Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review
Abstract
:1. Introduction
2. Yeasts Producing 2-PE
3. Biochemical Pathways for 2-PE Production
4. Factors Affecting the Rate of 2-PE Production in Yeasts
5. 2-PE Production via Fermentation of Yeasts on Synthetic Media
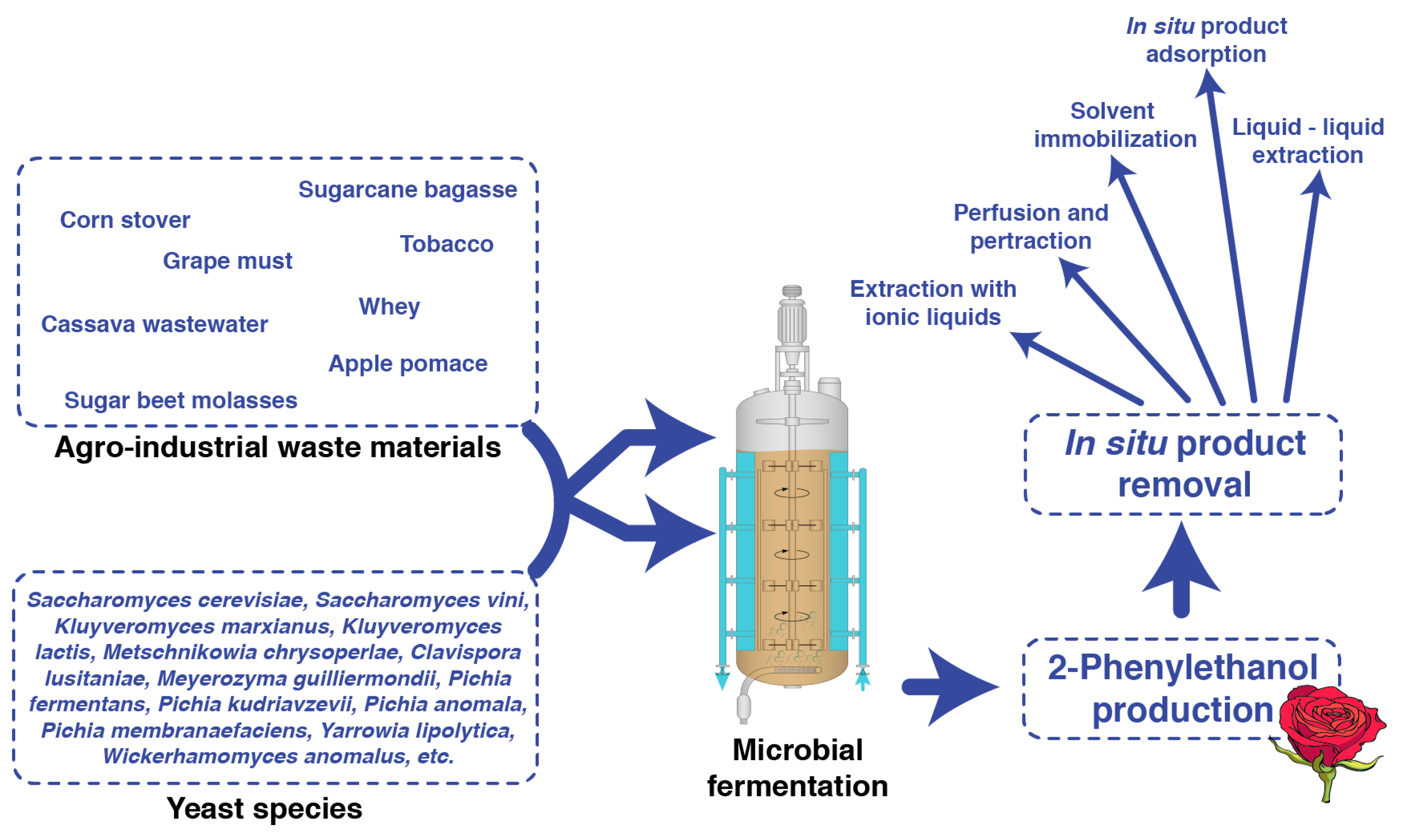
6. 2-PE Production via Fermentation on Agro-Industrial Waste and By-Products
6.1. Whey
6.2. Grape Must
6.3. Corn Stover
6.4. Sugar Beet Molasses (SBM)
6.5. Sugarcane Bagasse (SCB)
6.6. Tobbaco
6.7. Cassava Wastewater (CWW)
6.8. Other Agro-Industrial Waste
7. Cytotoxicity of 2-PE
8. Conventional Strategies to Increase the 2-PE Production
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chantasuban, T.; Santomauro, F.; Gore-Lloyd, D.; Parsons, S.; Henk, D.; Scott, R.J.; Chuck, C. Elevated production of the aromatic fragrance molecule, 2-phenylethanol, using Metschnikowia pulcherrima through both de novo and ex novo conversion in batch and continuous modes. J. Chem. Technol. Biotechnol. 2018, 93, 2118–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, L.; Kong, S.; Liu, Z.; Li, X.; Pan, H. Metabolic Engineering of Escherichia coli for Production of 2-Phenylethanol and 2-Phenylethyl Acetate from Glucose. J. Agric. Food Chem. 2018, 66, 5886–5891. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, J.; Flôres, S.H.; Pizzolato, T.M.; Peralba, M.D.C.; Ayub, M.A.Z. Bioconversion of l-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J. Microbiol. Biotechnol. 2007, 23, 1273–1279. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on phenylethyl alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef]
- Majdabadi, N.; Falahati, M.; Heidarie-Kohan, F.; Farahyar, S.; Rahimi-Moghaddam, P.; Ashrafi-Khozani, M.; Razavi, T.; Mohammadnejad, S. Effect of 2-Phenylethanol as Antifungal Agent and Common Antifungals (Amphotericin B, Fluconazole, and Itraconazole) on Candida Species Isolated from Chronic and Recurrent Cases of Candidal Vulvovaginitis. Assay Drug Dev. Technol. 2018, 16, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 2019, 111, 1499–1506. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chiang, S.H.; Ju, H.Y.; Chen, Y.M.; Liao, M.Y.; Liu, Y.C.; Shieh, C.J. Enzymatic synthesis of rose aromatic ester (2-phenylethyl acetate) by lipase. J. Sci. Food Agric. 2012, 92, 2141–2147. [Google Scholar] [CrossRef]
- Celińska, E.; Kubiak, P.; Białas, W.; Dziadas, M.; Grajek, W. Yarrowia lipolytica: The novel and promising 2-phenylethanol producer. J. Ind. Microbiol. Biotechnol. 2013, 40, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Quintelas, C.; Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. NIR spectroscopy applied to the determination of 2-phenylethanol and l-phenylalanine concentrations in culture medium of Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2019, 94, 812–818. [Google Scholar] [CrossRef]
- Oliveira, S.M.M.; Gomes, S.D.; Sene, L.; Christ, D.; Piechontcoski, J. Production of natural aroma by yeast in wastewater of cassava starch industry. Eng. Agric. 2015, 35, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Fed-Batch and Sequential-Batch Approaches to Enhance the Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate in Solid-State Fermentation Residue-Based Systems. J. Agric. Food Chem. 2019, 67, 3389–3399. [Google Scholar] [CrossRef]
- Conde-Báez, L.; López-Molina, A.; Gómez-Aldapa, C.; Pineda-Muñoz, C.; Conde-Mejía, C. Economic projection of 2-phenylethanol production from whey. Food Bioprod. Process. 2019, 115, 10–16. [Google Scholar] [CrossRef]
- Lukito, B.R.; Wu, S.; Saw, H.J.J.; Li, Z. One-Pot Production of Natural 2-Phenylethanol from l-phenylalanine via Cascade Biotransformations. ChemCatChem 2019, 11, 831–840. [Google Scholar] [CrossRef]
- Mu, L.; Hu, X.; Liu, X.; Zhao, Y.; Xu, Y. Production of 2-phenylethanol by microbial mixed cultures allows resource recovery of cane molasses wastewater. Fresenius Environ. Bull. 2014, 23, 1356–1365. [Google Scholar]
- Sakai, M.; Hirata, H.; Sayama, H.; Sekiguchi, K.; Itano, H.; Asai, T.; Dohra, H.; Hara, M.; Watanabe, N. Production of 2-phenylethanol in roses as the dominant floral scent compound from l-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci. Biotechnol. Biochem. 2007, 10, 2408–2419. [Google Scholar] [CrossRef]
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Leigh Francis, I.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel wine yeast with ARO4 and TYR1 mutations that overproduce ‘floral’ aroma compounds 2-phenylethanol and 2-phenylethyl acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Schrader, J. An aqueous-organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast. Appl. Microbiol. Biotechnol. 2006, 71, 440–443. [Google Scholar] [CrossRef]
- Mierzejewska, J.; Dąbkowska, K.; Chreptowicz, K.; Sokołowska, A. Hydrolyzed corn stover as a promising feedstock for 2-phenylethanol production by nonconventional yeast. J. Chem. Technol. Biotechnol. 2019, 94, 777–784. [Google Scholar] [CrossRef]
- Dai, J.; Xia, H.; Yang, C.; Chen, X. Sensing, Uptake and Catabolism of l-phenylalanine During 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Q.; Guan, A.; Meng, C.; Shi, X.; Guo, Y. Synergistic inhibition effect of 2-phenylethanol and ethanol on bioproduction of natural 2-phenylethanol by Saccharomyces cerevisiae and process enhancement. J. Biosci. Bioeng. 2011, 112, 26–31. [Google Scholar] [CrossRef]
- Conde-Báez, L.; Castro-Rosas, J.; Villagómez-Ibarra, J.R.; Páez-Lerma, J.B.; Gómez-Aldapa, C. Evaluation of Waste of the Cheese Industry for the Production of Aroma of Roses (Phenylethyl Alcohol). Waste Biomass Valorization 2017, 8, 1343–1350. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Sternicka, M.K.; Kowalska, P.D.; Mierzejewska, J. Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett. Appl. Microbiol. 2018, 66, 153–160. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process engineering for bioflavour production with metabolically active yeasts—A mini-review. Yeast 2015, 32, 123–143. [Google Scholar] [CrossRef]
- Eshkol, N.; Sendovski, M.; Bahalul, M.; Katz-Ezov, T.; Kashi, Y.; Fishman, A. Production of 2-phenylethanol from l-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009, 106, 534–542. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, S.; Li, Y.; Shi, G. Improvement of 2-phenylethanol production in Saccharomyces cerevisiae by evolutionary and rational metabolic engineering. PLoS ONE 2021, 16, e0258180. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, M.; Guo, X.; Liu, Z.; He, X. Reconstruction of metabolic module with improved promoter strength increases the productivity of 2-phenylethanol in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jesús Rodríguez-Romero, J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-phenylethylacetate production by nonconventional yeasts using tequila vinasses as a substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef] [PubMed]
- Mameeva, O.G.; Ostapchuk, A.M.; Podgorsky, V.S. The 2-Phenylethanol and Ethanol Production by Yeast Saccharomyces cerevisiae. Microbiol. Biotechnol. 2010, 1, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Song, Y.; Jin, Y.; Liu, H.; Zhang, H.; Sun, Y.; Liu, G. Biosynthesis of 2-phenylethanol using tobacco waste as feedstock. Biocatal. Biotransform. 2013, 31, 292–298. [Google Scholar] [CrossRef]
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De novo biosynthesis of 2-phenylethanol in engineered Pichia pastoris. Enzyme Microb. Technol. 2020, 133, 109459. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, X.; Guo, X.; He, X. Regulation of crucial enzymes and transcription factors on 2-phenylethanol biosynthesis via Ehrlich pathway in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 129–139. [Google Scholar] [CrossRef]
- Sendovski, M.O.R.; Nir, N.; Fishman, A. Bioproduction of 2-Phenylethanol in a Biphasic Ionic liquid Aqueous system. J. Agric. Food Chem. 2010, 58, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Koma, D.; Yamanaka, H.; Moriyoshi, K.; Ohmoto, T.; Sakai, K. Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway. Appl. Environ. Microbiol. 2012, 78, 6203–6216. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jiang, J.; Bai, Y.; Fan, T.P.; Zhao, Y.; Zheng, X.; Cai, Y. Mimicking a New 2-Phenylethanol Production Pathway from Proteus mirabilis JN458 in Escherichia coli. J. Agric. Food Chem. 2018, 66, 3498–3504. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium. Biotechnol. Lett. 2003, 25, 531–536. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2010; Volume 8, p. e0132. [Google Scholar] [CrossRef] [Green Version]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Wielechowska, M.; Główczyk-Zubek, J.; Rybak, E.; Mierzejewska, J. Production of natural 2-phenylethanol: From biotransformation to purified product. Food Bioprod. Process. 2016, 100, 275–281. [Google Scholar] [CrossRef]
- de Lima, L.A.; Diniz, R.H.S.; de Queiroz, M.V.; Fietto, L.G.; da Silveira, W.B. Screening of Yeasts Isolated from Brazilian Environments for the 2-Phenylethanol (2-PE) Production. Biotechnol. Bioprocess Eng. 2018, 23, 326–332. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zong, H.; Ji, H.; Zhuge, B.; Dong, Z. Bioconversion of l-phenylalanine to 2-phenylethanol by the novel stress-tolerant yeast Candida glycerinogenes WL2002-5. Bioengineered 2016, 7, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adame-Soto, P.J.; Aréchiga-Carvajal, E.T.; López, M.G.; González-Herrera, S.M.; Moreno-Jiménez, M.R.; Urtiz-Estrada, N.; Rutiaga-Quiñones, O.M. Potential production of 2-phenylethanol and 2-phenylethylacetate by non-Saccharomyces yeasts from Agave durangensis. Ann. Microbiol. 2019, 69, 989–1000. [Google Scholar] [CrossRef]
- Fan, G.; Cheng, L.; Fu, Z.; Sun, B.; Teng, C.; Jiang, X.; Li, X. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Dai, J.; Li, K.; Song, N.; Yao, W.; Xia, H.; Yang, Q.; Zhang, X.; Li, X.; Wang, Z.; Yao, L.; et al. Zygosaccharomyces rouxii, an Aromatic Yeast Isolated from Chili Sauce, Is Able to Biosynthesize 2-Phenylethanol via the Shikimate or Ehrlich Pathways. Front. Microbiol. 2020, 11, 597454. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, X.; Shen, Q.; Wang, K.; Zhu, T. Optimisation of biotransformation conditions for production of 2-phenylethanol by a Saccharomyces cerevisiae CWY132 mutant. Nat. Prod. Res. 2011, 25, 754–759. [Google Scholar] [CrossRef]
- Fukuda, K.; Watanabe, M.; Asano, K. Altered Regulation of Aromatic Amino Acid Biosynthesis in β-Phenylethyl-alcohol-overproducing Mutants of Sake Yeast Saccharomyces cerevisiae. Agric. Biol. Chem. 1990, 54, 3151–3156. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Cho, B.R.; Hahn, J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, S.W.; Oh, M.K. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb. Technol. 2014, 61–62, 44–47. [Google Scholar] [CrossRef]
- Miller, K.K.; Alper, H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262. [Google Scholar] [CrossRef]
- Basu, S.; Bose, C.; Ojha, N.; Das, N.; Das, J.; Pal, M.; Khurana, S. Evolution of bacterial and fungal growth media. Bioinformation 2015, 11, 182–184. [Google Scholar] [CrossRef]
- Santos, F.P.; de Magalhães, D.C.M.M.; Nascimento, J.d.S.; Ramos, G.L.d.P.A. Use of products of vegetable origin and waste from hortofruticulture for alternative culture media. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Qian, X.; Yan, W.; Zhang, W.; Dong, W.; Ma, J.; Ochsenreither, K.; Jiang, M.; Xin, F. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2019, 39, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-phenylethanol (rose aroma) production potential of an isolated pichia kudriavzevii through solid-state fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.H. Economic aspects of cheese making as influenced by whey processing options. Int. Dairy J. 2005, 15, 537–545. [Google Scholar] [CrossRef]
- Viana, T.; Loureiro-Dias, M.C.; Prista, C. Efficient fermentation of an improved synthetic grape must by enological and laboratory strains of Saccharomyces cerevisiae. AMB Express 2014, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Grape Must Composition Overview. In Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Maicas, S.; Mateo, J.J. Sustainability of wine production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Mannitol bioproduction from surplus grape musts and wine lees. LWT 2021, 151, 112083. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, L.; Liu, W.J.; Fang, M.Q.; Wang, N. Assessment of the nutritive value of whole corn stover and its morphological fractions. Asian-Australas. J. Anim. Sci. 2014, 27, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Lu, J.; Zhao, J.; Qu, Y. Characteristics of corn stover pretreated with liquid hot water and fed-batch semi-simultaneous saccharification and fermentation for bioethanol production. PLoS ONE 2014, 9, e95455. [Google Scholar] [CrossRef] [PubMed]
- Maung, T.A.; Gustafson, C.R. The economic feasibility of sugar beet biofuel production in central North Dakota. Biomass Bioenergy 2011, 35, 3737–3747. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Zhou, M.; Wang, H.; Chen, L.; Li, Z.; Cai, D.; Wen, Z.; Ma, X.; Chen, S. Efficient synthesis of 2-phenylethanol from l-phenylalanine by engineered Bacillus licheniformis using molasses as carbon source. Appl. Microbiol. Biotechnol. 2020, 104, 7507–7520. [Google Scholar] [CrossRef]
- Mustafa, G.; Arshad, M.; Bano, I.; Abbas, M. Biotechnological applications of sugarcane bagasse and sugar beet molasses. Biomass Convers. Biorefinery 2020, 1–13. [Google Scholar] [CrossRef]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of desugarized sugar beet molasses for the production of poly(3-hydroxybutyrate) by halophilic Bacillus megaterium uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Khattab, S.M.R.; Watanabe, T. Bioethanol from Sugarcane Bagasse: Status and Perspectives. In Bioethanol Production from Food Crops; Academic Press: Cambridge, MA, USA, 2019; pp. 187–212. [Google Scholar] [CrossRef]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Bioproduction of 2-phenylethanol and 2-phenethyl acetate by Kluyveromyces marxianus through the solid-state fermentation of sugarcane bagasse. Appl. Microbiol. Biotechnol. 2018, 102, 4703–4716. [Google Scholar] [CrossRef] [Green Version]
- Novotny, T.E.; Bialous, S.A.; Burt, L.; Curtis, C.; Luiza da Costa, V.; Iqtidar, S.U.; Liu, Y.; Pujari, S.; Tursan d’Espaignet, E. The environmental and health impacts of tobacco agriculture, cigarette manufacture and consumption. Bull. World Health Organ. 2015, 93, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Mañunga, T.; Barrios-Pérez, J.D.; Zaiat, M.; Rodríguez-Victoria, J.A. Evaluation of pretreatment methods and initial pH on mixed inoculum for fermentative hydrogen production from cassava wastewater. Biofuels 2019, 1–8. [Google Scholar] [CrossRef]
- Stark, D.; Zala, D.; Münch, T.; Sonnleitner, B.; Marison, I.W.; Von Stockar, U. Inhibition aspects of the bioconversion of l-phenylalanine to 2-phenylethanol by Saccharomyces cerevisiae. Enzyme Microb. Technol. 2003, 32, 212–223. [Google Scholar] [CrossRef]
- Larroude, M.; Rossignol, T.; Nicaud, J.M.; Ledesma-Amaro, R. Synthetic biology tools for engineering Yarrowia lipolytica. Biotechnol. Adv. 2018, 36, 2150–2164. [Google Scholar] [CrossRef]
- Larroude, M.; Nicaud, J.M.; Rossignol, T. Yarrowia lipolytica chassis strains engineered to produce aromatic amino acids via the shikimate pathway. Microb. Biotechnol. 2020, 14, 2420–2434. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Mierzejewska, J. Enhanced bioproduction of 2-phenylethanol in a biphasic system with rapeseed oil. New Biotechnol. 2018, 42, 56–61. [Google Scholar] [CrossRef]
- Combes, J.; Clavijo Rivera, E.; Clément, T.; Fojcik, C.; Athès, V.; Moussa, M.; Allais, F. Solvent selection strategy for an ISPR (In Situ/In stream product recovery) process: The case of microbial production of p-coumaric acid coupled with a liquid-liquid extraction. Sep. Purif. Technol. 2021, 259, 118170. [Google Scholar] [CrossRef]
- Gao, F.; Daugulis, A.J. Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by Kluyveromyces marxianus. Biotechnol. Bioeng. 2009, 104, 332–339. [Google Scholar] [CrossRef]
- Hua, D.; Lin, S.; Li, Y.; Chen, H.; Zhang, Z.; Du, Y.; Zhang, X.; Xu, P. Enhanced 2-phenylethanol production from l-phenylalanine via in situ product adsorption. Biocatal. Biotransform. 2010, 28, 259–266. [Google Scholar] [CrossRef]
- Serp, D.; Von Stockar, U.; Marison, I.W. Enhancement of 2-phenylethanol productivity by Saccharomyces cerevisiae in two-phase fed-batch fermentations using solvent immobilization. Biotechnol. Bioeng. 2003, 82, 103–110. [Google Scholar] [CrossRef] [PubMed]

| Synthetic Culture Media | Agro-Industrial Waste and By-Products | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Synthetic-Based Media | Agro-Industrial Waste and By-Products | ||||||
|---|---|---|---|---|---|---|---|
| Strain | Media Major Constituents + l-Phe Content | 2-PE Production | Ref. | Strain | Media Type + l-Phe Content | 2-PE Production | Ref. |
| S. cerevisiae | |||||||
| JM2014 | Medium 8 + 5 g/L l-Phe | 3.6 g/L | [43] | JM2014 WUT4 WUT34 WUT35 | WGP medium + 0.5% l-Phe | 2 g/L | [24] |
| CWY132 | 34.16 g/L glucose + 5g/L l-Phe | 3.76 g/L | [49] | NS | Tobacco waste + 0 g/L l-Phe | 1.55 g/L | [33] |
| JHY315 | 20 g/L glucose + 10 g/L l-Phe | 4.8 g/L | [51] | NS | Cassava wastewater + 5.5 g/L l-Phe | 1.33 g/L | [11] |
| K. marxianus | |||||||
| CCT 7735 | 3 g/L glucose + 4 g/L l-Phe | 3.44 g/L | [44] | ITD00262 | Sweet whey + 0 g/L l-Phe | 0.96 g/L | [23] |
| Acid whey + 0 g/L l-Phe | 0.7 g/L | ||||||
| Curd whey + 0 g/L l-Phe | 0.47 g/L | ||||||
| BY25569 | 20 g/L glucose + 0 g/L l-Phe | 1.3 g/L | [52] | CBS 6556 | Grape must + 3 g/L l-Phe | 0.77 g/L | [4] |
| CBS 600 | Sugar beet molasses + 7 g/L l-Phe | 0.89 g/L | [39] | ||||
| Pichia kudriavzevii | |||||||
| YF1702 | 50 g/L glucose + 10.7 g/L l-Phe | 5.09 g/L | [47] | WUT7 | WGP medium + 0.5% l-Phe | Between 1 and 2 g/L | [24] |
| CECT 10467 | Sugarcane bagasse + 2% l-Phe | 27.2 mg2-PE g−1DS | [58] | ||||
| CECT 13184 | Rice husk | 5.7 mg2-PE g−1DS | [57] | ||||
| Brewer’s spent grain | 6.5 mg2-PE g−1DS | ||||||
| Soy fiber | 11.5 mg2-PE g−1DS | ||||||
| Rice fiber | 8.5 mg2-PE g−1DS | ||||||
| Asparagus tails | 7.7 mg2-PE g−1DS | ||||||
| Orange peel | 17.2 mg2-PE g−1DS | ||||||
| Banana peel | 17.2 mg2-PE g−1DS | ||||||
| Green apple pomace | 17.4 mg2-PE g−1DS | ||||||
| Red apple pomace | 25.2 mg2-PE g−1DS | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.-M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. https://doi.org/10.3390/foods11010109
Mitri S, Koubaa M, Maroun RG, Rossignol T, Nicaud J-M, Louka N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods. 2022; 11(1):109. https://doi.org/10.3390/foods11010109
Chicago/Turabian StyleMitri, Sara, Mohamed Koubaa, Richard G. Maroun, Tristan Rossignol, Jean-Marc Nicaud, and Nicolas Louka. 2022. "Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review" Foods 11, no. 1: 109. https://doi.org/10.3390/foods11010109
APA StyleMitri, S., Koubaa, M., Maroun, R. G., Rossignol, T., Nicaud, J.-M., & Louka, N. (2022). Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods, 11(1), 109. https://doi.org/10.3390/foods11010109









