Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Extraction of Pomegranate Phenolic Compounds
2.4. Quantification of Total Phenolic Content
2.5. Quantification of Total Flavonoid Content
2.6. Determination of Total Anthocyanin Content
2.7. HPLC Analysis of Phenolic Compounds by UV Detection
2.8. Antioxidant Activity Measurement Using the DPPH Radical Scavenging Assay
2.9. Hydrogen Peroxide (H2O2) Scavenging Assay
2.10. Ferric Antioxidant Power (FRAP) Assay
2.11. β-Carotene-Linoleic Acid Bleaching (BCB) Assay
2.12. Low Density Lipoprotein Isolation
2.13. Copper-Mediated LDLs Oxidation
2.14. Biochemical Markers of Lipid Peroxidation
Conjugated Diene Formation and α-Tocopherol Disappearance
2.15. Paraoxonase 1 (PON 1) Protein Expression and Activity Measurement
2.16. Cell Culture
2.17. Determination of Reactive Oxygen Species (ROS) in J82 Cells
2.18. Determination of Thiobarbituric Acid Reactive Substances (TBARS) in J82 Cells
2.19. Data and Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic, Flavonoid, and Anthocyanin Content
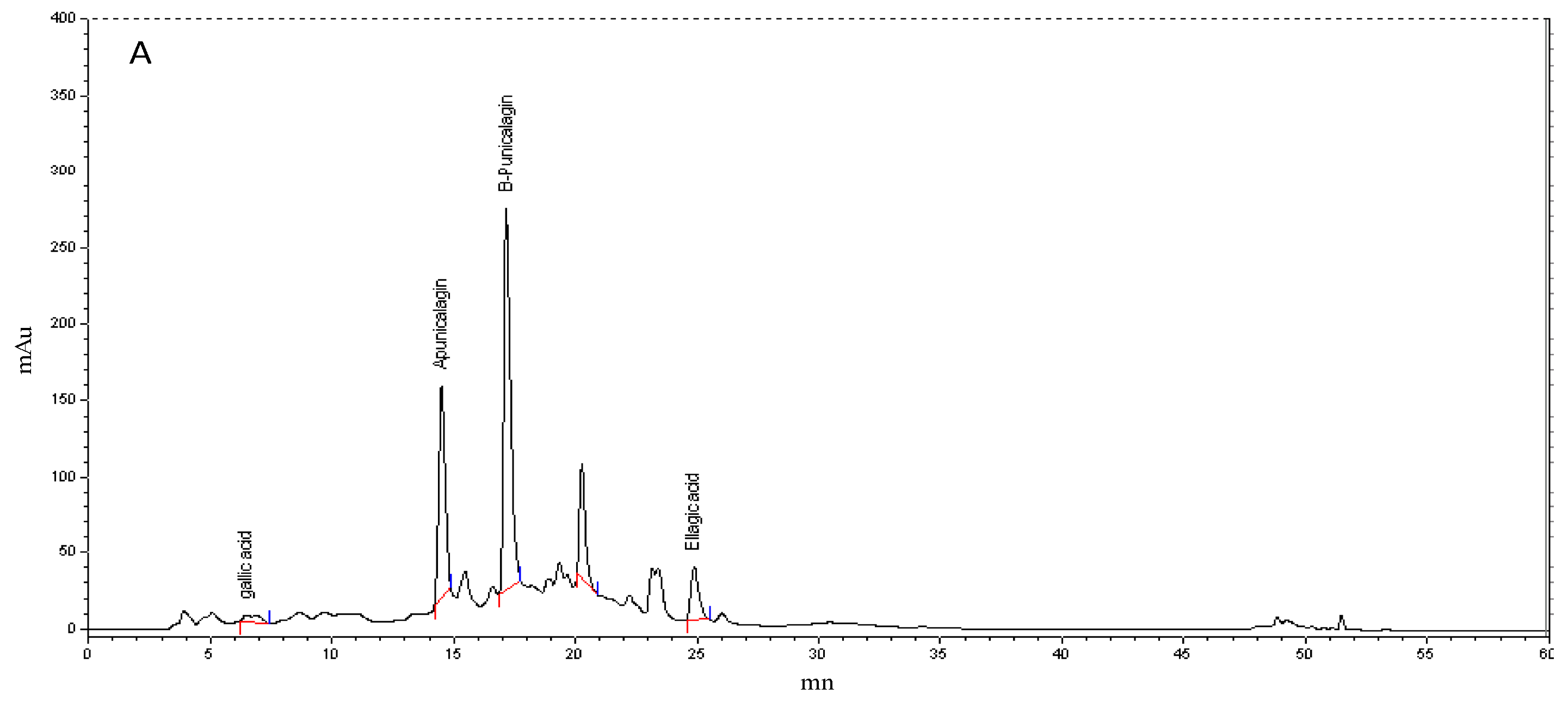
3.2. Polyphenol HPLC Analysis
3.3. Antioxidant Activities
3.4. LDL Oxidation and Paraoxonase 1 (PON1) Activity
3.5. Effect of Phenolic-Rich Pomegranate Extracts on Antioxidant Activities in J82 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Sabraoui, T.; Khider, T.; Nasser, B.; Eddoha, R.; Moujahid, A.; Benbachir, M.; Essamadi, A. Determination of Punicalagins Content, Metal Chelating, and Antioxidant Properties of Edible Pomegranate (Punica granatum L.) Peels and Seeds Grown in Morocco. Int. J. Food Sci. 2020, 2020, 8885889. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Calhau, C. The Bioactivity of Pomegranate: Impact on Health and Disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Benzie, I.F.F. (Eds.) Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxidative Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Li, J. Pomegranate peel extract polyphenols induced apoptosis in human hepatoma cells by mitochondrial pathway. Food Chem. Toxicol. 2016, 93, 158–166. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef]
- Kojadinovic, M.I.; Arsic, A.C.; Debeljak-Martacic, J.D.; Ristić, A.K.; Kardum, N.D.; Popovic, T.B.; Glibetic, M.D. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1798–1804. [Google Scholar] [CrossRef]
- Razani, Z.; Dastani, M.; Kazerani, H.R. Cardioprotective Effects of Pomegranate (Punica granatum) Juice in Patients with Ischemic Heart Disease. Phytother. Res. 2017, 31, 1731–1738. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef]
- Alexandre, E.; Silva, S.; Santos, S.; Silvestre, A.J.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, M. Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci. 2015, 12, 100–106. [Google Scholar] [CrossRef]
- Mastrogiovanni, F.; Bernini, R.; Basiricò, L.; Bernabucci, U.; Campo, M.; Romani, A.; Santi, L.; Lacetera, N. Antioxidant and anti-inflammatory effects of pomegranate peel extracts on bovine mammary epithelial cells BME-UV1. Nat. Prod. Res. 2020, 34, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Chaves, F.M.; Pavan, I.C.B.; Da Silva, L.G.S.; De Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum. Nutr. 2020, 75, 54–62. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; Dhouib, S.; Sakli, F. UV Protection and Dyeing Properties of Wool Fabrics Dyed with Aqueous Extracts of Madder Roots, Chamomiles, Pomegranate Peels, and Apple Tree Branches Barks. J. Nat. Fibers 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Tehrani-Bagha, A.R.; Gharanjig, K.; Hashemi, E. Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J. Clean. Prod. 2019, 231, 1463–1473. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Martínez, J.J.; Hernández, F.; Abdelmajid, H.; Legua, P.; Martínez, R.; El Amine, A.; Melgarejo, P. Physico-chemical characterization of six pomegranate cultivars from Morocco: Processing and fresh market aptitudes. Sci. Hortic. 2012, 140, 100–106. [Google Scholar] [CrossRef]
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, S2675–S2684. [Google Scholar] [CrossRef]
- Aviram, M.; Kaplan, M.; Rosenblat, M.; Fuhrman, B. Dietary Antioxidants and Paraoxonases against LDL Oxidation and Atherosclerosis Development. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Lee, C.-K.; Liao, C.-W.; Meng, S.-W.; Wu, W.-K.; Chiang, J.-Y.; Wu, M.-S. Lipids and Lipoproteins in Health and Disease: Focus on Targeting Atherosclerosis. Biomedicines 2021, 9, 985. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Duan, X.-J.; Huang, H.-L.; Zhang, Y.; Wang, B.-G. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). Environ. Boil. Fishes 2006, 19, 97–108. [Google Scholar] [CrossRef]
- Bhatti, M.Z.; Ali, A.; Ahmad, A.; Saeed, A.; Malik, S.A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res. Notes 2015, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-Scavenging Activity and Ferric Reducing Ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J. Phenolic Constituents from the Lichen Parmotrema stuppeum (Nyl.) Hale and Their Antioxidant Activity. Z. Nat. C 2000, 55, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Sattler, W.; Mohr, D.; Stocker, R. [50] Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 233, pp. 469–489. [Google Scholar]
- Berrougui, H.; Grenier, G.; Loued, S.; Drouin, G.; Khalil, A. A new insight into resveratrol as an atheroprotective compound: Inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis 2009, 207, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Cloutier, M.; Isabelle, M.; Khalil, A. Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis 2006, 184, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Jaouad, L.; de Guise, C.; Berrougui, H.; Cloutier, M.; Isabelle, M.; Fulop, T.; Payette, H.; Khalil, A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1′s free sulfhydryl groups. Atherosclerosis 2006, 185, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; de la Llera-Moya, M.; Dallal, G.E.; Horvath, K.V.; Schaefer, E.J.; Rothblat, G.H. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J. Lipid Res. 2005, 46, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sánchez, R.; Posadas-Romero, C.; Mendoza-Pérez, E.; Caracas-Portilla, N.A.; Cardoso-Saldaña, G.; Medina-Urrutia, A.; Jorge-Galarza, E.; Juárez-Rojas, J.G. Cholesterol Efflux and Metabolic Abnormalities Associated With Low High-Density-Lipoprotein-Cholesterol and High Triglycerides in Statin-Treated Coronary Men With Low-Density Lipoprotein-Cholesterol <70 mg/dl. Am. J. Cardiol. 2021, 109, 636–641. [Google Scholar]
- Yang, Q.-Q.; Cheng, L.-Z.; Zhang, T.; Yaron, S.; Jiang, H.-X.; Sui, Z.-Q.; Corke, H. Phenolic profiles, antioxidant, and antiproliferative activities of turmeric (Curcuma longa). Ind. Crops Prod. 2020, 152, 112561. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, Sensitive, and Specific Thiobarbituric Acid Method for Measuring Lipid Peroxidation in Animal Tissue, Food, and Feedstuff Samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Effect of an extraction solvent on the antioxidant quality of Pinus densiflora needle extract. J. Pharm. Anal. 2019, 9, 193–200. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Layla, A. Pomegranate Bioactive Molecules and Health Benefits. In Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Tehranifar, A.; Zarei, M.; Nemati, Z.; Esfandiyari, B.; Vazifeshenas, M.R. Investigation of physico-chemical properties and antioxidant activity of twenty Iranian pomegranate (Punica granatum L.) cultivars. Sci. Hortic. 2010, 126, 180–185. [Google Scholar] [CrossRef]
- Caliskan, O.; Bayazit, S. Phytochemical and antioxidant attributes of autochthonous Turkish pomegranates. Sci. Hortic. 2012, 147, 81–88. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Tao, X.; Men, X.-M.; Xu, Z.-W.; Wang, T. In Vitro and In Vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Ali, S.; El-Baz, F.; El-Emary, G.A.E.; Khan, E.; Amin Mohamed, A. HPLC-analysis of polyphenolic compounds and free radical scavenging activity of pomegranate fruit (Punica granatum L.). Int. J. Pharm. Clin. Res. 2014, 6, 348–355. [Google Scholar]
- Guo, S.; Deng, Q.; Xiao, J.; Xie, B.; Sun, Z. Evaluation of Antioxidant Activity and Preventing DNA Damage Effect of Pomegranate Extracts by Chemiluminescence Method. J. Agric. Food Chem. 2007, 55, 3134–3140. [Google Scholar] [CrossRef]
- Zaouay, F.; Mena, P.; Garcia-Viguera, C.; Mars, M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod. 2012, 40, 81–89. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.; Ali, H.; Sarwat, M.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić Martinović, L.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Kraljević Pavelić, S. Assessment of the Biological Activity and Phenolic Composition of Ethanol Extracts of Pomegranate (Punica granatum L.) Peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Murthy, K.; Jayaprakasha, G. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models. J. Agric. food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, Tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Mariod, A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Volkova, N.; Coleman, R.; Dreher, M.; Reddy, M.K.; Ferreira, D.; Rosenblat, M. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: Studies in vivo in atherosclerotic apolipoprotein e-deficient (E0) mice and In Vitro in cultured macrophages and lipoproteins. J. Agric. Food Chem. 2008, 56, 1148–1157. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Ricci, D.; Giamperi, L.; Bucchini, A.; Fraternale, D. Antioxidant activity of Punica granatum fruits. Fitoterapia 2006, 77, 310–312. [Google Scholar] [CrossRef]
- BinMowyna, M.; Binobead, M.; Badr, N.; AlSedairy, S.; Elredh, I.; Alqahtani, W. Effect of Saudi and Egyptian pomegranate polyphenols in regulating the activity of PON1, PON2 and lipid profile for preventing coronary heart disease. J. Regen. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef]
- Dorantes-Morales, A.; Estrada-Luna, D.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Luna-Luna, M.; Flores-Castillo, C.; Vargas-Alarcón, G.; Fragoso, J.M.; Pérez-Méndez, Ó.; Carreón-Torres, E. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules 2020, 25, 3297. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Martínez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; López-Rodríguez, G.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2017, 57, 383–389. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef]
- Ripple, M.O.; Henry, W.F.; Schwarze, S.R.; Wilding, G.; Weindruch, R. Effect of antioxidants on androgen-induced AP-1 and NF-kappaB DNA-binding activity in prostate carcinoma cells. J. Natl. Cancer Inst. 1999, 91, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Aviram, M. Pomegranate phytosterol (β-sitosterol) and polyphenolic antioxidant (punicalagin) addition to statin, significantly protected against macrophage foam cells formation. Atherosclerosis 2013, 226, 110–117. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Suh, H.-J.; Kim, Y.M.; Boo, Y.C. Anti-Inflammatory Effects of Pomegranate Peel Extract in THP-1 Cells Exposed to Particulate Matter PM10. Evid.-Based Complement. Altern. Med. 2016, 2016, 6836080. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaid, M.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-mediated Oxidative Stress and Markers of Photoaging in Immortalized HaCaT Keratinocytes by Pomegranate Polyphenol Extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef]
- Elango, S.; Balwas, R.; Padma, V.V. Gallic Acid Isolated from Pomegranate Peel Extract Induces Reactive Oxygen Species Mediated Apoptosis in A549 Cell Line. J. Cancer Ther. 2011, 2, 638–645. [Google Scholar] [CrossRef][Green Version]





| Plant Extract | Polyphenols (mg GAE/g dw) | Flavonoids (mg QE/g dw) | Total Anthocyanin (mg cy-3-glu/100 g dw) | α-Punicalagin (mg/g dw) | β-Punicalagin (mg/g dw) | Gallic Acid (mg/g dw) | Ellagic Acid (mg/g dw) |
|---|---|---|---|---|---|---|---|
| PPPE | 283.86 ± 17.89 * | 185.37 ± 3.05 *** | 102.97 ± 9.19, ns | 148.95 ± 2.43 *** | 302.38 ± 7.26 *** | 5.87 ± 0.08 | 18.85 ± 0.41 |
| PAPE | 166.90 ± 18.10 | 57.43 ± 0.41 | 81.26 ± 18.39 | 40.40 ± 2.67 | 3.03 ± 0.44 | 3.88 ± 0.04 | 14.43 ± 0.21 |
| Plant Extract | Antioxidant Activities | |||
|---|---|---|---|---|
| DPPH (IC50 Values, µg/mL) | H2O2 (IC50 Values, µg/mL) | FRAP (mg AAE/g dw) | BCB (%) | |
| PPPE | 12.49 ± 0.60 * | 19.96 ± 0.02 *** | 374.83 ± 16.85 *** | 86.83 ± 1.22 *** |
| PAPE | 21.58 ± 4.44 | 37.06 ± 0.05 | 189.83 ± 5.29 | 55.64 ± 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process. Foods 2021, 10, 2219. https://doi.org/10.3390/foods10092219
Benchagra L, Berrougui H, Islam MO, Ramchoun M, Boulbaroud S, Hajjaji A, Fulop T, Ferretti G, Khalil A. Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process. Foods. 2021; 10(9):2219. https://doi.org/10.3390/foods10092219
Chicago/Turabian StyleBenchagra, Lamiae, Hicham Berrougui, Mohamed Obaidul Islam, Mhamed Ramchoun, Samira Boulbaroud, Abdelouahed Hajjaji, Tamas Fulop, Gianna Ferretti, and Abdelouahed Khalil. 2021. "Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process" Foods 10, no. 9: 2219. https://doi.org/10.3390/foods10092219
APA StyleBenchagra, L., Berrougui, H., Islam, M. O., Ramchoun, M., Boulbaroud, S., Hajjaji, A., Fulop, T., Ferretti, G., & Khalil, A. (2021). Antioxidant Effect of Moroccan Pomegranate (Punica granatum L. Sefri Variety) Extracts Rich in Punicalagin against the Oxidative Stress Process. Foods, 10(9), 2219. https://doi.org/10.3390/foods10092219









