Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation and Sampling
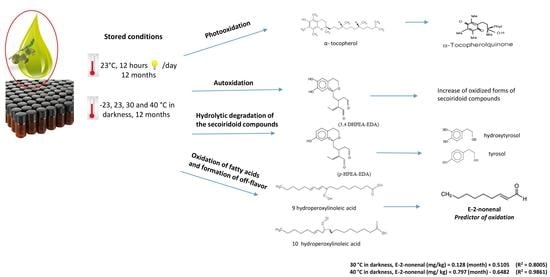
2.2.1. Storage Conditions
2.2.2. Preparation of Samples Stored in Darkness
2.2.3. Preparation of Samples Stored at Light
2.2.4. Sampling
2.3. Quality Parameters
2.4. Determination of Phenolic Compounds
2.5. Total Phenolic Content
2.6. Hydrophilic Orac Assay (H-ORACFL)
2.7. Tocopherol Content
2.8. Volatile Compounds
Determination of Volatile Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Quality Parameters
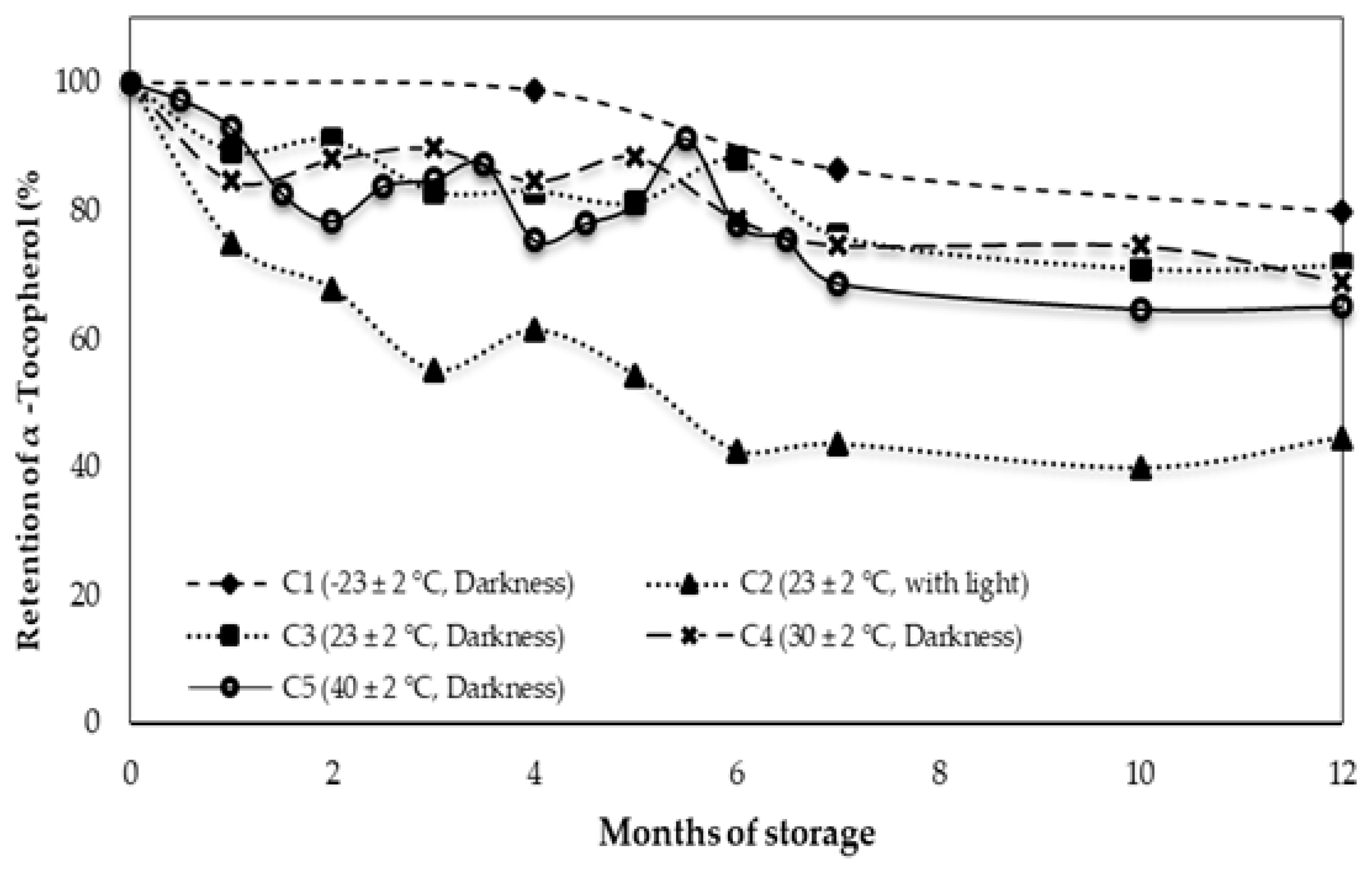
3.2. Behavior of Alpha Tocopherol during Storage
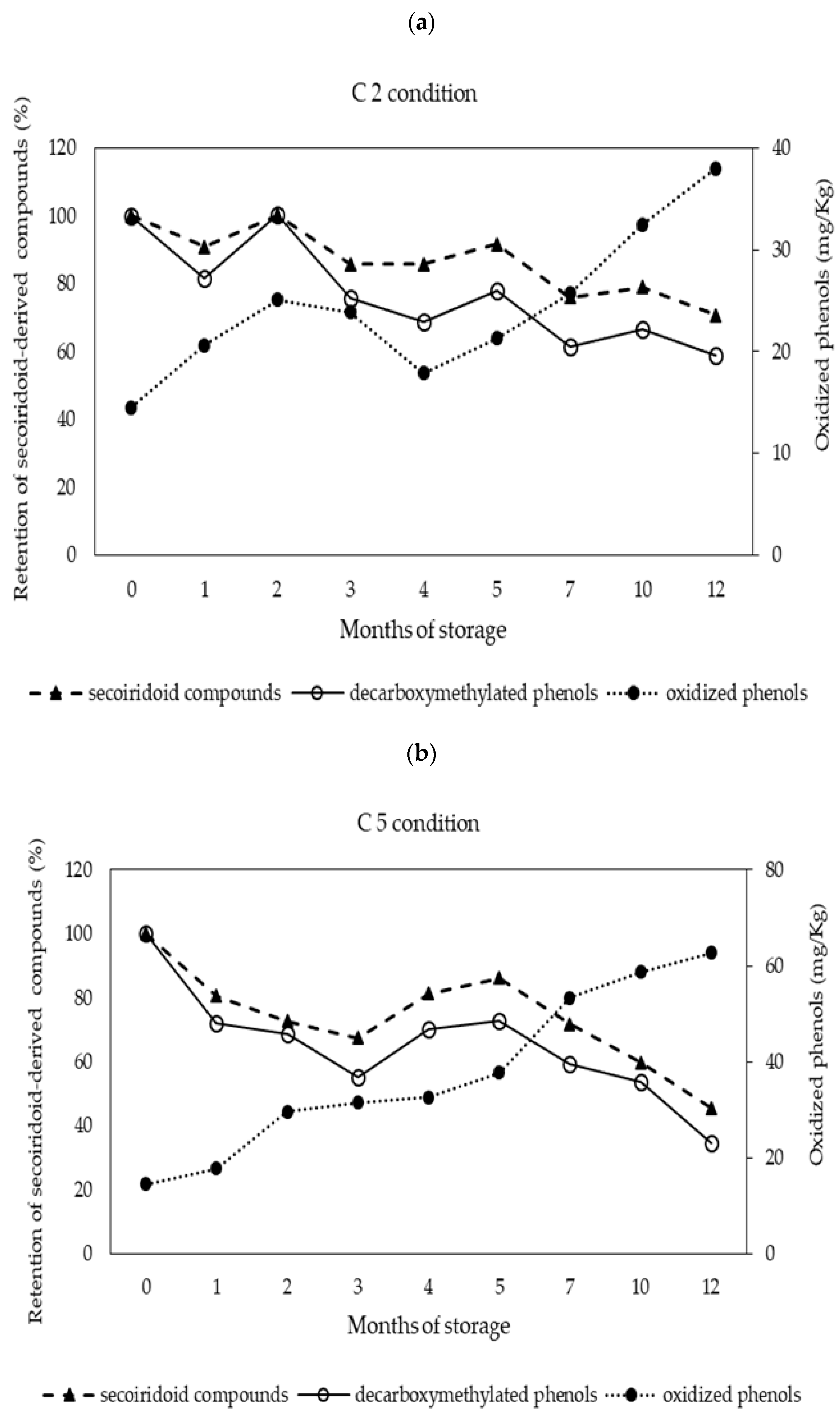
3.3. Behavior of Phenolic Compounds during Storage
3.4. Antioxidant Capacity
3.5. Behavior of Volatile Compounds during Storage
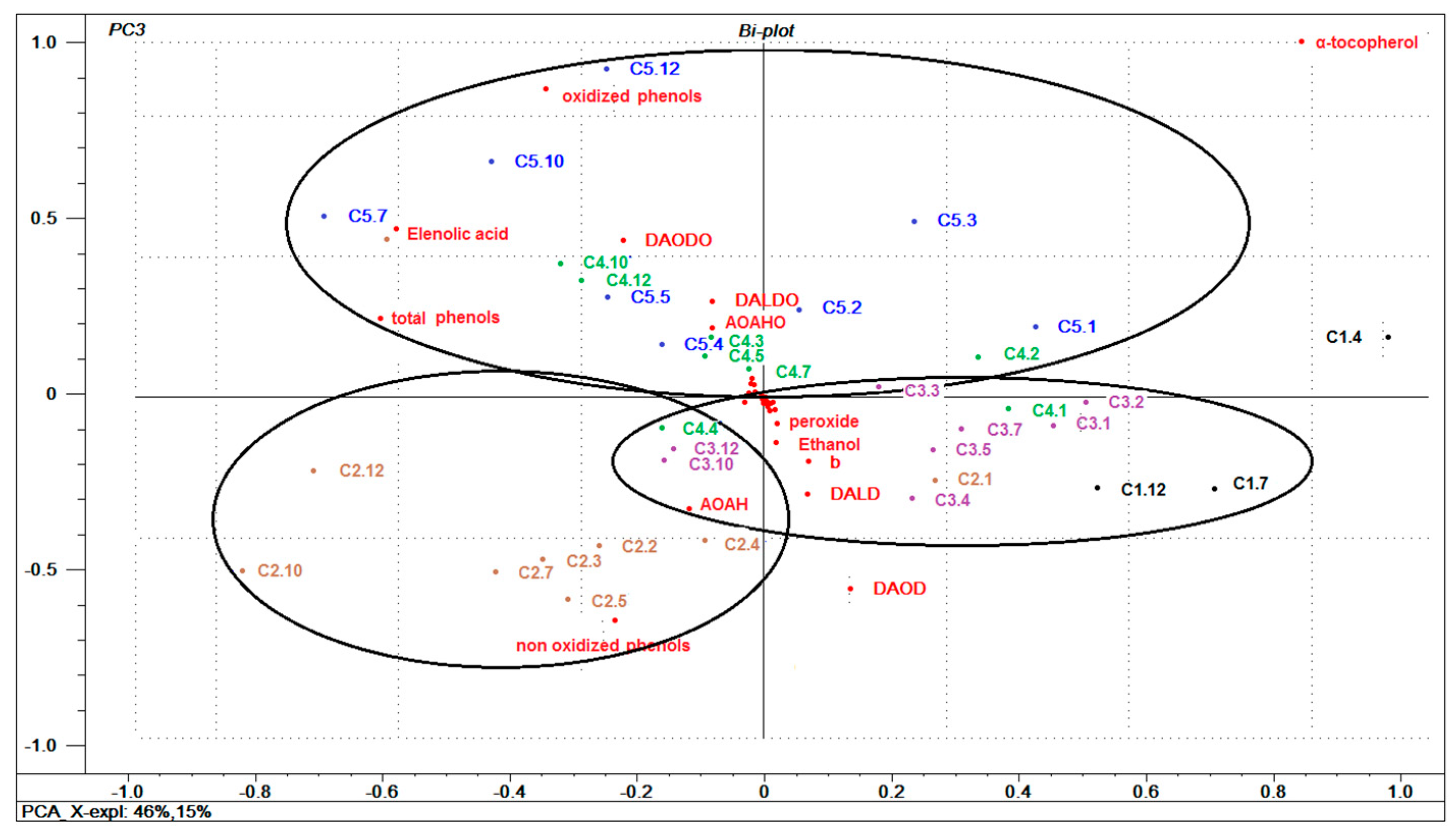
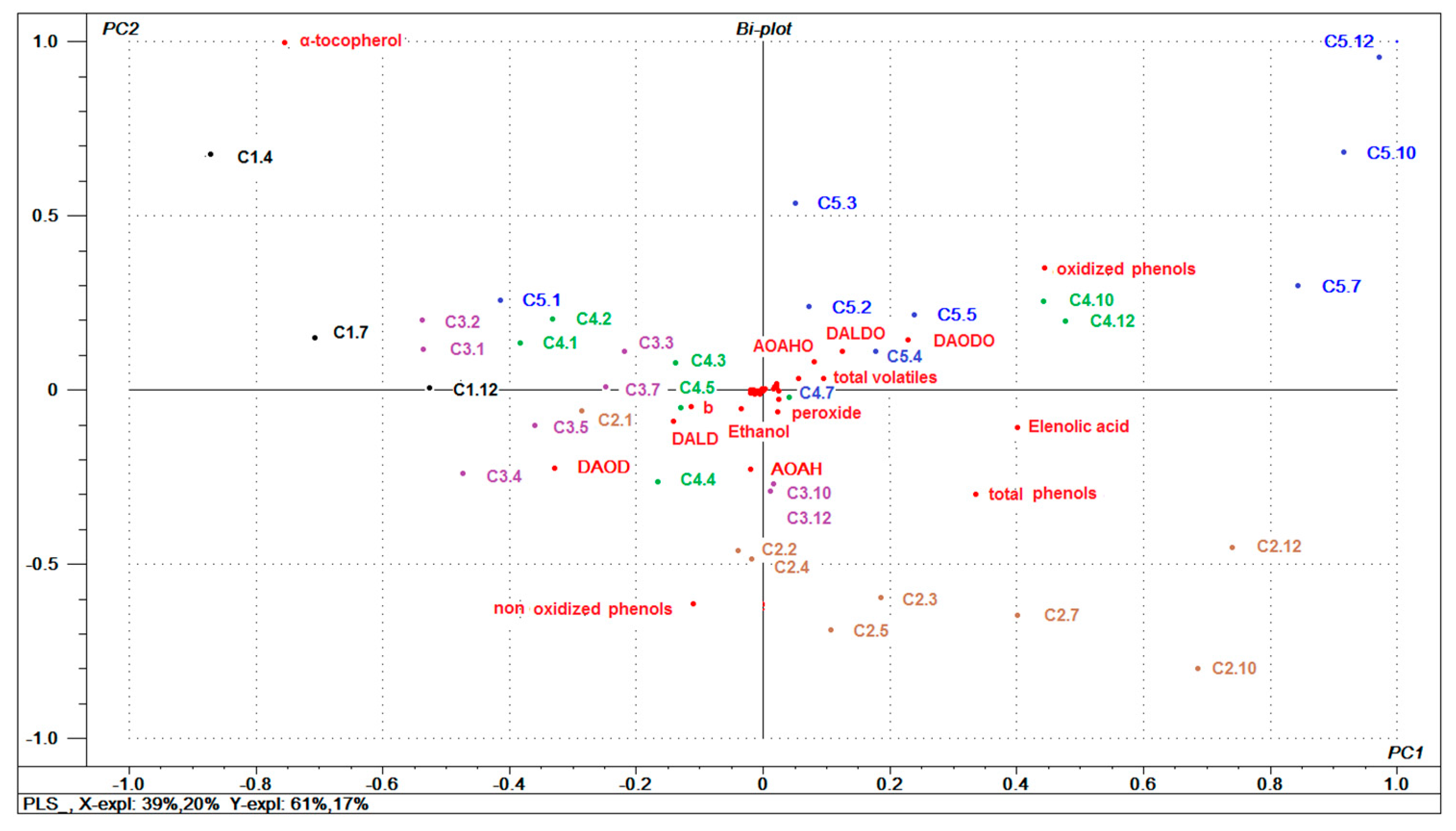
3.6. General Multivariate Characterization of Samples using PCA
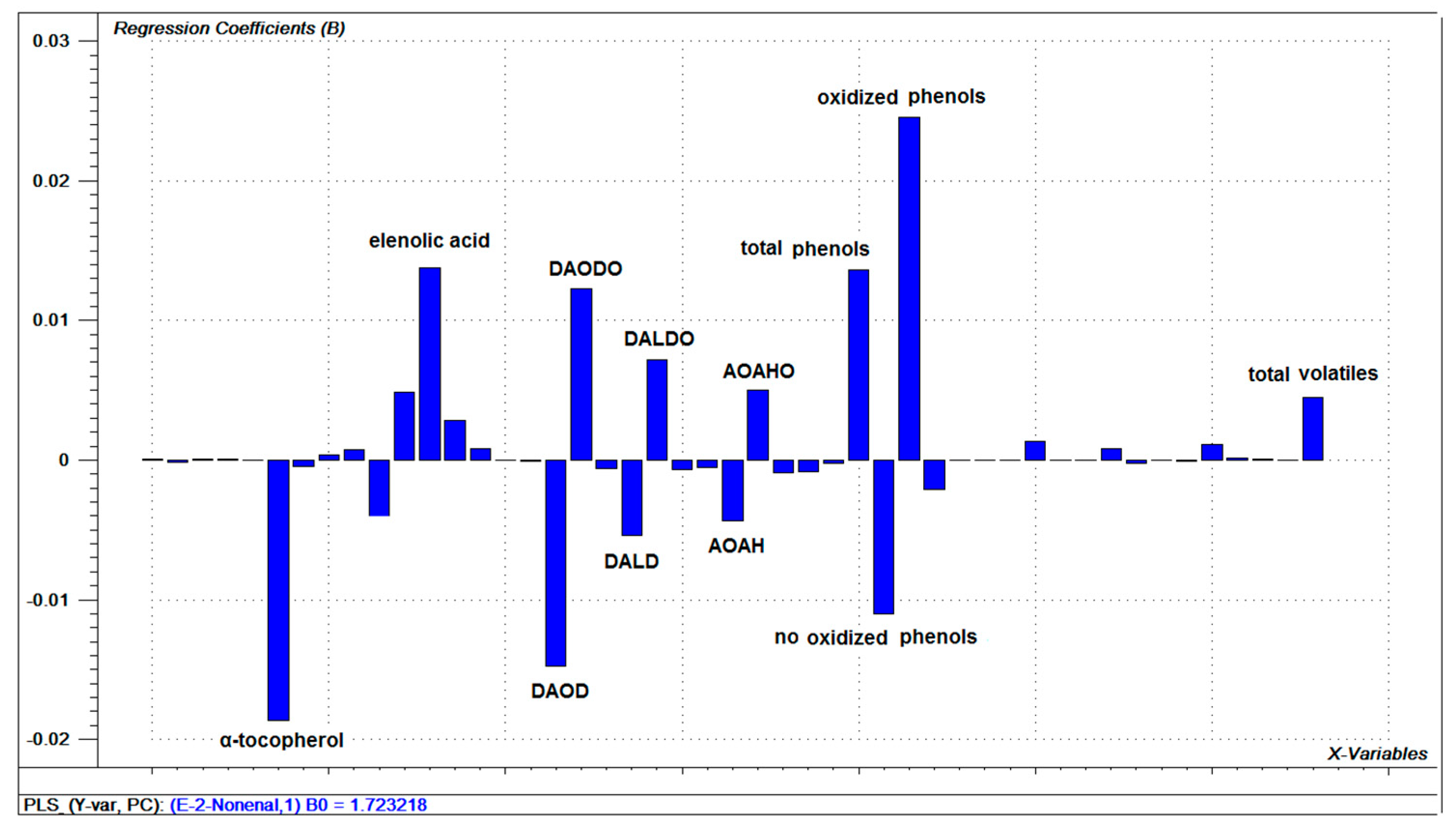
3.7. Relationships between Oils Variables and (E)-2-nonenal
3.8. (E)-2-nonenal as an Oxidation Marker
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; García-González, D.L.; Sikorska, E. Monitoring virgin olive oil shelf-life by fluorescence spectroscopy and sensory characteristics: A multidimensional study carried out under simulated market conditions. Foods 2020, 9, 1846. [Google Scholar] [CrossRef]
- International Olive Council (IOC), Standard. COI/T.15/NC No 3/Rev. 15. Trade Standard Applying to Olive Oil and Olive Pomace oils; International Olive Council: Madrid, Spain, 2019. [Google Scholar]
- Australian Standard. AS 5264-2011: Olive Oils and Olive Pomace Oils; Committee FT-034: Sydney, Australia, 2011. [Google Scholar]
- International Olive Council. Standard. COI/BPS/Doc.No1. In Best Practice Guidelines for the Storage of Olive Oils and Olive-Pomace Oils for Human Consumption; International Olive Council: Madrid, Spain, 2018. [Google Scholar]
- Ayton, J.; Mailer, R.J.; Graham, K. The Effect of Storage Conditions on Extra Virgin Olive Oil Quality; Rural Industries Research and Development Corporation RIRDC: Barton, Australia, 2012. [Google Scholar]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.T.; Aparicio-Ruiz, R.; Aparicio, R. Chromatographic methodologies: Compounds for olive oil odor issues. In Handbook of Olive Oil; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 261–309. [Google Scholar]
- Frankel, E.N. Chemistry of autoxidation: Mechanism, products and flavor significance. In Flavor Chemistry of Fats and Oils; Min, D.B., Smouse, T.H., Eds.; The American Oil Chemists Society AOCS: Champaign, IL, USA, 1985; pp. 1–37. [Google Scholar]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Criado-Navarro, I.; Ledesma-Escobar, C.; López-Bascón, M.; Priego-Capote, F. The decrease in the health benefits of extra virgin olive oil during storage is conditioned by the initial phenolic profile. Food Chem. 2020, 336, 127730. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M. Monitoring the phenolic compounds of Greek extra-virgin olive oils during storage. Food Chem. 2016, 200, 255–262. [Google Scholar] [CrossRef]
- Tsolakou, A.; Diamantakos, P.; Kalaboki, I.; Mena-Bravo, A.; Priego-Capote, F.; Abdallah, I.M.; Kaddoumi, A.; Melliou, E.; Magiatis, P. Oleocanthalic acid, a chemical marker of olive oil aging and exposure to a high storage temperature with potential neuroprotective activity. J. Agric. Food Chem. 2018, 66, 7337–7346. [Google Scholar] [CrossRef]
- Lolis, A.; Badeka, A.; Kontominas, M. Quality retention of extra virgin olive oil, Koroneiki cv. packaged in bag-in-box containers under long term storage: A comparison to packaging in dark glass bottles. Food Packag. Shelf Life 2020, 26, 100549. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Guillaume, C.; Gertz, C.; Ravetti, L. Pyropheophytin a and 1,2-diacyl-glycerols over time under different storage conditions in natural olive oils. J. Am. Oil Chem. Soc. 2014, 91, 697–709. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control. 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Mailer, R.J.; Graham, K. Effect of Storage Containers on Olive Oil Quality; Publication No. 09/160; Rural Research and Development Corporation (RIRDC): Barton, Australia, 2009. [Google Scholar]
- Méndez, A.I.; Falqué, E. Effect of storage time and container type on the quality of extra-virgin olive oil. Food Control. 2007, 18, 521–529. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Giansante, L.; Di Loreto, G.; Di Giacinto, L. Shelf life of extra-virgin olive oils: First efforts toward a prediction model. J. Food Process. Preserv. 2018, 42, e13663. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Changes induced by uv radiation during virgin olive oil storage. J. Agric. Food Chem. 2006, 54, 4790–4794. [Google Scholar] [CrossRef]
- Lobo-Prieto, A.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Tracking sensory characteristics of virgin olive oils during storage: Interpretation of their changes from a multiparametric perspective. Molecules 2020, 25, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanoudaki, E.; Williams, M.; Harwood, J. Changes in virgin olive oil characteristics during different storage conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 906–914. [Google Scholar] [CrossRef]
- Bubola, K.B.; Koprivnjak, O.; Sladonja, B.; Belobrajić, I. Influence of storage temperature on quality parameters, phenols and volatile compounds of Croatian virgin olive oils. Grasas Aceites 2014, 65, e034. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M.; Kali, K. Changes occurring in the volatile composition of Greek virgin olive oils during storage: Oil variety influences stability. Eur. J. Lipid Sci. Technol. 2014, 117, 514–522. [Google Scholar] [CrossRef]
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servili, M. Quality evolution of extra-virgin olive oils according to their chemical composition during 22 months of storage under dark conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Servili, M.; Urbani, S.; Sordini, B.; Veneziani, G.; Daidone, L.; Selvaggini, R. Overall quality evolution of extra virgin olive oil exposed to light for 10 months in different containers. Food Chem. 2021, 351, 129297. [Google Scholar] [CrossRef]
- American Oil Chemists’s Society. Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Romero, N.; Jimenez, P. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef]
- International Olive Council. Standard. COI/T.20/Doc No 29. In Determination of Biophenols in Olive Oils by HPLC; International Olive Council: Madrid, Spain, 2009. [Google Scholar]
- Grossi, M.; Palagano, R.; Bendini, A.; Riccò, B.; Servili, M.; González, D.G.; Toschi, T.G. Design and in-house validation of a portable system for the determination of free acidity in virgin olive oil. Food Control. 2019, 104, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation. In Handbook of Olive Oil; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 479–522. [Google Scholar]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How heating affects extra virgin olive oil quality indexes and chemical composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, M.L. Análisis de la Calidad del Aceite de Oliva Virgen: Relación Entre la Estabilidad Oxidativa y la Composición Fenólica; Degree in Agricultural Engineering, Agricultural Operations, U. of Sevilla and Instituto de la Grasa, CSIC: Seville, Spain, 2016. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Barbero, L.M. Estudio de la Alteración Oxidativa del Aceite de Oliva Virgen: Compuestos Fenólicos; Degree in Pharmacy, U. Sevilla: Seville, Spain, 2017. [Google Scholar]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [Green Version]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Senizza, B.; Pollini, L.; Lucini, L.; Blasi, F. Untargeted metabolomics to evaluate the stability of extra-virgin olive oil with added Lycium barbarum carotenoids during storage. Foods 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef] [Green Version]
- Artajo, L.S.; Romero-Fabregat, M.-P.; Morelló, J.R.; Motilva, M.J. Enrichment of refined olive oil with phenolic compounds: Evaluation of their antioxidant activity and their effect on the bitter index. J. Agric. Food Chem. 2006, 54, 6079–6088. [Google Scholar] [CrossRef]
- Morales, M.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Cao, J.; Deng, L.; Zhu, X.-M.; Fan, Y.; Hu, J.-N.; Li, J.; Deng, Z. Novel approach to evaluate the oxidation state of vegetable oils using characteristic oxidation indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef]
- Aidos, I.; Lourenclo, S.; Padt, A.; Luten, J.; Boom, R. Stability of crude herring oil produced from fresh byproducts: Influence of temperature during storage. J. Food Sci. 2002, 67, 3314–3320. [Google Scholar] [CrossRef]
- Salas, J.J.; Harwood, J.L.; Martínez-Force, E. Lipid metabolism in olive: Biosynthesis of triacylglycerols and aroma components. In Handbook of Olive Oil; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 97–127. [Google Scholar]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. 2-Nonenal newly found in human body odor tends to increase with aging. J. Investig. Dermatol. 2001, 116, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, G.; Morales, M.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, A.J.J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Coutelieris, F.; Kanavouras, A. Experimental and theoretical investigation of packaged olive oil: Development of a quality indicator based on mathematical predictions. J. Food Eng. 2006, 73, 85–92. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Rodrigues, N.; Renard, C.M.; Pereira, J.A. Volatile changes in cv. verdeal transmontana olive oil: From the drupe to the table, including storage. Food Res. Int. 2018, 106, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Youssef, O.; Guido, F.; Manel, I.; Ben Youssef, N.; Luigi, C.P.; Mohamed, H.; Daoud, D.; Mokhtar, Z. Volatile compounds and compositional quality of virgin olive oil from Oueslati variety: Influence of geographical origin. Food Chem. 2011, 124, 1770–1776. [Google Scholar] [CrossRef]
- Biedermann, M.; Bongartz, A.; Mariani, C.; Grob, K. Fatty acid methyl and ethyl esters as well as wax esters for evaluating the quality of olive oils. Eur. Food Res. Technol. 2008, 228, 65–74. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R. Effect of extraction conditions on sensory quality of virgin olive oil. J. Am. Oil Chem. Soc. 1999, 76, 295–300. [Google Scholar] [CrossRef]
| Free Fatty Acid (% m/m Oleic Acid) | K232 (Absorbance Units) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | C1 | C2 | C3 | C4 | C5 | p-Value | Month | C1 | C2 | C3 | C4 | C5 | p-Value |
| 0 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | - | 0 | 1.55 ± 0.01 | 1.55 ± 0.01 | 1.55 ± 0.01 | 1.55 ± 0.01 | 1.55 ± 0.01 | - |
| 2 | - | 0.19 ± 0.01 ab | 0.18 ± 0.00 a | 0.19 ± 0.00 ab | 0.20 ± 0.00 b | * | 2 | - | 1.69 ± 0.08 ab | 1.66 ± 0.02 a | 1.84 ± 0.02 bc | 1.88 ± 0.09 c | * |
| 3 | - | 0.19 ± 0.00 a | - | 0.21 ± 0.00 b | 0.22 ± 0.01 b | * | 3 | - | 1.72 ± 0.04 a | 1.85 ± 0.07 ab | 1.90 ± 0.07 ab | 1.94 ± 0.10 b | * |
| 4 | 0.17 ± 0.01 a | 0.16 ± 0.01 a | 0.17 ± 0.01 a | 0.18 ± 0.01 a | 0.21 ± 0.01 b | ** | 4 | 1.76 ± 0.08 a | 1.81 ± 0.11 ab | 1.99 ± 0.03 b | 1.95 ± 0.02 ab | 1.88 ± 0.09 ab | * |
| 5 | - | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.01 a | 0.20 ± 0.00 b | * | 5 | - | 1.83 ± 0.07 a | 1.93 ± 0.07 a | 1.92 ± 0.01 a | + | |
| 6 | - | 0.17 ± 0.00 a | 0.17 ± 0.01 a | 0.18 ± 0.01 b | 0.21 ± 0.01 c | ** | 6 | - | 1.84 ± 0.00 a | 2.05 ± 0.01 a | 1.95 ± 0.23 a | + | |
| 7 | 0.18 ± 0.01 a | 0.19 ± 0.01 ab | 0.20 ± 0.01 ab | 0.21 ± 0.00 b | 0.28 ± 0.02 c | ** | 7 | 1.68 ± 0.11 a | 1.78 ± 0.04 a | 1.89 ± 0.09 a | 1.85 ± 0.06 a | + | |
| 10 | - | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.22 ± 0.01 b | 0.29 ± 0.01 c | ** | 10 | - | 1.81 ± 0.02 a | 1.79 ± 0.10 a | 1.86 ± 0.05 a | 1.98 ± 0.01 b | * |
| 12 | 0.18 ± 0.01 a | 0.19 ± 0.00 b | 0.19 ± 0.01 b | 0.23 ± 0.00 c | 0.32 ± 0.00 d | ** | 12 | 1.60 ± 0.09 a | 1.80 ± 0.00 ab | 2.03 ± 0.05 b | 1.84 ± 0.06 a | 1.96 ± 0.03 ab | * |
| Peroxide Value (meq O2/kg) | K270 (Absorbance Units) | ||||||||||||
| Month | C1 | C2 | C3 | C4 | C5 | p-Value | Month | C1 | C2 | C3 | C4 | C5 | p-Value |
| 0 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | - | 0 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | - |
| 2 | - | 5.5 ± 0.2 c | 4.4 ± 0.0 a | 5.1 ± 0.2 bc | 4.8 ± 0.1 ab | ** | 2 | 0.11 ± 0.01 c | 0.08 ± 0.01 a | 0.09 ± 0.00 ab | 0.11 ± 0.01 bc | ** | |
| 3 | - | 6.7 ± 0.5 b | 4.6 ± 0.1 a | 4.9 ± 0.0 a | 5.3 ± 0.6 a | ** | 3 | 0.12 ± 0.00 c | 0.09 ± 0.00 a | 0.10 ± 0.01 ab | 0.11 ± 0.01 b | ** | |
| 4 | 3.5 ± 0.1 a | 6.7 ± 0.1 d | 4.8 ± 0.1 b | 5.2 ± 0.2 c | 4.6 ± 0.0 b | ** | 4 | 0.09 ± 0.01 a | 0.13 ± 0.00 c | 0.09 ± 0.01 a | 0.10 ± 0.00 a | 0.12 ± 0.00 b | ** |
| 5 | - | 7..2 ± 0.7 b | 5.3 ± 0.2 a | 5.1 ± 0.1 a | 5.0 ± 0.2 a | ** | 5 | 0.13 ± 0.01 b | 0.09 ± 0.00 a | 0.10 ± 0.00 a | 0.12 ± 0.01 b | ** | |
| 6 | - | 7.4 ± 0.00 b | 5.3 ± 0.4 a | 5.1 ± 0.0 a | 4.4 ± 0.0 a | * | 6 | 0.13 ± 0.00 c | 0.09 ± 0.01 a | 0.11 ± 0.01 b | 0.13 ± 0.00 c | ** | |
| 7 | 3.4 ± 0.1 a | 7.9 ± 0.01 c | 5.6 ± 0.8 b | 5.1 ± 0.3 b | 4.3 ± 0.6 ab | ** | 7 | 0.09 ± 0.01 a | 0.13 ± 0.01 c | 0.09 ± 0.00 a | 0.11 ± 0.01 b | 0.15 ± 0.01 c | ** |
| 10 | - | 8.5 ± 0.8 c | 5.1 ± 0.1 ab | 5.7 ± 0.2 b | 4.0 ± 0.4 a | ** | 10 | 0.15 ± 0.01 c | 0.09 ± 0.01 a | 0.12 ± 0.00 b | 0.17 ± 0.01 d | ** | |
| 12 | 3.3 ± 0.1 a | 8.2 ± 0.8 d | 5.9 ± 0.2 c | 5.3 ± 0.1 bc | 4.4 ± 0.2 b | ** | 12 | 0.09 ± 0.00 a | 0.15 ± 0.01 c | 0.10 ± 0.01 a | 0.13 ± 0.00 b | 0.18 ± 0.01 d | ** |
| Phenolic Compound (mg/kg) | Fresh EVOO (Month 0) | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|
| Elenolic acid | 76.5 ± 1.8 a | 73.1 ± 3.6 a | 107.9 ± 0.4 c | 107.4 ± 4.9 c | 113.6 ± 5.4 c | 98.5 ± 3.5 b |
| Hydroxytyrosol | 3.1 ± 3.0 a | 3.2 ± 0.1 a | 6.0 ± 0.3 c | 5.1 ± 0.1 b | 6.9 ± 0.1 d | 8.8 ± 0.3 e |
| Tyrosol | 5.1 ± 0.3 a | 5.0 ± 0.2 a | 6.0 ± 0.3 c | 5.4 ± 0.2 ab | 5.7 ± 0.1 bc | 6.5 ± 0.1 d |
| Vanillic acid | 0.4 ± 0.0 b | 0.3 ± 0.0 a | 0.3 ± 0.0 ab | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a |
| p-Coumaric acid | 1.8 ± 0.1 c | 0.9 ± 0.1 b | 0.7 ± 0.0 a | 0.8 ± 0.0 ab | 0.8 ± 0.0 a | 0.8 ± 0.0 ab |
| 3,4-DHPEA-EDA | 74.7 ± 3.3 e | 64.9 ± 1.6 d | 42.7 ± 0.9 b | 52.2 ± 0.7 c | 40.4 ± 0.8 b | 25.3 ± 1.9 a |
| 3,4-DHPEA-EDA, oxidized form | 2.7 ± 0.3 a | 2.4 ± 0.2 a | 15.8 ± 1.3 d | 6.8 ± 0.4 b | 14.1 ± 0.3 c | 25.3 ± 0.7 e |
| 3,4-DHPEA-EDA-DOA | 3.5 ± 1.0 b | 1.5 ± 0.2 a | 0.6 ± 0.1 a | 0.7 ± 0.0 a | 0.5 ± 0.1 a | 0.4 ± 0.1 a |
| p-HPEA-EDA | 34.7 ± 2.7 e | 29.1 ± 0.7 d | 21.8 ± 0.9 b | 24.9 ± 1.0 c | 21.0 ± 0.5 b | 12.7 ± 0.5 a |
| p-HPEA-EDA, oxidized form | 2.3 ± 0.2 a | 1.8 ± 0.0 a | 8.3 ± 0.9 c | 4.6 ± 0.3 b | 9.4 ± 0.3 d | 17.2 ± 0.3 e |
| Pinoresinol a | 15.8 ± 1.2 c | 15.1 ± 0.8 bc | 14.2 ± 0.5 abc | 13.7 ± 0.3 ab | 12.8 ± 0.2 a | 12.7 ± 1.1 a |
| p-HPEA-EDA-DLA | 4.3 ± 0.4 ab | 4.6 ± 0.3 b | 4.0 ± 0.3 ab | 3.7 ± 0.1 ab | 3.5 ± 0.3 a | 4.2 ± 0.8 ab |
| 3,4-DHPEA-EA-AH, oxidized form | 9.6 ± 0.7 a | 10.8 ± 0.4 ab | 13.9 ± 0.8 c | 11.0 ± 0.6 b | 15.5 ± 0.5 d | 20.3 ± 0.9 e |
| 3,4-DHPEA-EA-AH | 27.8 ± 2.9 b | 30.2 ± 0.3 bc | 33.3 ± 0.7 c | 38.7 ± 1.4 d | 30.3 ± 1.2 bc | 23.5 ± 0.6 a |
| Luteolin | 7.7 ±0.7 d | 8.3 ± 0.2 d | 6.1 ± 0.2 bc | 6.6 ± 0.2 c | 5.4 ± 0.1 ab | 5.2 ± 0.2 a |
| Apigenin | 2.4 ± 0.3 a | 2.8 ± 0.2 b | 2.4 ± 0.1 a | 2.4 ± 0.1 ab | 2.3 ± 0.0 a | 2.3 ± 0.1 a |
| Methyl luteolin | 1.3 ± 0.2 a | 1.5 ± 0.1 b | 1.3 ± 0.1 a | 1.3 ± 0.0 ab | 1.2 ± 0.0 a | 1.2 ± 0.0 a |
| Total phenols | 273.5 ± 8.2 bc | 255.7 ± 5.1 a | 285.3 ± 4.1 c | 285.6 ± 6.2 c | 283.5 ± 7.8 c | 265.2 ± 7.1 ab |
| non-oxidized phenols | 259.0 ± 7.5 cd | 240.6 ± 4.9 b | 247.3 ± 1.8 bc | 263.3 ± 5.8 d | 244.5 ± 7.7 b | 202.5 ± 5. 6 a |
| Oxidized phenols | 14.5 ± 0.9 a | 15.0 ± 0.5 a | 38.0 ± 2.8 c | 22.3 ± 0.9 b | 39.0 ± 0.3 c | 62.8 ± 1.8 d |
| α-Tocopherol (mg/kg) | 180.2 ± 2.4 d | 144.0 ± 10.6 c | 80.3 ± 4.0 a | 128.8 ± 2.6 bc | 124.3 ± 0.6 b | 117.3 ± 1.2 b |
| ORAC (μmol TE/g) | 5.5 ± 0.2 b | 3.7 ± 0.3 a | 3.6 ± 0.2 a | 4.1 ± 0.4 a | 3.9 ± 0.3 a | 3.9 ± 0.4 a |
| Volatile Compounds | Fresh EVOO (Month 0) | C1 | C2 | C3 | C4 | C5 | Sensory Atributes | Odor Thresholdin Oil (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Ethanol | 14.74 ± 0.71 bcd | 15.81 ± 1.57 d | 14.37 ± 0.68 bc | 15.68 ± 0.37 cd | 13.2 ± 0.51 b | 10.46 ± 0.27 a | Apple, sweet | 30 |
| Ethylpropanoate | 0.1 ± 0.01 b | 0.1 ± 0.01 b | 0.09 ± 0.01 ab | 0.1 ± 0.01 b | 0.09 ± 0.01 ab | 0.08 ± 0.01 a | Strawberry, apple, fruity | 0.10 |
| 4-methyl-pentan-2-one | 0.13 ± 0.01 a | 0.16 ± 0.01 b | 0.16 ± 0.01 b | 0.16 ± 0.01 b | 0.16 ± 0.01 b | 0.16 ± 0.01 b | Strawberry, fruity, sweet, ethereal | 0.30 |
| Butylacetate | 0.05 ± 0.01 b | 0.04 ± 0.00 ab | 0.04 ± 0.00 ab | 0.04 ± 0.01 ab | 0.05 ± 0.01 b | 0.04 ± 0.01 a | Green, fruity, pungent, sweet | 0.10 |
| Hexanal | 6.26 ± 0.1 a | 6.52 ± 0.01 a | 6.97 ± 0.34 b | 7.08 ± 0.11 b | 7.66 ± 0.04 c | 8.6 ± 0.16 d | Green apple, grass | 0.075 |
| 2-methyl-butan-1-ol | 0.12 ± 0 b | 0.12 ± 0.01 b | 0.11 ± 0.01 b | 0.12 ± 0.01 b | 0.11 ± 0.01 b | 0.09 ± 0 a | Winey, spicy | 0.48 |
| 3-methyl-butan-1-ol | 0.27 ± 0 bc | 0.29 ± 0.01 d | 0.27 ± 0.01 c | 0.28 ± 0.01 cd | 0.26 ± 0.01 b | 0.21 ± 0.01 a | Woody, sweet | 0.10 |
| (E)-2-hexenal | 15.28 ± 0.24 a | 18.7 ± 0.25 b | 18.34 ± 1.2 b | 19.36 ± 0.53 b | 18.94 ± 0.47 b | 18.26 ± 0.39 b | Bitter almonds, green- fruity | 0.42 |
| Hexan-1-ol | 5.76 ± 0.18 bc | 5.85 ± 0.04 bc | 5.89 ± 0.4 c | 5.55 ± 0.14 bc | 5.5 ± 0.13 b | 5.0 ± 0.15 a | Fruity, soft, aromatic | 0.40 |
| (E)-2-nonenal | 0.12 ± 0.12 a | 0.21 ± 0.15 a | 1.93 ± 0.29 b | 0.48 ± 0.29 a | 2.03 ± 0.56 b | 4.58 ± 1.07 c | Fatty, rancid, paper-like, penetrating, waxy, beany | 0.90 |
| (E)-2-hexen-1-ol | 7.51 ± 0.05 a | 7.85 ± 0.03 b | 7.76 ± 0.10 b | 7.88 ± 0.09 b | 7.79 ± 0.1 b | 7.49 ± 0.07 a | Green grass, leaves, fruity, astringent, bitter | 5.00 |
| Acetic acid | 6.15 ± 0.27 a | 6.58 ± 0.22 a | 7.24 ± 0.33 b | 7.27 ± 0.19 b | 7.56 ± 0.14 b | 8.04 ± 0.24 c | Sour, vinegary | 0.50 |
| Propionic acid | 0.21 ± 0.02 a | 0.2 ± 0.01 a | 0.26 ± 0.02 b | 0.38 ± 0.03 c | 0.47 ± 0.02 d | 0.56 ± 0.01 e | Pungent, sour, mould | 0.72 |
| Octan-1-ol | 0.18 ± 0.01 a | 0.14 ± 0.01 a | 0.15 ± 0.04 a | 0.13 ± 0.04 a | 0.17 ± 0.04 a | 0.18 ± 0.03 a | - | - |
| Butanoic acid | 0.33 ± 0.01 c | 0.17 ± 0.02 ab | 0.16 ± 0.01 ab | 0.15 ± 0.02 a | 0.18 ± 0.02 ab | 0.2 ± 0.03 b | Rancid, cheese, sweat | 0.65 |
| Total Volatiles | 57.23 ± 0.60 a | 62.74 ± 1.99 b | 63.74 ± 1.07 b | 64.64 ± 0.93 b | 64.17 ± 1.26 b | 63.94 ± 1.66 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods 2021, 10, 2161. https://doi.org/10.3390/foods10092161
Caipo L, Sandoval A, Sepúlveda B, Fuentes E, Valenzuela R, Metherel AH, Romero N. Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods. 2021; 10(9):2161. https://doi.org/10.3390/foods10092161
Chicago/Turabian StyleCaipo, Leeanny, Ana Sandoval, Betsabet Sepúlveda, Edwar Fuentes, Rodrigo Valenzuela, Adam H. Metherel, and Nalda Romero. 2021. "Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles)" Foods 10, no. 9: 2161. https://doi.org/10.3390/foods10092161
APA StyleCaipo, L., Sandoval, A., Sepúlveda, B., Fuentes, E., Valenzuela, R., Metherel, A. H., & Romero, N. (2021). Effect of Storage Conditions on the Quality of Arbequina Extra Virgin Olive Oil and the Impact on the Composition of Flavor-Related Compounds (Phenols and Volatiles). Foods, 10(9), 2161. https://doi.org/10.3390/foods10092161