Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods
Abstract
:1. Introduction
2. Microorganisms and Foodborne Illnesses Related to Infant Formula and Complementary Food
3. Global Food Safety Standards for Complementary Foods: CODEX Alimentarius & ISO
4. Food Safety Standards for Complementary Food in the United States
Hazard Analysis and Critical Control Point (HACCP) and Food Safety Modernization Act (FSMA)
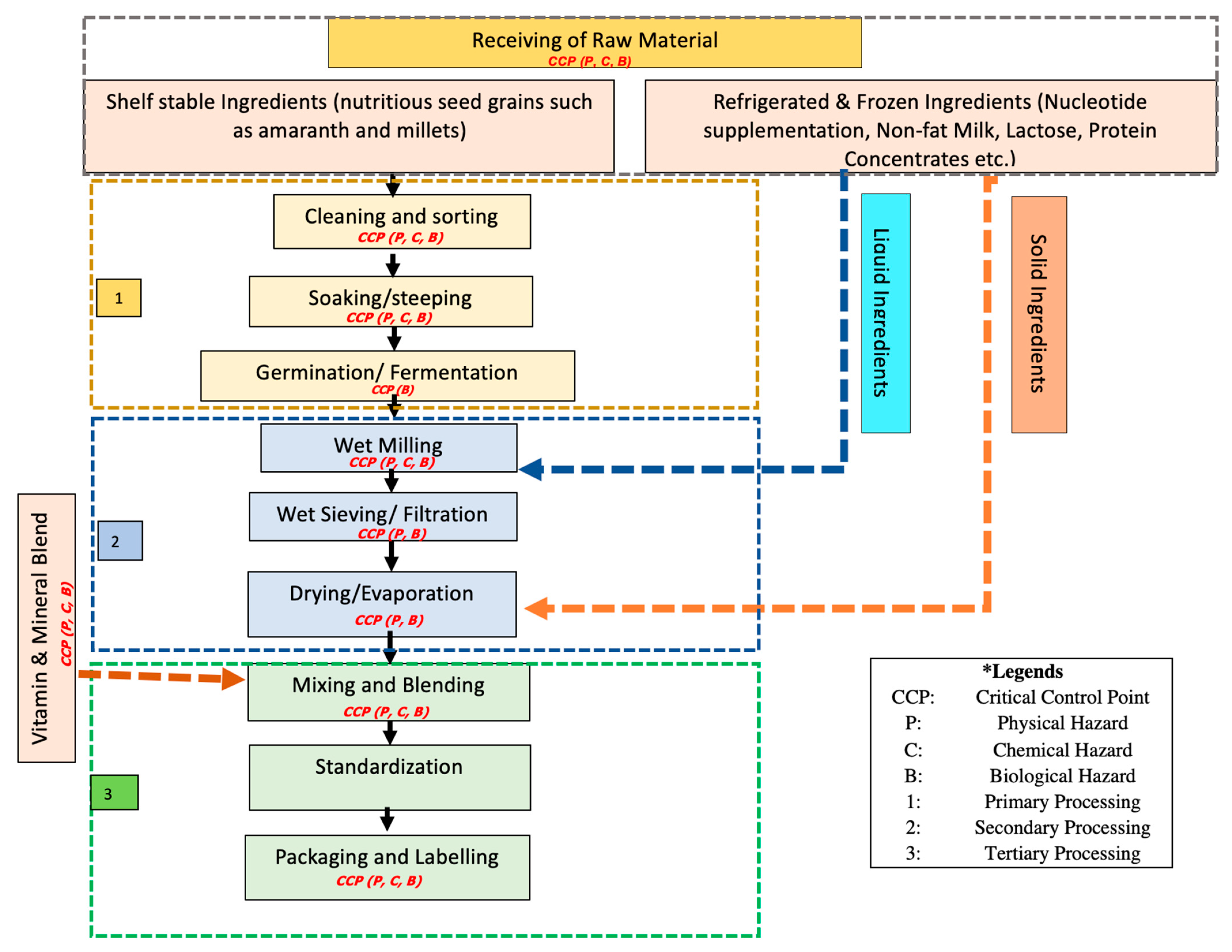
5. HARPC Food Safety Plan for Complementary Food
5.1. Food Processing Steps
5.1.1. Receiving and Storage of Ingredients & Packaging Material
5.1.2. Processing of Raw Materials
5.1.3. Cleaning and Sorting of Raw Material
5.1.4. Soaking/Steeping of Grains
5.1.5. Fermentation
5.1.6. Wet Milling
5.1.7. Wet Sieving
5.1.8. Evaporation
5.1.9. Spray Drying
5.1.10. Mixing and Blending
5.1.11. Standardization
5.1.12. Packaging
5.1.13. Labelling
5.2. Hazard Analysis
5.3. Monitoring & Corrective Actions
5.4. Preventive Controls for Complementary Food
5.5. Verification, Validation and Recordkeeping Procedures
5.6. Types of Recall Associated with Infant Formula/Complementary Food
5.7. Applicability of Regulation and Conduct of Audits
6. Impact of COVID-19
7. Future Potentials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewey, K.G. The Challenge of Meeting Nutrient Needs of Infants and Young Children during the Period of Complementary Feeding: An Evolutionary Perspective. J. Nutr. 2013, 143, 2050–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Xu, X. Sprouted grains-based fermented products. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–173. [Google Scholar]
- Harker, C.S. Introductory Nutrition, 3rd ed.; The C.V. Mosby Co.; Times Mirror/Mosby College Publishing: St. Louis, MO, USA, 1976; p. 576. [Google Scholar]
- Li, W.C.; Chow, C.F. Adverse child health impacts resulting from food adulterations in the Greater China Region: Adverse child health impacts resulting from food adulterations. J. Sci. Food Agric. 2017, 97, 3897–3916. [Google Scholar] [CrossRef] [PubMed]
- U.S Department of Health & Human Services. People at Risk: Children under Five. 2019. Available online: https://www.foodsafety.gov/people-at-risk/children-under-five (accessed on 2 May 2020).
- Cook, J. Global Regulatory Requirements for Baby Food. 2015. Available online: https://www.sgs.com/en/news/2015/12/global-regulatory-requirements-for-baby-food (accessed on 4 May 2020).
- Cook, J. Infant Formula—Recent Issues and Regulatory Changes. 2015. Available online: https://www.sgs.com/en/news/2015/12/infant-formula-recent-issues-and-regulatory-changes (accessed on 4 May 2020).
- Soon, J.M.; Brazier, A.K.M.; Wallace, C.A. Determining common contributory factors in food safety incidents—A review of global outbreaks and recalls 2008–2018. Trends Food Sci. Technol. 2020, 97, 76–87. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Ensure Safe Food from Production to Consumption. In Ensuring Safe Food: From Production to Consumption; National Academies Press: Washington, DC, USA, 1998.
- Skovgaard, N. Foodborne Disease Outbreaks, Guidelines for investigation and control. Int. J. Food Microbiol. 2009, 135, 184–185. [Google Scholar] [CrossRef]
- Food & Agriculture Organization World Health Organization Codex Alimentarius. Codex Standard for Follow-Up Formula, Codex Stan 156-1987. 2017. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B156-1987%252FCXS_156e.pdf (accessed on 25 September 2020).
- Carsetti, R.; Quintarelli, C.; Quinti, I.; Piano Mortari, E.; Zumla, A.; Ippolito, G.; Locatelli, F. The immune system of children: The key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc. Health 2020, 4, 414–416. [Google Scholar] [CrossRef]
- Koletzko, B.; Shamir, R.; Ashwell, M. Quality and safety aspects of infant nutrition. Ann. Nutr. Metab. 2012, 60, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsythe, S. Powdered Infant Formula. In The Microbiological Safety of Low Water Activity Foods and Spices; Springer: Manhattan, NY, USA, 2014; pp. 177–211. [Google Scholar]
- Anselmo, A.C.; Xu, X.; Buerkli, S.; Zeng, Y.; Tang, W.; McHugh, K.J.; Behrens, A.M.; Rosenberg, E.; Duan, A.R.; Sugarman, J.L.; et al. A heat-stable microparticle platform for oral micronutrient delivery. Sci. Transl. Med. 2019, 11, 3680. [Google Scholar] [CrossRef]
- Food & Drug Administration. Outbreaks of Foodborne Illness; FDA: Washington, DC, USA, 2016. Available online: https://www.fda.gov/food/recalls-outbreaks-emergencies/outbreaks-foodborne-illness (accessed on 25 September 2020).
- Henry, M.; Fouladkhah, A. Outbreak History, Biofilm Formation, and Preventive Measures for Control of Cronobacter sakazakii in Infant Formula and Infant Care Settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.J.; Petrosyan, M.; Ford, H.R.; Prasadarao, N.V. Enterobacter sakazakii: An Emerging Pathogen in Infants and Neonates. Surg Infect 2008, 9, 533–539. [Google Scholar] [CrossRef]
- Cahill, S.M.; Wachsmuth, I.K.; Costarrica, M.d.L.; Embarek, P.K.B. Powdered Infant Formula as a Source of Salmonella Infection in Infants. Clin. Infect. Dis. 2008, 46, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.Q.; Condell, O.; Power, K.; Butler, F.; Tall, B.D.; Fanning, S. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: A review of our current understanding of the biology of this bacterium: A review of Cronobacter species. J. Appl. Microbiol. 2012, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Haim, M.S.; Lilly, T., Jr. BAM Chapter 17: Clostridium Botulinum, 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-17-clostridium-botulinum (accessed on 25 September 2020).
- Ashurst, J.V.; Dawson, A. Klebsiella Pneumonia In: Stat Pearls Treasure Island (FL); Stat Pearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519004 (accessed on 29 September 2020).
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441868 (accessed on 29 September 2020).
- McDowell, R.H.; Sands, E.M.; Friedman, H. Bacillus Cereus; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459121 (accessed on 25 September 2020).
- Center for Veterinary Medicine, FDA. Chemical Hazards. FDA. 2021. Available online: https://www.fda.gov/animal-veterinary/biological-chemical-and-physical-contaminants-animal-food/chemical-hazards (accessed on 25 August 2021).
- Branigan, T. Chinese Figures Show Fivefold Rise in Babies Sick from Contaminated Milk. The Guardian. 2008. Available online: https://www. Theguardian.com/world/2008/dec/02/china (accessed on 7 May 2020).
- Center for Veterinary Medicine, FDA. Physical Hazards. FDA. 2021. Available online: https://www.fda.gov/animal-veterinary/biological-chemical-and-physical-contaminants-animal-food/physical-hazards (accessed on 25 August 2021).
- Corley, H. Current Baby Food and Formula Recalls. 2020. Available online: https://www.verywellfamily.com/baby-food-and-baby-formula-recalls-293993 (accessed on 8 June 2020).
- Rowan. Baby Food Recall List. 2020. Available online: https://thebabyswag.com/baby-and-toddler-food-recalls (accessed on 8 June 2020).
- Keller & Heckman. Beech-Nut Exits Market Following Voluntary Recall. The National Law Review. 2016. Available online: https://www.natlawreview.com/article/beech-nut-exits-market-following-voluntary-recall (accessed on 25 August 2021).
- Naden, C. New International Standard for Determining Infant Formula Ingredients Just Published. 2018. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/news/2018/09/Ref2320.html (accessed on 5 May 2020).
- Henson, S.; Humphrey, J. Private standards in global agri-food chains. In Private Standards and Global Governance; Edward Elgar Publishing: Cheltenham, UK, 2012. [Google Scholar]
- Food & Drug Administration. Guidance for Industry: Frequently Asked Questions about FDA’s Regulation of Infant Formula; FDA: Washington, DC, USA, 2006. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-frequently-asked-questions-about-fdas-regulation-infant-formula (accessed on 25 September 2020).
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21 Part 106; FDA: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=106&showFR=1 (accessed on 25 September 2020).
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21 Part 107; FDA: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=107/ (accessed on 25 September 2020).
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21 Part 110; FDA: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=110/ (accessed on 25 September 2020).
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21 Part 113; FDA: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=113/ (accessed on 25 September 2020).
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21 Part 117; FDA: Washington, DC, USA, 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=117/ (accessed on 25 September 2020).
- Orriss, G.D.; Whitehead, A.J. Hazard analysis and critical control point (HACCP) as a part of an overall quality assurance system in international food trade. Food Control. 2000, 11, 345–351. [Google Scholar] [CrossRef]
- Safe Food Alliance. The History of HACCP. Safe Food Alliance. 2019. Available online: https://safefoodalliance.com/haccp/the-history-of-haccp (accessed on 12 May 2020).
- Food & Agriculture Organization. Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application; FAO: Rome, Italy, 1997; Available online: http://www.fao.org/3/y1579e/y1579e03.htm (accessed on 15 May 2020).
- Belden, C.; Orden, D. Review of the FDA Food Safety Modernization Act (FSMA): What It Means, Where It Is Headed, and Why It Matters; Virginia Tech Publishing: Blacksburg, VA, USA, 2011. [Google Scholar]
- Center for Food Safety and Applied Nutrition Food Safety Modernization Act (FSMA). 2020. Available online: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/food-safety-modernization-act-fsma (accessed on 15 May 2020).
- DeWaal, C.S.; Grooters, S.V.; Plunkett, D.W. The food safety modernization act—A series on what is essential for a food professional to know: Article 5. Surveillance. Food Prot. Trends 2013, 33, 327–332. [Google Scholar]
- King, H.; Ades, G. Hazard Analysis and Risk-Based Preventive Controls (HARPC): The New GMP for Food Manufacturing—Food Safety Magazine. Food Saf. 2015. Available online: https://www.foodsafetymagazine.com/magazine-archive1/octobernovember-2015/hazard-analysis-and-risk-based-preventive-controls-harpc-the-new-gmp-for-food-manufacturing (accessed on 30 September 2020).
- Food & Drug Administration. Draft Guidance for Industry: Hazard Analysis and Risk-Based Preventive Controls for Human Food; FDA: Washington, DC, USA, 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-hazard-analysis-and-risk-based-preventive-controls-human-food (accessed on 25 September 2020).
- Quality Assurance & Food Safety. Today’s HACCP in FDA- and USDA-regulated Facilities. 2018. Available online: https://www.qualityassurancemag.com/article/haccp-fda-usda-regulated-facilities (accessed on 11 May 2020).
- Maloy, Laurel. Explaining Differences Between HACCP and HARPC. 2015. Available online: https://www.foodonline.com/doc/explaining-differences-between-haccp-and-harpc-0001/ (accessed on 30 September 2020).
- Kumari, P.; Sangeetha, N. Effect of Processing and Drying Methods on the Nutritional Characteristic of the Multi-cereals and Legume Flour. J. Food Process. Technol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Olajide, J.O.; Oke, M.O.; Olaniyan, S.A.; Grace, F.O. Production and Quality Evaluation of Complementary Food from Malted Millet, Plantain and Soybean Blends. J. Sci. Eng. Res. 2016, 7, 12. [Google Scholar]
- Mbithi-Mwikya, S.; Van Camp, J.; Mamiro, P.R.S.; Ooghe, W.; Kolsteren, P.; Huyghebaert, A. Evaluation of the Nutritional Characteristics of a Finger Millet Based Complementary Food. J. Agric. Food Chem. 2002, 50, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Food & Drug Administration. Food Safety Plan Builder; FDA: Washington, DC, USA, 2014. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/food-safety-plan-builder (accessed on 25 September 2020).
- Connor, A. New Safety Standards Set for Baby Food. The New York Times. 2014. Available online: https://www.nytimes.com/2014/06/10/us/new-safety-standards-set-for-baby-food.html (accessed on 3 May 2020).
- Mead Johnson Nutrition. Enfamil Infant Formula. Enfamil US. 2020. Available online: https://www.enfamil.com/products/enfamil-infant/12-5-oz-powder-can-case-6 (accessed on 30 September 2020).
- Similac Abbott Global Similac® Advance® Baby Formula. 2020. Available online: https://similac.com/baby-formula/similac-advance (accessed on 25 September 2020).
- Hejazi, S.N. Development of Innovative Probiotic Finger Millet- and Amaranth-based Weaning Products; McGill Library: Montreal, QC, Canada, 2016. [Google Scholar]
- Almeida, R.C.C.; Matos, C.O.; Almeida, P.F. Implementation of a HACCP system for on-site hospital preparation of infant formula. Food Control 1999, 10, 181–187. [Google Scholar] [CrossRef]
- Garza, C.; Black, R.E.; Brown, K.H.; Cash, R.A.; Harper, J.; Keausch, G.; Pelto, G.H. Processing Techniques Suitable for Weaning Foods. In Nutrition Issues in Developing Countries: Part I: Diarrheal Diseases: Part II: Diet and Activity During Pregnancy and Lactation; National Academies Press: Washington, DC, USA, 1992. [Google Scholar]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Rosentrater, K.A.; Evers, A.D. Wet milling. In Kent’s Technology of Cereals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 839–860. [Google Scholar] [CrossRef]
- Daeschner, H.W. Wet sieving with precision electroformed sieves. Powder Technol. 1969, 2, 349–355. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Dali, N.S.M.; Bakar, N.A.; Aziz, N.A.; Yusof, Y.A.; Taip, F.S. The influence of dry-blending operational parameters on homogeneity of milk formula powder. Acta Hortic. 2017, 1152, 399–408. [Google Scholar] [CrossRef]
- Jablonski, J.E.; Yu, L.; Malik, S.; Sharma, A.; Bajaj, A.; Balasubramaniam, S.L.; Bleher, R.; Weiner, R.G.; Duncan, T.V. Migration of Quaternary Ammonium Cations from Exfoliated Clay/Low-Density Polyethylene Nanocomposites into Food Simulants. ACS Omega 2019, 4, 13349–13359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Office of Food Additive Safety. Preparation of Food Contact Notifications for Food Contact Substances in Contact with Infant Formula and/or Human Milk: Guidance for Industry. 2019. Available online: https://www.fda.gov/media/124714/download (accessed on 3 May 2020).
- Environmental Working Group. Toxic Plastics Chemical in Infant Formula. 2007. Available online: https://www.ewg.org/research/toxic-plastics-chemical-infant-formula (accessed on 6 June 2020).
- Lingle, R. Gerber’s New Recyclable Baby Food Pouch: 10 Things to Know. Plastics Today. 2020. Available online: https://www.plasticstoday.com/packaging/gerber-s-new-recyclable-baby-food-pouch-10-things-know/202662377162786/ (accessed on 30 September 2020).
- Clear and Well Comprehensive Safe and Natural Baby Food Guide. Available online: https://clearandwell.com/comprehensive-safe-and-natural-baby-food-guide (accessed on 26 June 2020).
- Food & Drug Administration. Food Safety for Infants & Toddlers; FDA: Washington, DC, USA, 2014. Available online: https://www.fda.gov/food/people-risk-foodborne-illness/food-safety-infants-toddlers (accessed on 30 September 2020).
- Food & Drug Administration. Hazard Analysis Critical Control Point (HACCP); FDA: Washington, DC, USA, 1997. Available online: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/hazard-analysis-critical-control-point-haccp (accessed on 25 September 2020).
- World Health Organization. Coronavirus Disease (COVID-19) Advice for the Public. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 29 September 2020).
- World Health Organization. COVID-19 and Food Safety: Guidance for Competent Authorities Responsible for National Food Safety Control Systems; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications-detail-redirect/covid-19-and-food-safety-guidance-for-competent-authorities-responsible-for-national-food-safety-control-systems (accessed on 29 September 2020).
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2020; Available online: https://covid19.who.int (accessed on 25 September 2020).
- Center of Disease Control and Prevention. 2020. “Food and Coronavirus Disease 2019 (COVID-19)|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/food-and-COVID-19.html (accessed on 20 July 2020).
- Food & Agriculture Organization World Health Organization. COVID-19|Codex Alimentarius FAO-WHO; FAO: Rome, Italy, 2019; WHO: Geneva, Switzerland, 2020; Available online: http://www.fao.org/fao-who-codexalimentarius/thematic-areas/COVID-19/en (accessed on 26 July 2020).
- ISO. ISO—COVID-19; ISO: Geneva, Switzerland, 2020; Available online: https://www.iso.org/covid19 (accessed on 25 September 2020).
- Food & Drug Administration. COVID-19 Frequently Asked Questions; FDA: Washington, DC, USA, 2019. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-frequently-asked-questions/ (accessed on 25 September 2020).

| Category | Age Group | Food |
|---|---|---|
| Neonate | Birth to 28 days | Breast Milk/Infant formula |
| Infant | 1 month–6 months | Breast Milk/Infant formula |
| 6 months–24 months | Complementary Food | |
| Children | 2 to 12 years | Standard food |
| Adolescent | 12 to 16 years | Standard food |
| Name of Company and Product | Date of Recall | Reason for Recall/ Outbreak |
|---|---|---|
| Beech-Nut Nutrition | 9 June 2021 | High arsenic levels |
| Calcilo Formula | 17 September 2019 | Inconsistency in aroma and color |
| Mountain Mel’s Peaceful Baby Herbal Tea | 29 August 2019 | Contamination with Salmonella |
| Heinz Turkey Stew Baby Food | December 2019 | Possibility of being contaminated with insects |
| Parent’s Choice Advantage Infant Formula | 21 June 2019 | Potential presence of metal foreign matter |
| Lactalis Formula | 11 December 2017 | Contamination with Salmonella |
| PC Organic Baby Food | 7 February 2017 | Growth of Clostridium botulinum |
| H-E-B Baby Food | 18 November 2016 | Small piece of rubber found in one product |
| Essential Nutrient | Minimum Content per 100 Available Calories | Maximum Content per 100 Available Calories |
|---|---|---|
| Protein | 3 g with nutritional with quality equivalent to or greater than casein. | 5.5 g |
| Fat | 3 g (linolenic acid should not be less than 300 mg per 100 calories) | 6 g |
| Carbohydrates | Nutritionally available carbohydrates added in accordance to required energy density | - |
| Vitamins | ||
| Vitamin A | 167.5 mg | 502.5 mg |
| Vitamin D | 26.8 mg | 80.4 mg |
| Vitamin C (Ascorbic acid) | 8 mg | - |
| Vitamin B1 (Thiamine) | 40 µg | - |
| Vitamin B2 | 60 µg | - |
| Nicotinamide | 250 µg | - |
| Vitamin B6 | 45 µg | - |
| Folic Acid | 4 µg | - |
| Pantothenic acid | 300 µg | - |
| Vitamin B12 | 0.15 µg | - |
| Vitamin K1 | 4 µg | - |
| Biotin | 1.5 µg | - |
| Minerals | ||
| Sodium | 20 mg | 85 mg |
| Potassium | 80 mg | - |
| Chloride | 55 mg | - |
| Calcium | 90 mg | - |
| Phosphorous | 60 mg | - |
| Magnesium | 6 mg | - |
| Iron | 1 mg | 2 mg |
| Iodine | 5 µg | - |
| Zinc | 0.5 mg | - |
| Global Safety Standard Certification | Description |
|---|---|
| International Organization for Standardization (ISO 22000) | The ISO 22000 standard describes the food safety management system requirements for any organization involved in the food chain, such as ingredient producers, retailers, catering services, transportation, etc. Any organization can pursue certification and registration if it conforms with this standard. |
| Safe Quality Food (SQF) 1000/2000 | It is a Hazard Analysis and Critical Control Points (HACCP)-based certification system for food safety and quality of ingredients, packaging, farming, packing houses, etc. |
| British Retail Consortium (BRCGS) | This standard has been developed in collaboration with the industry for provision of product safety and quality. |
| PrimusGFS | This certification program is a farm-focused Global Food Safety Initiative (GFSI). |
| Global Good Agricultural Practices (GAP) | This certification program covers primarily agricultural crops, such as fruits, vegetables, hops, tea, etc. |
| International Featured Standards (IFS) | This certification program covers the processes in the supply chain by doing risk-based assessments. |
| Food Safety Management Certification (FSSC) 22000 | This certification is based on ISO 22000, ISO 9001, ISO/TS22003 and ISO 22003 and confirms food safety and quality of the organization certified. |
| Regulation | Description |
|---|---|
| 21 CFR Part 106 | Current Good Manufacturing Practices (cGMP), production and in process control system, prevention of adulteration by workers, facilities, equipment or utensils, ingredients, microorganism, packaging and labelling, audit of cGMP, record keeping, registration, submission and notification. |
| 21 CFR Part 107 | General provision and applicability, labelling, exemptions, nutrient requirements, recalls, elements of infant formula recall, notification requirements and record retention. |
| 21 CFR Part 110 | cGMP, packaging, holding of human food, production and process control. |
| 21 CFR Part 113 | Thermally processed low acid food packaged in hermetically sealed containers. |
| 21 CFR Part 117 | cGMP, hazard analysis and risk-based preventive controls for human food, requirements applying to records that must be established and maintained and the supply chain program |
| 21 CFR Part 120 | HACCP, The regulation primarily pertains to juice products. |
| Hazard Analysis and Critical Control Point (HACCP) | Hazard Analysis and Risk-Based Preventive Controls (HARPC) |
|---|---|
| HACCP is based on the Codex Alimentarius and guidelines given by the National Advisory Committee on Microbiological Criteria for Food. It is required by meat, poultry, seafood and juice industries. | HARPC is a food safety plan based on the Food Safety Modernization Act (FSMA). |
| HACCP only covers chemical, biological and physical hazards. | In addition to chemical, biological and physical hazards, HARPC also considers radiological hazards, natural toxins, pesticides and drug residues, parasites, allergens and unapproved food and color additives, and non-intentional and intentional economically motivated hazards. |
| Critical control points are required for the processes. | Critical control points are required for processes and other points as required for the food safety. (21CFR117.135) |
| According to HACCP plan, the critical limits are set at CCPs. | HARPC also has a set of parameters and values in terms of maximum and minimum values. Optimization of these values minimizes the occurrence of hazards. (21CFR117.135 c (1)) |
| HACCP requires all the set process controls to be verified. | In HARPC, verification is required for all preventive and process controls. Supplier verification is also required. |
| Recall is not required in the plan. | According to HARPC, a written recall plan is mandatory when a hazard is identified. The written plan should include procedures which explains the aftermath steps and relevant responsibility. |
| The HACCP is required to be reviewed at least once a year or when required. | The HARPC plan can be reviewed once every three years or when required. |
| Source | Potential Hazards | Quality Procedure & Preventive Control |
|---|---|---|
| Receiving raw material (ingredients, packaging and labelling material) | Biological because of growth of pathogens and chemical because of possible allergen cross-contact | Allergen preventive control, supply chain preventive control |
| Storage of raw material | Chemical hazard because of possible oxidative rancidity | Sanitation preventive control |
| Weighing and mixing of ingredients | Biological hazards because of environmental pathogens and physical hazards because of chances of metal inclusion from metal-metal contact during mixing | Sanitation and process preventive control |
| Processing (homogenization, evaporation and spray drying) | Biological hazards can occur if the set temperature is not reached, and physical hazards can occur if there is metal to metal contact during processing | Process preventive control |
| Packaging | Chemical hazard because of allergen cross-contact | Allergen preventive control |
| Final Product | Biological because of growth of pathogens and chemical hazards because of possible allergen cross-contact | Allergen preventive control, supply chain preventive control |
| Storage of final product | Chemical hazard because of possible oxidative rancidity | Sanitation preventive control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Krishnaswamy, K.; Mustapha, A. Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods. Foods 2021, 10, 2199. https://doi.org/10.3390/foods10092199
Malik S, Krishnaswamy K, Mustapha A. Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods. Foods. 2021; 10(9):2199. https://doi.org/10.3390/foods10092199
Chicago/Turabian StyleMalik, Sargun, Kiruba Krishnaswamy, and Azlin Mustapha. 2021. "Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods" Foods 10, no. 9: 2199. https://doi.org/10.3390/foods10092199
APA StyleMalik, S., Krishnaswamy, K., & Mustapha, A. (2021). Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods. Foods, 10(9), 2199. https://doi.org/10.3390/foods10092199







