genes (Figure 2). In the phylogenetic analysis, L. casei, L. chiayiensis, and L. zeae were very similar, which is similar to a previous study that three species were clustered adjacent to each other using a phylogenetic tree based on core genome MLST [5]. Therefore, it was confirmed that L. casei and L. zeae were differentiated by pan-genome analysis based on whole-genome sequences.
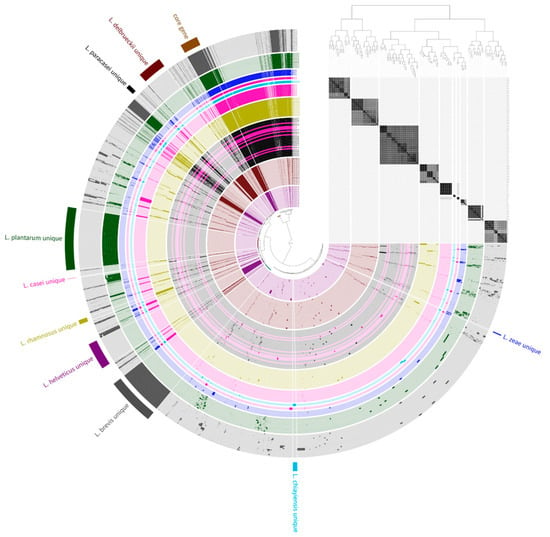
Figure 1.
Pan-genome distribution of 141 genome sequences. The blue, sky blue, pink, green, gray, purple, yellow, red, and black color bars represent L. zeae, L. chiayiensis, L. casei, L. plantarum, L. brevis, L. helveticus, L. rhamnosus, L. delbrueckii, and L. paracasei genomes, respectively. The dark and bright of the bar mean gene presence and absence, respectively. The phylogenetic tree constructed based on the gene cluster frequency is on the right.
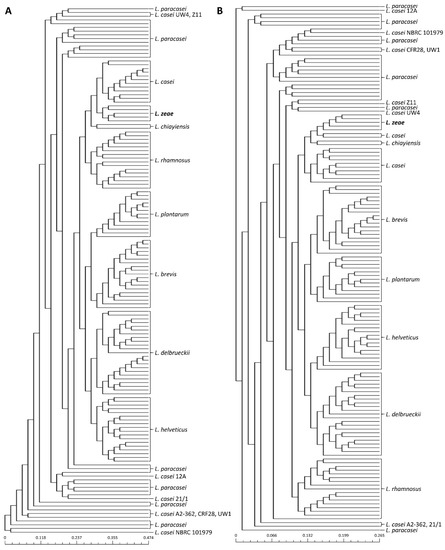
Figure 2.
Phylogeny analysis based on (A) core-genome and (B) pan-genome matrix of 141 genome sequences.
The 17,978 pan-genome obtained from 141 genomes is composed of 144 core genes and 4271 unique genes. The core, accessory, and unique gene clusters were further annotated into COG categories. The core-genome was mostly preserved in the following: transcription, ribosomal structure & biogenesis (38.6%), and nucleotide transport & metabolism (6.7%). Also, the genes common to five genome sequences of L. zeae were classified to COG categories, mainly functioning in the defense system (16.7%), amino acid transport and metabolism (11.1%), and cell wall/membrane/envelop biogenesis (11.1%). Among the 13,563 accessory genes, there were 18 genes common to five L. zeae genome sequences (94.0–100% sequence identity). The 18 genes were aligned with 72,899,005 bacterial sequences through blast analysis. As a result, three genes existed in other microorganisms such as Enterococcus durans, Pediococcus damnosus, and P. acidilactici, and the remaining 15 genes were specifically present in L. zeae. Only 15 genes presented in five genome sequences in L. zeae. Among these, the gene specific to L. zeae was finally selected as glycosyltransferase family 8 (accession no. KRK10099.1) in consideration of the GC content and length. Glycosyltransferase was also present in other lactic acid bacteria. The glycosyltransferase of L. zeae was compared with other bacterial strains, and as a result, it showed the highest homology with Enterococcus gallinarum (36% identity) and less than 34% homology with other Lacticaseibacillus strains (Table S1). This gene was proven to be a gene specific to L. zeae because of its low sequence similarity of less than 36% with other species.
Glycosyltransferases are associated with bacterial stress response, biofilm formation, and sucrose metabolism [26]. This enzyme is also associated with exopolysaccharides (EPS) biosynthesis, which can be part of an important process related to probiotic characteristics such as auto-aggregation, colonization, and survival [27]. In the classification system, glycosyltransferases are divided into 111 families according to their amino acid sequences and differ in function and structure based on the family type (https://www.cazy.org accessed on 4 December 2020). A previous study has reported that lactobacilli encoded various families of glycosyltransferases that have different sequences depending on the species or strains [28]. The same family of glycosyltransferase can also have different sequences with diverse functions depending on their evolutionary origin or acquisition [29,30]. The glycosyltransferase family 8 (accession no. KRK10099.1) found in this study was conserved in genomes of L. zeae with high amino acid sequence similarity (>99%), whereas it showed low similarity in other species. As shown in Figure S1, since the sequences did not match consecutively, designing a primer at any position within this gene does not result in amplification in other bacteria. Therefore, we confirmed that this gene was specifically present in L. zeae.
3.2. Specificity Test
PCR is a well-known and powerful tool to accurately and rapidly detect lactic acid bacteria [31]. The accuracy of PCR depends on the specificity and sensitivity of the gene or primer used in the experiment. Previous studies have reported differentiating L. zeae using the 16S rRNA gene sequence and housekeeping genes such as yycH and dnaK gene as PCR markers [6,32]. However, these genes have high sequence similarities (about 80–100%) among other lactic acid bacterial strains and require an additional sequencing process that is costly and time-consuming. Therefore, this study developed a PCR method that can rapidly and accurately detect L. zeae by targeting a novel unique gene obtained from the pan-genome analysis.
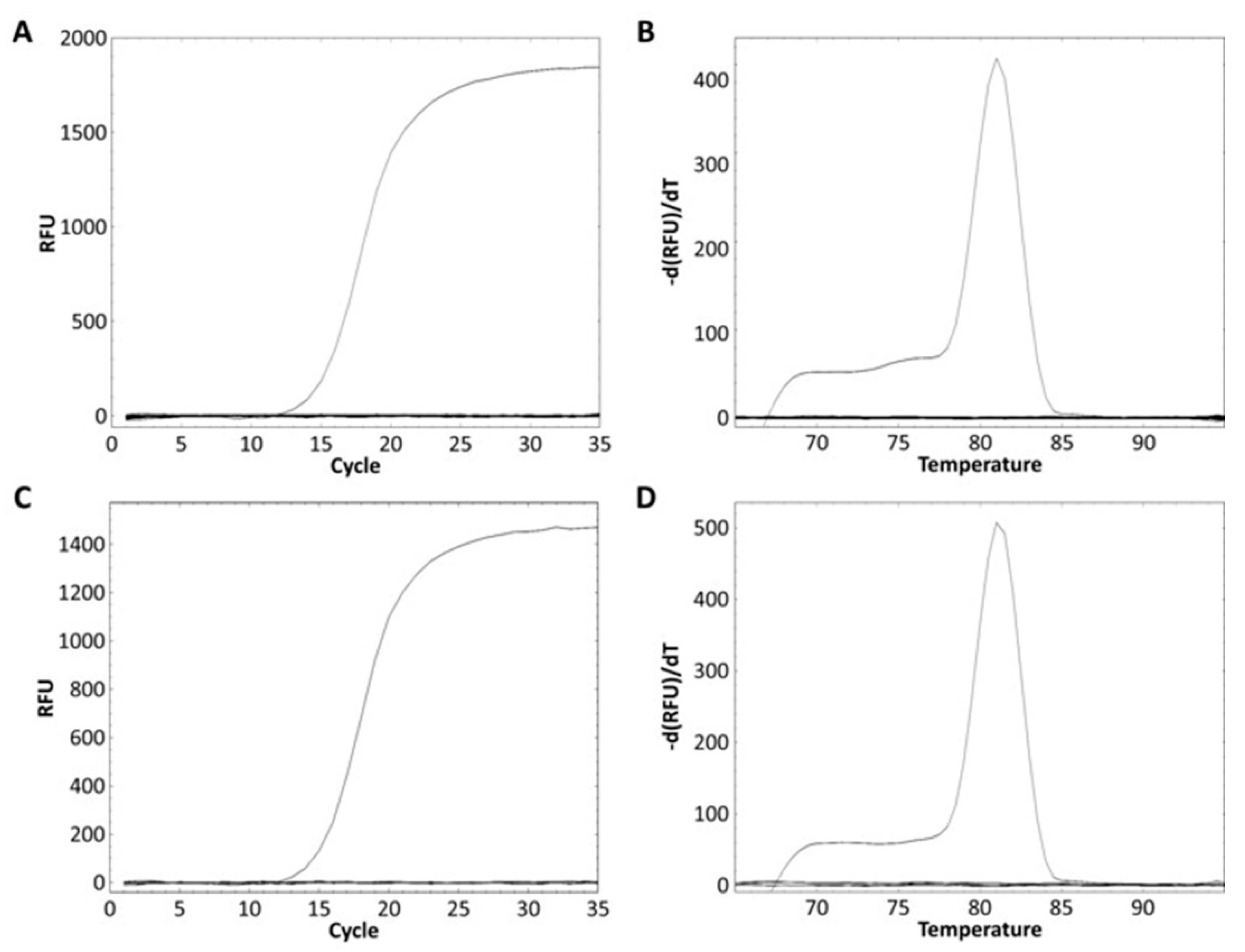
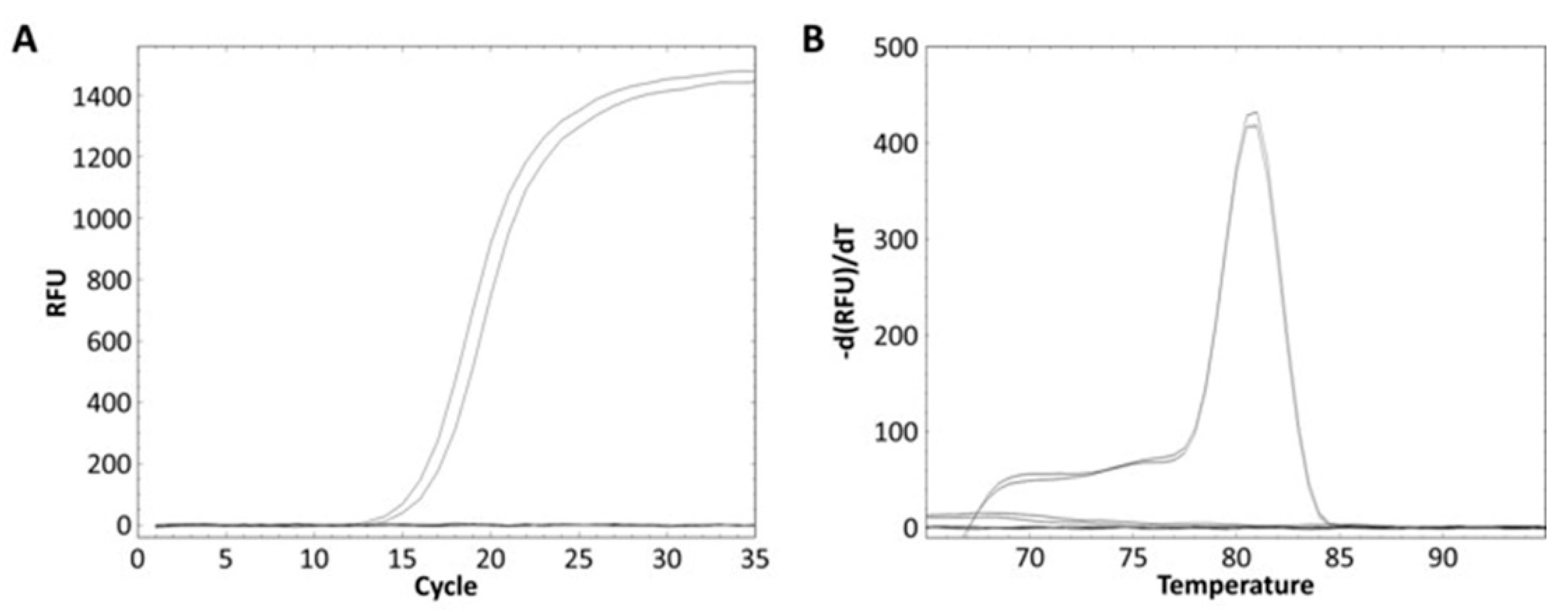
The L. zeae specific primer was designed from the glycosyltransferase family 8 gene obtained from the pan-genome analysis (Table 3). The specificity of the primer was performed using pure and mixed DNA of nine species of L. zeae, L. casei, L. paracasei, L. chiayiensis, L. rhamnosus, L. helveticus, L. plantarum, L. delbrueckii, and L. brevis. L. zeae KACC 11461 presented a Ct value of 13.84 (Figure 3A), and amplicon presented Tm of 81 °C (Figure 3B). Other pure cultured strains did not show amplification for real-time PCR; and the mixed DNA of nine species was amplified only in a well containing L. zeae with a Ct value of 14.88 (Figure 3C), and amplicon presented Tm of 81 °C (Figure 3D). Other mixed cultures did not produce any amplification curve. Therefore, our method successfully amplified the glycosyltransferase family 8 gene in pure and mixed cultures of nine species, suggesting the possibility of identification of L. zeae in complex microbial samples.

Table 3.
Primer information for L. zeae designed in this study.
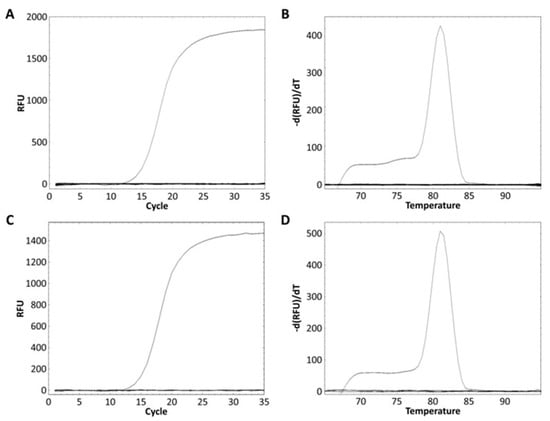
Figure 3.
Real-time PCR discrimination of L. zeae from the other eight species using the primer. (A) Amplification curve and (B) melting curve obtained from a pure culture. (C) Amplification curve and (D) melting curve obtained from the mixed DNA prepared by extracting DNA from the cells of nine species and mixing 25 ng each.
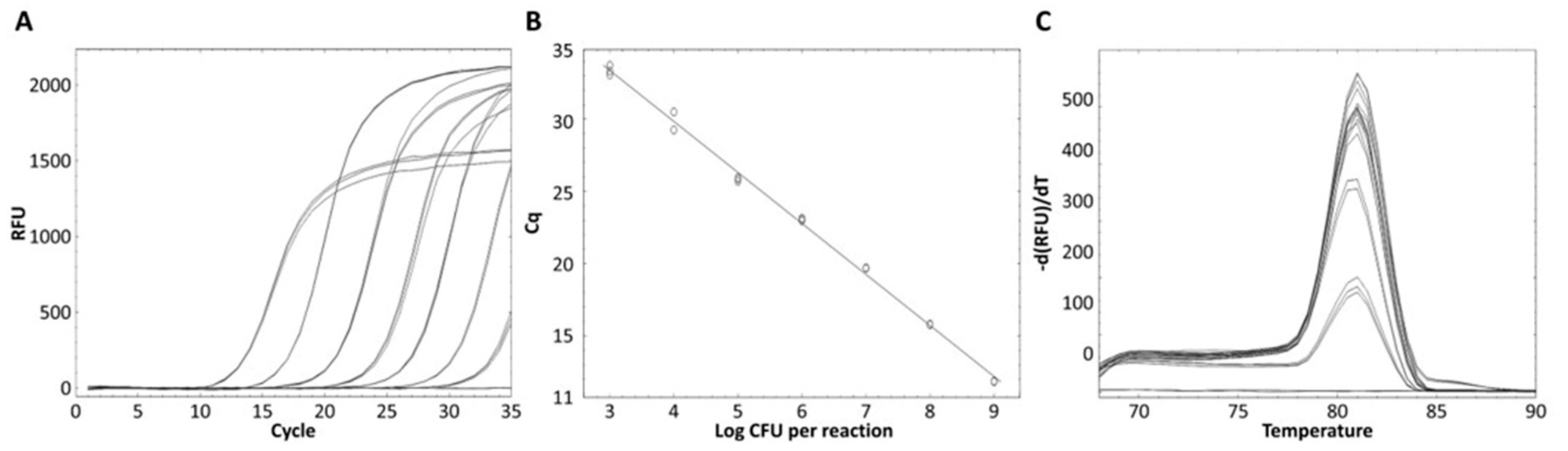
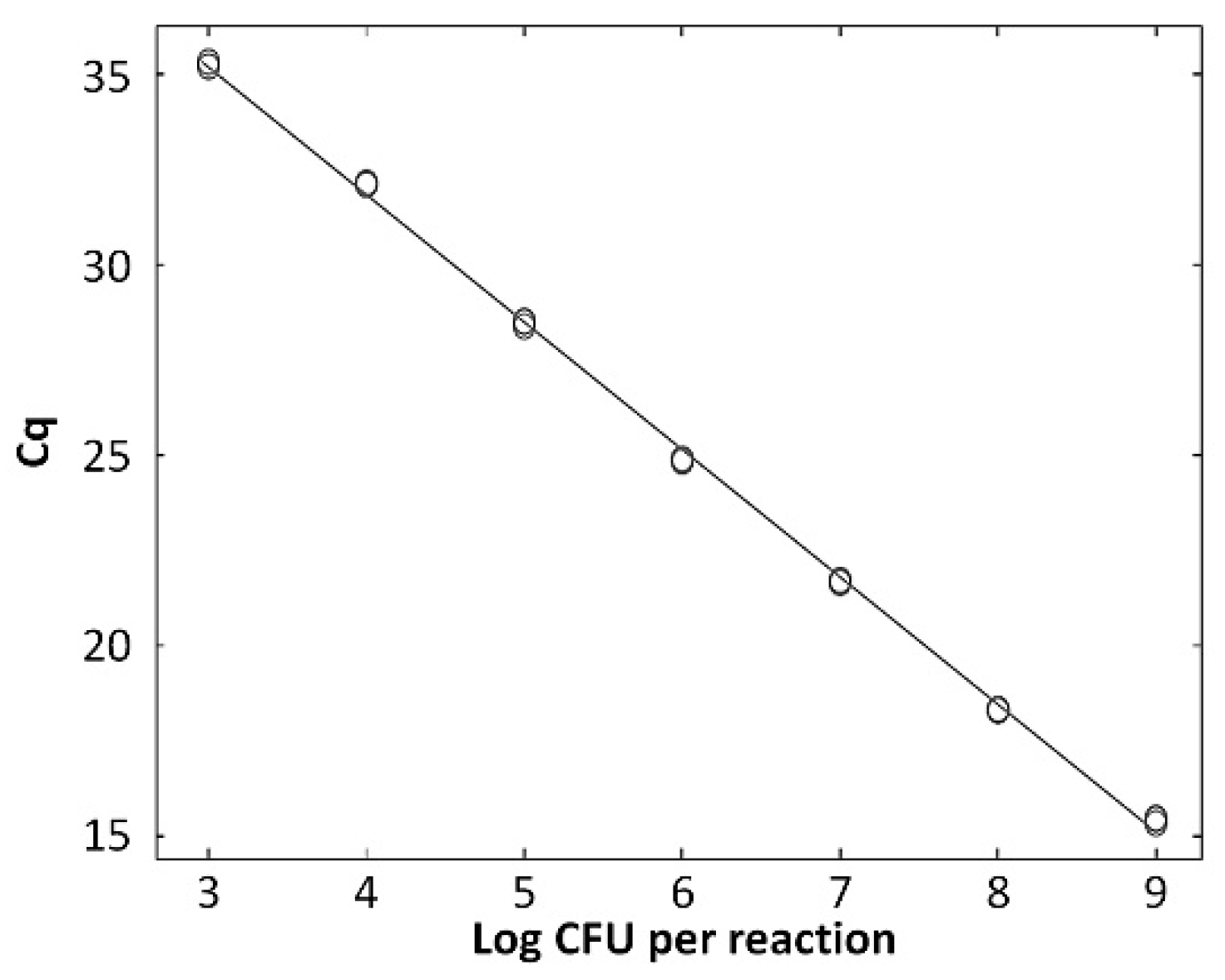
The standard curve for L. zeae was constructed using serial dilutions in the range from 103 to 109 CFU/mL per reaction. The coefficients of correlation (R2) were 0.997, and amplification efficiency was 92.0%. The standard curve had a slope of −3.530 (Figure 4). A previous study reported that a standard curve with an R2 value ≥ 0.98 and slope value in the range of −3.1 to −3.6 is a high-efficiency real-time PCR assay [33]. Therefore, our real-time PCR method is considered a highly efficient method for the identification of L. zeae.
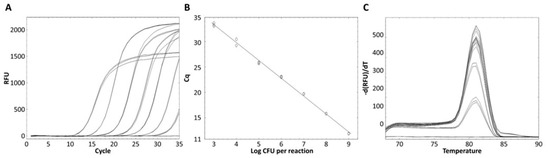
Figure 4.
Quantified standard curve of L. zeae. (A) Amplification plot and (B) melting curve obtained by dilutions of L. zeae from 103 to 109 CFU/mL. (C) Quantified standard curve using the equation y = −3.530x + 36.906 (R2 = 0.997).
3.3. Evaluation of Real-Time PCR Developed in This Study
Real-time PCR was performed using 61 bacterial strains to evaluate whether the designed primer could exclusively detect L. zeae. L. zeae KACC 11461 and LI220 presented Ct values of 16.31 and 17.09, respectively, and all amplicon presented Tm of 81 °C (Figure 5). Other bacterial strains did not show amplification for real-time PCR, demonstrating 100% specificity. The amplified L. zeae strains were confirmed using the conventional identification method of 16S rRNA gene sequencing. 16S rRNA gene sequencing presented three different candidates: L. casei, L. zeae, and L. rhamnosus, instead of providing one species (Table 4). This is consistent with previous studies that reported closely related species were difficult to distinguish using 16S rRNA gene sequences due to the sequence similarity [13,34]. Therefore, it was shown that the real-time PCR developed in this study more accurately distinguished L. zeae than 16S rRNA gene sequencing, which is mainly used for microbial identification. Because this species is rare in the environment and food, it was difficult in this study to find an isolate and only a limited number of isolates could be used for PCR analysis. Also, our data has a shortcoming in that a low number of Lacticaseibacillus species (5 out of 26 species) represented in this study because species that have been described very recently have not been easily accessible isolates. However, since the study used a primer designed with genes analyzed using most of the available genomes, specificity and accuracy could be proven.
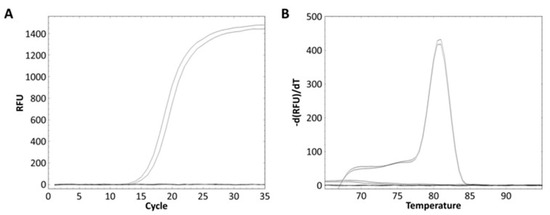
Figure 5.
Evaluation of real-time PCR method against 61 bacterial strains. (A) All strains except L. zeae KACC 11461 and LI220 showed no amplified and (B) only these products obtained melting curves.

Table 4.
Comparison of 16S rRNA gene sequencing and real-time PCR.
The quantification of genomic DNA in a food sample was conducted by artificially adding L. zeae strain to milk. The real-time PCR method developed in this study could successfully be used to identify L. zeae at a concentration of 103 to 109 CFU/mL in milk (Figure 6). Samples artificially inoculated with L. zeae (Ct values: 14.97 to 34.78) had a slightly higher Ct value than the pure culture of L. zeae strain, which seemed to slightly affect the efficiency of real-time PCR. This may be due to the presence of PCR inhibitors from fast and protein in food [21]. Therefore, our real-time PCR method was able to identify L. zeae in the food matrix.
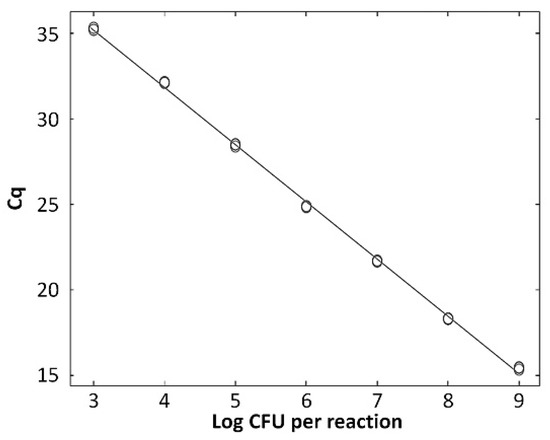
Figure 6.
Result for the detection of L. zeae in spiked milk sample. Standard curve generated by plotting Cq values with logarithm of the number of L. zeae strain artificially inoculated per milliliter of milk. Standard curve equation is y = −3.343 x + 45.215 (R2 = 0.999).
4. Conclusions
In this study, the glycosyltransferase family 8 gene was revealed as a unique gene of L. zeae using a pan-genome analysis. The primer targeting the glycosyltransferase family 8 gene showed high specificity for 61 bacterial strains and was able to rapidly and efficiently distinguish and quantify L. zeae. It also showed higher accuracy than conventional identification methods targeting 16S rRNA gene sequences. Therefore, this method could be further applied to screen L. zeae in complex microbial communities in food samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10092112/s1, Figure S1: Sequence alignment for glycosyltransferase between L. zeae and other strains, Table S1: Similarity for glycosyltransferase between L. zeae and other strains.
Author Contributions
Conceptualization, E.K. and H.-Y.K.; methodology, E.K. and H.-Y.K.; investigation, S.-M.Y.; data curation, E.K.; visualization, S.-M.Y.; writing—original draft preparation, E.K.; writing—review & editing, H.-Y.K.; supervision, H.-Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number 2020R1A6A3A01100168).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Liu, D.D.; Gu, C.T. Proposal to reclassify lactobacillus zhaodongensis, lactobacillus zeae, lactobacillus argentoratensis and lactobacillus buchneri subsp. Silagei as lacticaseibacillus zhaodongensis comb. nov., lacticaseibacillus zeae comb. nov., lactiplantibacillus argento. Int. J. Syst. Evol. Microbiol. 2020, 70, 6414–6417. [Google Scholar] [CrossRef]
- Tindall, B.J. The type strain of Lactobacillus casei is ATCC 393, ATCC 334 cannot serve as the type because it represents a different taxon, the name Lactobacillus paracasei and its subspecies names are not rejected and the revival of the name “Lactobacillus zeae” cont. Int. J. Syst. Evol. Microbiol. 2008, 58, 1764–1765. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Li, S.W.; Huang, L.; Watanabe, K. Identification and classification for the Lactobacillus casei group. Front. Microbiol. 2018, 9, 1974. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.C.; Liou, J.S.; Lee, A.Y.; Blom, J.; Lin, Y.C.; Huang, L.; Watanabe, K. Genome-based reclassification of lactobacillus casei: Emended classification and description of the species lactobacillus zeae. Int. J. Syst. Evol. Microbiol. 2020, 70, 3755–3762. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, M.T.; Huang, L. Use of highly variable gene (yycH) as DNA marker to resolve interspecific relationships within the Lactobacillus casei group and a target for developing novel species-specific PCR primers. Eur. Food Res. Technol. 2014, 239, 719–724. [Google Scholar] [CrossRef]
- Lee, C.M.; Sieo, C.C.; Cheah, Y.K.; Abdullah, N.; Ho, Y.W. Discrimination of probiotic Lactobacillus strains for poultry by repetitive sequenced-based PCR fingerprinting. J. Sci. Food Agric. 2012, 92, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Meterelliyöz, M. Practical identification of human originated Lactobacillus species by amplified ribosomal DNA restriction analysis (ARDRA) for probiotic use. Mol. Biol. Rep. 2015, 42, 1323–1332. [Google Scholar] [CrossRef]
- Venturi, M.; Guerrini, S.; Vincenzini, M. Stable and non-competitive association of Saccharomyces cerevisiae, Candida milleri and Lactobacillus sanfranciscensis during manufacture of two traditional sourdough baked goods. Food Microbiol. 2012, 31, 107–115. [Google Scholar] [CrossRef]
- Herbel, S.R.; Lauzat, B.; von Nickisch-Rosenegk, M.; Kuhn, M.; Murugaiyan, J.; Wieler, L.H.; Guenther, S. Species-specific quantification of probiotic lactobacilli in yoghurt by quantitative real-time PCR. J. Appl. Microbiol. 2013, 115, 1402–1410. [Google Scholar] [CrossRef]
- Bottari, B.; Felis, G.E.; Salvetti, E.; Castioni, A.; Campedelli, I.; Torriani, S.; Bernini, V.; Gatti, M. Effective identification of Lactobacillus casei group species: Genome-based selection of the gene mutL as the target of a novel multiplex PCR assay. Microbiology 2017, 163, 950–960. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Cho, E.J.; Kim, H.Y. Novel real-time PCR assay for Lactobacillus casei group species using comparative genomics. Food Microbiol. 2020, 90, 103485. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Lim, B.; Park, S.H.; Rackerby, B.; Kim, H.Y. Design of PCR assays to specifically detect and identify 37 Lactobacillus species in a single 96 well plate. BMC Microbiol. 2020, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Huang, L. Rapid species- and subspecies-specific level classification and identification of Lactobacillus casei group members using MALDI Biotyper combined with ClinProTools. J. Dairy Sci. 2018, 101, 979–991. [Google Scholar] [CrossRef]
- Rantsiou, K.; Kathariou, S.; Winkler, A.; Skandamis, P.; Saint-Cyr, M.J.; Rouzeau-Szynalski, K.; Amézquita, A. Next generation microbiological risk assessment: Opportunities of whole genome sequencing (WGS) for foodborne pathogen surveillance, source tracking and risk assessment. Int. J. Food Microbiol. 2018, 287, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Wassie, T. Isolation and identification of lactic acid bacteria from raw cow milk. Int. J. Adv. Res. Biol. Sci. 2016, 3, 44–49. [Google Scholar]
- Eren, A.M.; Esen, O.C.; Quince, C.; Vineis, J.H.; Morrison, H.G.; Sogin, M.L.; Delmont, T.O. Anvi’o: An advanced analysis and visualization platformfor ’omics data. PeerJ 2015, 2015, e1319. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA-an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeb, S.; Gulfam, S.M.; Bokhari, H. Comparative core/pan genome analysis of Vibrio cholerae isolates from Pakistan. Infect. Genet. Evol. 2020, 82, 104316. [Google Scholar] [CrossRef]
- Gómez-Rojo, E.M.; Romero-Santacreu, L.; Jaime, I.; Rovira, J. A novel real-time PCR assay for the specific identification and quantification of Weissella viridescens in blood sausages. Int. J. Food Microbiol. 2015, 215, 16–24. [Google Scholar] [CrossRef]
- Singh, P.; Mustapha, A. Multiplex real-time PCR assays for detection of eight Shiga toxin-producing Escherichia coli in food samples by melting curve analysis. Int. J. Food Microbiol. 2015, 215, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, H.B.; Yang, S.M.; Kim, D.; Kim, H.Y. Real-time PCR assay for detecting Lactobacillus plantarum group using species/subspecies-specific genes identified by comparative genomics. LWT 2021, 138, 110789. [Google Scholar] [CrossRef]
- Wuyts, S.; Wittouck, S.; De Boeck, I.; Allonsius, C.N.; Pasolli, E.; Segata, N.; Lebeer, S. Large-Scale Phylogenomics of the Lactobacillus casei Group Highlights Taxonomic Inconsistencies and Reveals Novel Clade-Associated Features. mSystems 2017, 2, e00061-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Romero, E.; Rodríguez-Medina, N.; Beltrán-Rojel, M.; Silva-Sánchez, J.; Barrios-Camacho, H.; Pérez-Rueda, E.; Garza-Ramos, U. Genome misclassification of Klebsiella variicola and Klebsiella quasipneumoniae isolated from plants, animals and humans. Salud Publica Mex. 2018, 60, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarangi, A.N.; Mukherjee, M.; Bhowmick, S.; Tripathy, S. Reanalysis of Lactobacillus paracasei Lbs2 strain and large-scale comparative genomics places many strains into their correct taxonomic position. Microorganisms 2019, 7, 487. [Google Scholar] [CrossRef] [Green Version]
- Schwab, C.; Walter, J.; Tannock, G.W.; Vogel, R.F.; Gänzle, M.G. Sucrose utilization and impact of sucrose on glycosyltransferase expression in Lactobacillus reuteri. Syst. Appl. Microbiol. 2007, 30, 433–443. [Google Scholar] [CrossRef]
- Dertli, E.; Mayer, M.J.; Colquhoun, I.J.; Narbad, A. EpsA is an essential gene in exopolysaccharide production in Lactobacillus johnsonii FI9785. Microb. Biotechnol. 2016, 9, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Breton, C.; Šnajdrová, L.; Jeanneau, C.; Koča, J.; Imberty, A. Structures and mechanisms of glycosyltransferases. Glycobiology 2006, 16, 29R–37R. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, H.; Hahn, M.G.; Mohnen, D.; Xu, Y. Evolution and function of the plant cell wall synthesis-related glycosyltransferase family 8. Plant Physiol. 2010, 153, 1729–1746. [Google Scholar] [CrossRef] [Green Version]
- Renouf, V.; Claisse, O.; Lonvaud-Funel, A. rpoB gene: A target for identification of LAB cocci by PCR-DGGE and melting curves analyses in real time PCR. J. Microbiol. Methods 2006, 67, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lee, F.L. The dnaK gene as a molecular marker for the classification and discrimination of the Lactobacillus casei group. Antonie Leeuwenhoek 2011, 99, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Broeders, S.; Huber, I.; Grohmann, L.; Berben, G.; Taverniers, I.; Mazzara, M.; Roosens, N.; Morisset, D. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci. Technol. 2014, 37, 115–126. [Google Scholar] [CrossRef]
- Jeyaram, K.; Romi, W.; Singh, T.A.; Adewumi, G.A.; Basanti, K.; Oguntoyinbo, F.A. Distinct differentiation of closely related species of Bacillus subtilis group with industrial importance. J. Microbiol. Methods 2011, 87, 161–164. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).