The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains Preparation
2.2. Nanobubble Inactivation of Bacteria in Cell Suspension
2.3. Washing of Spinach Leaves
2.4. Bacterial Recovery from Spinach Leaves
2.5. Statistical Analysis
3. Results and Discussion
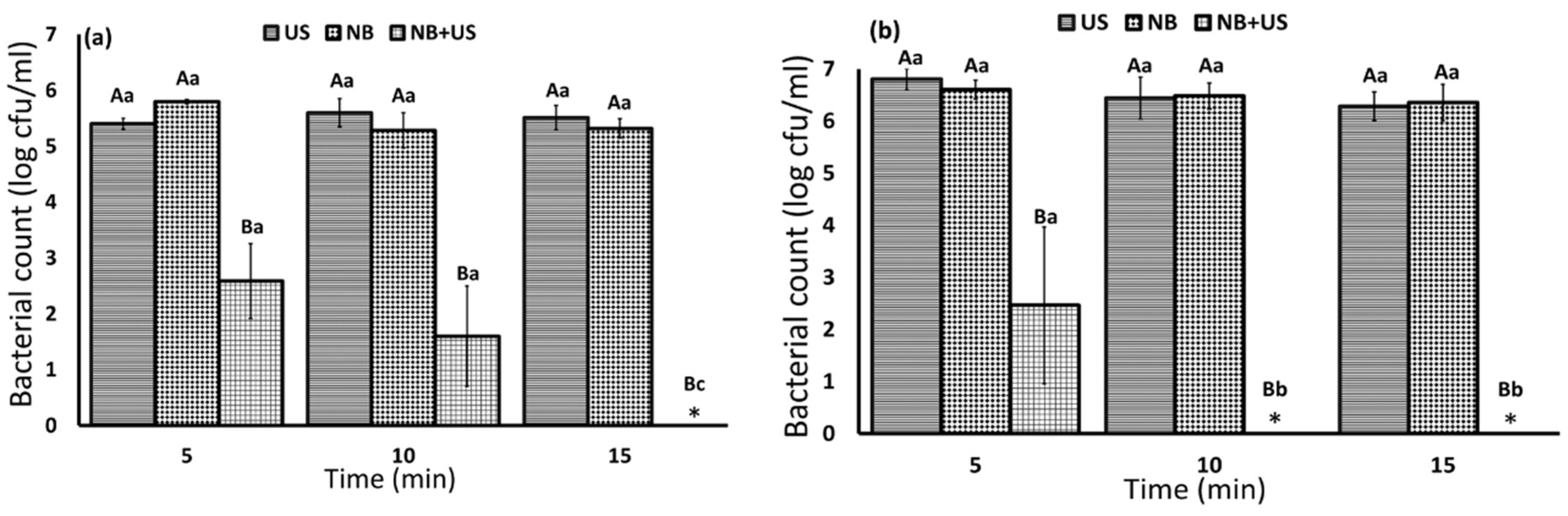
3.1. Bacterial Inactivation in Cell Suspension
3.2. The Effect of Nanobubbles and Ultrasound on Removing Bacteria from Spinach Leaves
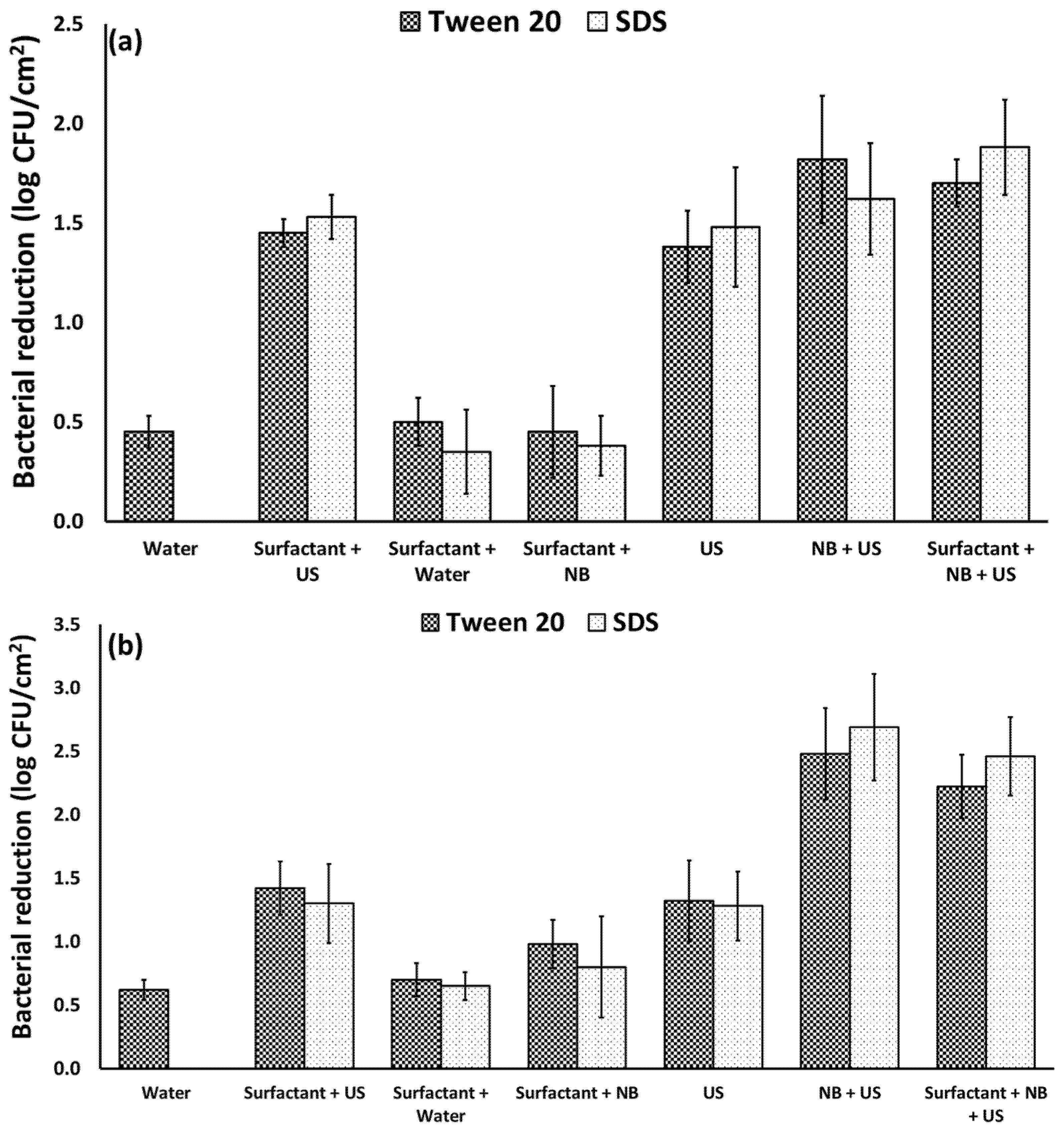
3.3. Effect of Surfactants on the Efficacy of Nanobubbles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M. The second world cancer research fund/american institute for cancer research Expert report. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Shiroodi, S.; Schwarz, M.H.; Nitin, N.; Ovissipour, R. Efficacy of Nanobubbles Alone or in Combination with Neutral Electrolyzed Water in Removing Escherichia coli O157: H7, Vibrio parahaemolyticus, and Listeria innocua Biofilms. Food Bioprocess Technol. 2021, 14, 287–297. [Google Scholar] [CrossRef]
- Frank, J.F. Microbial attachment to food and food contact surfaces. Adv. Food Nutr. Res. 2001, 43, 320–370. [Google Scholar]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Winkelströter, L.K.; dos Reis Teixeira, F.B.; Silva, E.P.; Alves, V.F.; De Martinis, E.C. Unraveling microbial biofilms of importance for food microbiology. Microb. Ecol. 2014, 68, 35–46. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Huang, K.; Nitin, N. Enhanced removal of Escherichia coli O157: H7 and Listeria innocua from fresh lettuce leaves using surfactants during simulated washing. Food Control 2017, 79, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.; Wu, F.; Shi, J.; Jun Xue, S.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Yaron, S.; Romling, U. Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microb. Biotechnol. 2014, 7, 496–516. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Zhu, J.; An, H.; Alheshibri, M.; Liu, L.; Terpstra, P.M.; Liu, G.; Craig, V.S. Cleaning with bulk nanobubbles. Langmuir 2016, 32, 11203–11211. [Google Scholar] [CrossRef]
- Akuzawa, H.; Amagai, K.; Funatsu, M.; Takakusagi, F.; Tabei, K.; Noda, Y. Study on cleaning of pipe inner wall by micro-bubble flow. Jpn. J. Multiph. Flow 2010, 24, 454–461. [Google Scholar] [CrossRef]
- Ghadimkhani, A.; Zhang, W.; Marhaba, T. Ceramic membrane defouling (cleaning) by air Nano Bubbles. Chemosphere 2016, 146, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, H.; Dong, Y.; Mao, H.; Sun, J.; Chen, S.; Craig, V.S.; Hu, J. Cleaning using nanobubbles: Defouling by electrochemical generation of bubbles. J. Colloid Interface Sci. 2008, 328, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Chen, J.; Luo, Y.; Zou, H.; Sun, B. Enhanced stable long-term operation of biotrickling filters treating VOCs by low-dose ozonation and its affecting mechanism on biofilm. Chemosphere 2016, 162, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hayakumo, S.; Arakawa, S.; Takahashi, M.; Kondo, K.; Mano, Y.; Izumi, Y. Effects of ozone nano-bubble water on periodontopathic bacteria and oral cells-in vitro studies. Sci. Technol. Adv. Mater. 2014, 15, 055003. [Google Scholar] [CrossRef] [Green Version]
- Ushida, A.; Koyama, T.; Nakamoto, Y.; Narumi, T.; Sato, T.; Hasegawa, T. Antimicrobial effectiveness of ultra-fine ozone-rich bubble mixtures for fresh vegetables using an alternating flow. J. Food Eng. 2017, 206, 48–56. [Google Scholar] [CrossRef]
- Rafeeq, S.; Shiroodi, S.; Schwarz, M.H.; Nitin, N.; Ovissipour, R. Inactivation of Aeromonas hydrophila and Vibrio parahaemolyticus by Curcumin-Mediated Photosensitization and Nanobubble-Ultrasonication Approaches. Foods 2020, 9, 1306. [Google Scholar] [CrossRef]
- Ajlouni, S.; Sibrani, H.; Premier, R.; Tomkins, B. Ultrasonication and fresh produce (Cos lettuce) preservation. J. Food Sci. 2006, 71, 62–68. [Google Scholar] [CrossRef]
- Huang, K.; Wrenn, S.; Tikekar, R.; Nitin, N. Efficacy of decontamination and a reduced risk of cross-contamination during ultrasound-assisted washing of fresh produce. J. Food Eng. 2018, 224, 95–104. [Google Scholar] [CrossRef]
- Demangeat, J.L. Gas nanobubbles and aqueous nanostructures: The crucial role of dynamization. Homeopathy 2015, 104, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 2019, 95, 118–130. [Google Scholar] [CrossRef]
- Zhang, X.H.; Maeda, N.; Craig, V.S. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir 2006, 22, 5025–5035. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafeeq, S.; Ovissipour, R. The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces. Foods 2021, 10, 2154. https://doi.org/10.3390/foods10092154
Rafeeq S, Ovissipour R. The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces. Foods. 2021; 10(9):2154. https://doi.org/10.3390/foods10092154
Chicago/Turabian StyleRafeeq, Shamil, and Reza Ovissipour. 2021. "The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces" Foods 10, no. 9: 2154. https://doi.org/10.3390/foods10092154




