Nanoencapsulation of Mandarin Essential Oil: Fabrication, Characterization, and Storage Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Methods
2.2.1. Preparation of Emulsion
2.2.2. Freeze-Drying Process
2.3. Properties of Emulsions
2.4. Properties of CEO Nanocapsules
2.4.1. Encapsulation Efficiency
2.4.2. Moisture Content, Hygroscopicity, Solubility, and Wettability
2.4.3. Bulk, Tapped, and Particle Density
2.4.4. Porosity, Cohesiveness, and Flowability
2.4.5. Color
2.4.6. Glass Transition Temperature (Tg)
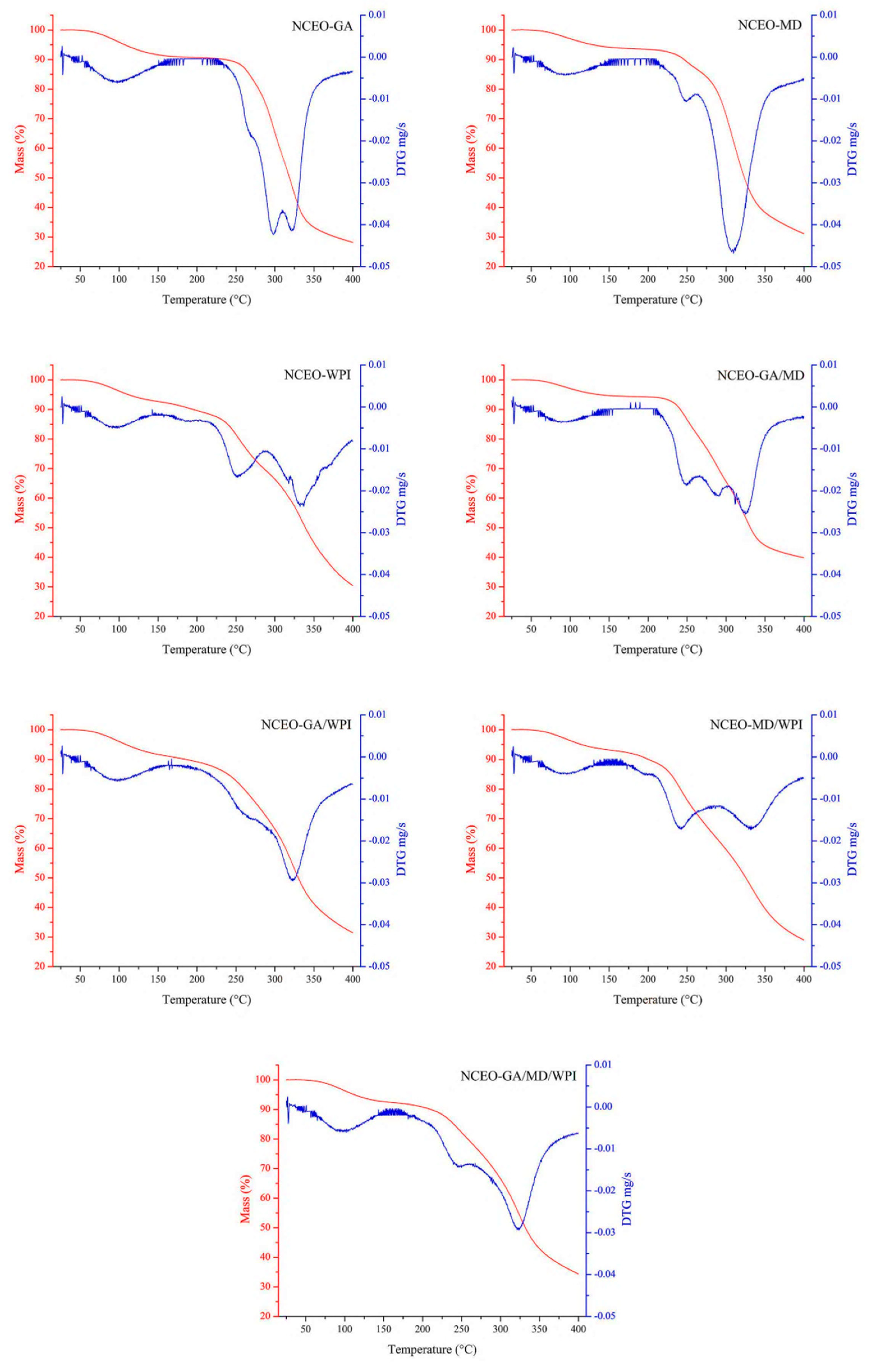
2.4.7. Thermogravimetric Analysis (TGA)
2.4.8. X-ray Diffraction (XRD)
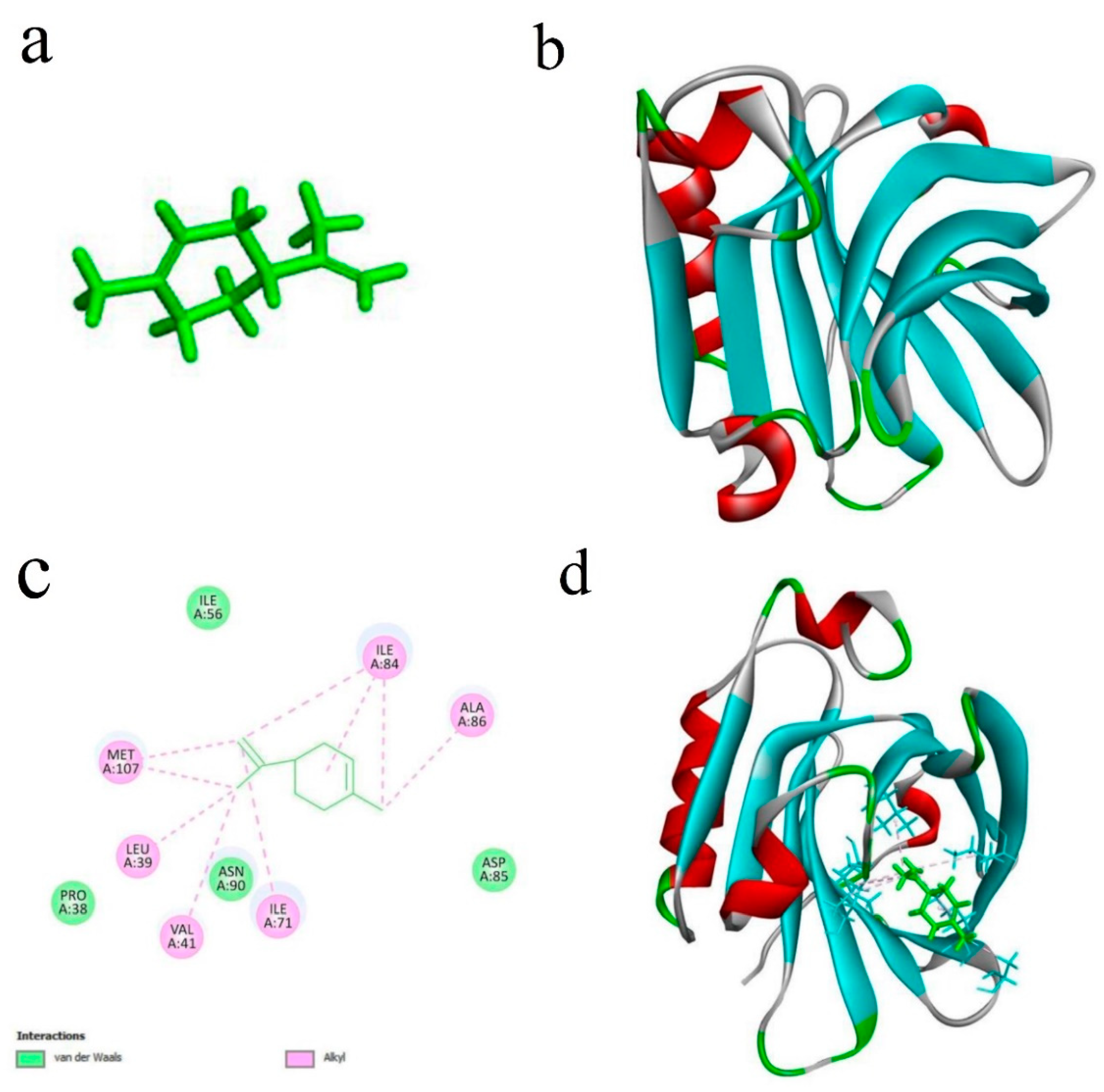
2.4.9. Molecular Docking Simulation
2.4.10. Scanning Electron Microscopy (SEM)
2.4.11. Storage Stability
2.5. Statistical Analysis
3. Results
3.1. MSD, PDI, and ζ-Potential of Fresh Emulsions
3.2. Properties of CEO Nanocapsules
3.2.1. Encapsulation Efficiency
3.2.2. Particle Size and PDI of Nanocapsules
3.2.3. Moisture Content
3.2.4. Hygroscopicity
3.2.5. Solubility
3.2.6. Wettability
3.2.7. Bulk, Tapped, and Particle Density
3.2.8. Porosity
3.2.9. Cohesiveness and Flowability
3.2.10. Color
3.2.11. Glass Transition Temperatures
3.2.12. Thermogravimetric Analysis
3.2.13. Crystallinity
3.2.14. Molecular Docking Simulation
3.2.15. Morphology of the Nanocapsules
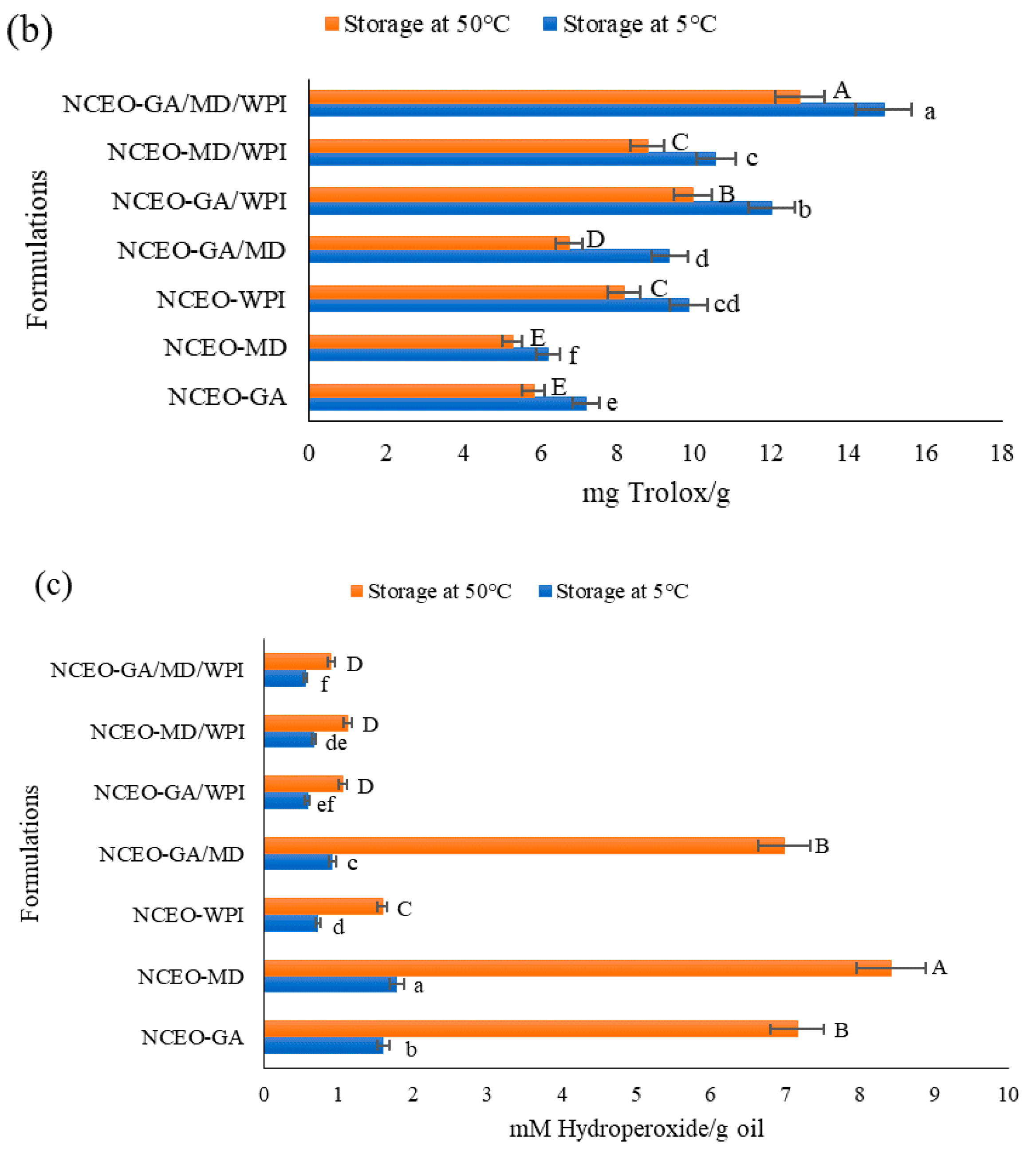
3.2.16. Stability of the Antioxidant Activity
3.2.17. Stability of the Antioxidant Capacity
3.2.18. Oxidative Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of antioxidant activity, antifungal activity, and oxidation stability of Citrus reticulata essential oil nanocapsules by clove and cinnamon essential oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT 2018, 95, 346–353. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Khalid, K.A.; Darwesh, O.M.; Ahmed, A.M. Peel Essential Oils of Citrus Types and Their Antimicrobial Activities in Response to Various Growth Locations. J. Essent. Oil Bear. Plants 2021, 1–20. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and Electrospraying-A review. Food Chem. 2020, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT 2020, 117, 108656. [Google Scholar] [CrossRef]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Mohammed, J.K.; Mahdi, A.A.; Al-Ansi, W.; Zhang, M.; Al-Adeeb, A.; Wei, M.; Phyo, H.M.; Yao, W. Physicochemical properties, microstructure, and storage stability of Pulicaria jaubertii extract microencapsulated with different protein biopolymers and gum arabic as wall materials. Int. J. Biol. Macromol. 2021, 187, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Zhang, J.; Li, L.; Luo, Z.; Wang, Y.; Yuan, Q.; Zhou, B.; Liu, H. Ginger essential oil-based microencapsulation as an efficient delivery system for the improvement of Jujube (Ziziphus jujuba Mill.) fruit quality. Food Chem. 2020, 306, 125628. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, K.; Shen, Y.; Niu, F.; Fu, Y. Influence of pure gum on the physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 442–448. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Zabot, G.L.; Cazarin, C.B.; Maróstica, M.R., Jr.; Meireles, M.A.A. Biopolymer-prebiotic carbohydrate blends and their effects on the retention of bioactive compounds and maintenance of antioxidant activity. Carbohydr. Polym. 2016, 144, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Li, M.; Mamet, T.; Li, C. Maltodextrin or gum Arabic with whey proteins as wall-material blends increased the stability and physiochemical characteristics of mulberry microparticles. Food Biosci. 2019, 31, 100445. [Google Scholar] [CrossRef]
- Meena, S.; Prasad, W.; Khamrui, K.; Mandal, S.; Bhat, S. Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum arabica and its in-vitro digestibility evaluation. Food Biosci. 2021, 41, 100990. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Ahmed, I.A.M.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Outuki, P.M.; de Francisco, L.M.B.; Hoscheid, J.; Bonifácio, K.L.; Barbosa, D.S.; Cardoso, M.L.C. Development of arabic and xanthan gum microparticles loaded with an extract of Eschweilera nana Miers leaves with antioxidant capacity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 103–112. [Google Scholar] [CrossRef]
- Lekshmi, R.K.; Tejpal, C.; Anas, K.; Chatterjee, N.; Mathew, S.; Ravishankar, C. Binary blend of maltodextrin and whey protein outperforms gum Arabic as superior wall material for squalene encapsulation. Food Hydrocoll. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Locali Pereira, A.R.; Gonçalves Cattelan, M.; Nicoletti, V.R. Microencapsulation of pink pepper essential oil: Properties of spray-dried pectin/SPI double-layer versus SPI single-layer stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123806. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; dos Santos, T.O.; de Paula, J.R.; Conceição, E.C.; Bara, M.T. Microencapsulation of essential oil from fruits of Pterodon emarginatus using gum arabic and maltodextrin as wall materials: Composition and stability. Dry. Technol. 2014, 32, 96–105. [Google Scholar] [CrossRef]
- Charve, J.; Reineccius, G.A. Encapsulation performance of proteins and traditional materials for spray dried flavors. J. Agric. Food. Chem. 2009, 57, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ji, X.; Lyu, F.; Liu, J.; Ding, Y. Heat stability and rheology of high-calorie whey protein emulsion: Effects of calcium ions. Food Hydrocoll. 2021, 114, 106583. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X. The protection effect of trehalose on the multinuclear microcapsules based on gelatin and high methyl pectin coacervate during freeze-drying. Food Hydrocoll. 2020, 105, 105807. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Wei, M.; Al-Adeeb, A.; Yao, W. Stabilization of water-in-oil emulsion of Pulicaria jaubertii extract by ultrasonication: Fabrication, characterization, and storage stability. Food Chem. 2021, 350, 129249. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ma, C.; Elkhedir, A.E.; Al-Maqtari, Q.A.; Al-Ansi, W.; Mahmud, A.; Wang, H. Application of argun fruit polysaccharide in microencapsulation of Citrus aurantium L. essential oil: Preparation, characterization, and evaluating the storage stability and antioxidant activity. J. Food Meas. Charact. 2020, 155–169. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; Al-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of fingered citron extract with gum arabic, modified starch, whey protein, and maltodextrin using spray drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef]
- Saifullah, M.; Yusof, Y.; Chin, N.; Aziz, M. Physicochemical and flow properties of fruit powder and their effect on the dissolution of fast dissolving fruit powder tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Bosco, S.J.D.; Francis, S.; Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol. 2015, 274, 37–43. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crop. Prod. 2019, 140, 111739. [Google Scholar] [CrossRef]
- Khalifa, I.; Nie, R.; Ge, Z.; Li, K.; Li, C. Understanding the shielding effects of whey protein on mulberry anthocyanins: Insights from multispectral and molecular modelling investigations. Int. J. Biol. Macromol. 2018, 119, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Stănciuc, N.; Oancea, A.M.; Aprodu, I.; Turturică, M.; Barbu, V.; Ioniţă, E.; Râpeanu, G.; Bahrim, G. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. J. Food Eng. 2018, 223, 197–207. [Google Scholar] [CrossRef]
- Kwangjai, J.; Cheaha, D.; Manor, R.; Sa-ih, N.; Samerphob, N.; Issuriya, A.; Wattanapiromsakul, C.; Kumarnsit, E. Modification of brain waves and sleep parameters by Citrus reticulata Blanco. cv. Sai-Nam-Phueng essential oil. Biomed. J. 2020. [Google Scholar] [CrossRef]
- Nidhi, P.; Rolta, R.; Kumar, V.; Dev, K.; Sourirajan, A. Synergistic potential of Citrus aurantium L. essential oil with antibiotics against Candida albicans. J. Ethnopharmacol. 2020, 262, 113135. [Google Scholar] [CrossRef] [PubMed]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of lemon essential oil in Chitosan-Hicap system. Part 1: Study on its physical and structural characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef]
- Belgheisi, S.; Motamedzadegan, A.; Milani, J.M.; Rashidi, L.; Rafe, A. Impact of ultrasound processing parameters on physical characteristics of lycopene emulsion. J. Food Sci. Technol. 2020, 58, 484–493. [Google Scholar] [CrossRef]
- Perera, U.M.S.P.; Rajapakse, N. Chitosan nanoparticles: Preparation, characterization, and applications. In Seafood Processing By-Products; Springer: Berlin/Heidelberg, Germany, 2014; pp. 371–387. [Google Scholar]
- de Barros Fernandes, R.V.; Borges, S.V.; Silva, E.K.; da Silva, Y.F.; de Souza, H.J.B.; do Carmo, E.L.; de Oliveira, C.R.; Yoshida, M.I.; Botrel, D.A. Study of ultrasound-assisted emulsions on microencapsulation of ginger essential oil by spray drying. Ind. Crop. Prod. 2016, 94, 413–423. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Alhelli, A.M.; Hussin, A.S.M. Process conditions of spray drying microencapsulation of Nigella sativa oil. Powder Technol. 2017, 315, 1–14. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Özbek, A.Z.; Ergönül, G.P. Optimisation of wall material composition of freeze–dried pumpkin seed oil microcapsules: Interaction effects of whey protein, maltodextrin, and gum Arabic by D–optimal mixture design approach. Food Hydrocoll. 2020, 107, 105909. [Google Scholar] [CrossRef]
- Yazicioglu, B.; Sahin, S.; Sumnu, G. Microencapsulation of wheat germ oil. J. Food Sci. Technol. 2015, 52, 3590–3597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korma, S.A.; Wei, W.; Ali, A.H.; Abed, S.M.; Zheng, L.; Jin, Q.; Wang, X. Spray-dried novel structured lipids enriched with medium-and long-chain triacylglycerols encapsulated with different wall materials: Characterization and stability. Food Res. Int. 2019, 116, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, S.; Ojagh, S.M.; Quek, S.Y.; Pourashouri, P.; Salaün, F. Nano-encapsulation of fish oil and garlic essential oil by a novel composition of wall material: Persian gum-chitosan. LWT 2019, 116, 108494. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Kang, Y.-R.; Lee, Y.-K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Juliano, P. Compression and compaction characteristics of selected food powders. Adv. Food Nutr. Res. 2005, 49, 233–300. [Google Scholar] [PubMed]
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. J. Food Eng. 2012, 111, 102–109. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Botrel, D.A.; Silva, E.K.; Borges, S.V.; de Oliveira, C.R.; Yoshida, M.I.; de Andrade Feitosa, J.P.; de Paula, R.C.M. Cashew gum and inulin: New alternative for ginger essential oil microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties. Food Sci. Technol. 2013, 33, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud-Thomassin, C.; Petit, J. Impact of the whey protein/casein ratio on the reconstitution and flow properties of spray-dried dairy protein powders. Powder Technol. 2021, 391, 275–281. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Palacios-González, E.; Lartundo-Rojas, L.; Garibay-Febles, V.; Yáñez-Fernández, J.; Hernandez-Sanchez, H.; Gutiérrez-López, G.; Alamilla-Beltran, L. Microstructural properties and distribution of components in microparticles obtained by spray-drying. J. Food Eng. 2015, 152, 105–112. [Google Scholar] [CrossRef]
- Bae, E.; Lee, S. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of walnut oil by spray drying: Effects of wall material and drying conditions on physicochemical properties of microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Ong, M.; Yusof, Y.; Aziz, M.; Chin, N.; Amin, N.M. Characterisation of fast dispersible fruit tablets made from green and ripe mango fruit powders. J. Food Eng. 2014, 125, 17–23. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Ting, S.; Jiang, J.; Liu, Y. Correlating emulsion properties to microencapsulation efficacy and nutrients retention in mixed proteins system. Food Res. Int. 2019, 115, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum arabic/maltodextrin microencapsulation confers peroxidation stability and antimicrobial ability to pepper seed oil. Food Chem. 2021, 337, 127748. [Google Scholar] [CrossRef]
- Dias, C.O.; dos Santos Opuski de Almeida, J.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Khadom, A.A.; Kadhim, M.M.; Anaee, R.A.; Mahood, H.B.; Mahdi, M.S.; Salman, A.W. Theoritical evaluation of Citrus Aurantium leaf extract as green inhibitor for chemical and biological corrosion of mild steel in acidic solution: Statistical, molecular dynamics, docking, and quantum mechanics study. J. Mol. Liq. 2021, 116978. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Moreno, T.; De Paz, E.; Navarro, I.; Rodríguez-Rojo, S.; Matías, A.; Duarte, C.; Sanz-Buenhombre, M.; Cocero, M. Spray drying formulation of polyphenols-rich grape marc extract: Evaluation of operating conditions and different natural carriers. Food Bioproc. Technol. 2016, 9, 2046–2058. [Google Scholar] [CrossRef]
- Sandhya, S.; Khamrui, K.; Prasad, W.; Kumar, M. Preparation of pomegranate peel extract powder and evaluation of its effect on functional properties and shelf life of curd. LWT 2018, 92, 416–421. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]



| Formulations Code | Gum Arabic (g) | Maltodextrin (g) | Whey Protein Isolate (g) | Water (g) |
|---|---|---|---|---|
| NCEO-GA | 24 | - | - | 68 |
| NCEO-MD | - | 24 | - | 68 |
| NCEO-WPI | - | - | 24 | 68 |
| NCEO-GA/MD | 12 | 12 | - | 68 |
| NCEO-GA/WPI | 12 | - | 12 | 68 |
| NCEO-MD/WPI | - | 12 | 12 | 68 |
| NCEO-GA/MD/WPI | 8 | 8 | 8 | 68 |
| Emulsions’ Code | MSD (nm) | PDI | Zeta-Potential (mV) |
|---|---|---|---|
| GA-based | 674.18 ± 10.07 a | 0.26 ± 0.03 a | −58.08 ± 0.28 b |
| MD-based | 628.59 ± 13.5 b | 0.23 ± 0.03 ab | −57.48 ± 0.24 a |
| WPI-based | 357.86 ± 12.39 e | 0.17 ± 0.05 c | −57.64 ± 0.33 ab |
| GA/MD-based | 578.58 ± 8.02 c | 0.25 ± 0.03 ab | −57.66 ± 0.26 ab |
| GA/WPI-based | 527.12 ± 8.25 d | 0.23 ± 0.04 ab | −57.69 ± 0.28 ab |
| MD/WPI-based | 372.18 ± 8.29 e | 0.23 ± 0.04 ab | −57.75 ± 0.26 ab |
| GA/MD/WPI-based | 529.64 ± 15.51 d | 0.20 ± 0.04 bc | −57.75 ± 0.28 ab |
| Formulations | Encapsulation Efficiency (%) | Particle Size (nm) | PDI |
|---|---|---|---|
| NCEO-GA | 37.17 ± 3.16 d | 782.09 ± 58.66 a | 0.47 ± 0.04 d |
| NCEO-MD | 36.29 ± 2.90 d | 695.49 ± 52.16 abc | 0.41 ± 0.03 c |
| NCEO-WPI | 65.55 ± 5.90 c | 427.35 ± 32.05 d | 0.26 ± 0.02 a |
| NCEO-GA/MD | 43.04 ± 3.23 d | 738.51 ± 55.39 ab | 0.36 ± 0.03 bc |
| NCEO-GA/WPI | 78.22 ± 6.65 b | 641.79 ± 48.13 c | 0.32 ± 0.02 b |
| NCEO-MD/WPI | 69.03 ± 4.83 c | 430.35 ± 32.28 d | 0.27 ± 0.02 a |
| NCEO-GA/MD/WPI | 92.08 ± 6.45 a | 674.95 ± 50.62 bc | 0.38 ± 0.03 c |
| Formulations | Moisture (%) | Hygroscopicity (%) | Solubility (%) | Wettability (s) |
|---|---|---|---|---|
| NCEO-GA | 5.71 ± 0.37 a | 12.90 ± 0.77 cd | 81.87 ± 3.68 b | 247.3 ± 17.5 c |
| NCEO-MD | 3.87 ± 0.29 c | 14.63 ± 0.95 ab | 93.58 ± 4.68 a | 108.0 ± 10.0 f |
| NCEO-WPI | 4.31 ± 0.26 c | 12.21 ± 0.61 d | 89.61 ± 4.93 ab | 305.3 ± 25.0 a |
| NCEO-GA/MD | 3.95 ± 0.26 c | 13.94 ± 0.77 abc | 84.12 ± 3.79 b | 172.0 ± 9.20 e |
| NCEO-GA/WPI | 4.12 ± 0.31 c | 15.42 ± 0.93 a | 83.25 ± 4.16 b | 286.0 ± 10.0 b |
| NCEO-MD/WPI | 3.93 ± 0.28 c | 12.05 ± 0.72 d | 86.51 ± 4.33 ab | 223.0 ± 14.4 d |
| NCEO-GA/MD/WPI | 4.98 ± 0.35 b | 13.84 ± 0.90 bc | 87.55 ± 4.82 ab | 242.3 ± 0.39 cd |
| Formulations | Bulk Density | Tapped Density | Particle Density |
|---|---|---|---|
| NCEO-GA | 0.26 ± 0.03 c | 0.43 ± 0.04 c | 1.79 ± 0.06 b |
| NCEO-MD | 0.26 ± 0.03 c | 0.46 ± 0.04 bc | 1.56 ± 0.08 c |
| NCEO-WPI | 0.36 ± 0.04 a | 0.50 ± 0.04 ab | 1.96 ± 0.09 a |
| NCEO-GA/MD | 0.28 ± 0.03 c | 0.52 ± 0.04 a | 1.67 ± 0.08 bc |
| NCEO-GA/WPI | 0.36 ± 0.04 a | 0.52 ± 0.05 a | 1.61 ± 0.06 c |
| NCEO-MD/WPI | 0.36 ± 0.03 a | 0.54 ± 0.05 a | 1.67 ± 0.09 bc |
| NCEO-GA/MD/WPI | 0.32 ± 0.04 b | 0.52 ± 0.04 a | 1.67 ± 0.06 bc |
| Formulations | Porosity (%) | Carr’s Index (%) | Hausner Ratio | Flowability |
|---|---|---|---|---|
| NCEO-GA | 75.86 ± 3.79 a | 38.95 ± 1.95 b | 1.64 ± 0.08 bc | Awful |
| NCEO-MD | 72.22 ± 3.97 abc | 43.75 ± 2.41 a | 1.78 ± 0.10 ab | Awful |
| NCEO-WPI | 74.45 ± 3.35 ab | 28.57 ± 1.29 d | 1.40 ± 0.06 d | Poor |
| NCEO-GA/MD | 68.75 ± 3.44 bc | 45.45 ± 2.27 a | 1.83 ± 0.09 a | Awful |
| NCEO-GA/WPI | 68.00 ± 3.74 bc | 30.00 ± 1.65 d | 1.43 ± 0.08 d | Poor |
| NCEO-MD/WPI | 67.39 ± 3.03 c | 34.29 ± 1.54 c | 1.52 ± 0.07 cd | Very poor |
| NCEO-GA/MD/WPI | 66.69 ± 3.33 c | 37.66 ± 1.88 b | 1.60 ± 0.08 c | Very poor |
| Formulations | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| NCEO-GA | 92.31 ± 0.20 d | 0.42 ± 0.01 e | 8.92 ± 0.12 c | 8.26 ± 0.12 c |
| NCEO-MD | 95.36 ± 0.20 f | −1.00 ± 0.11 a | 4.18 ± 0.29 a | 4.35 ± 0.21 a |
| NCEO-WPI | 88.69 ± 0.09 a | −0.13 ± 0.02 d | 22.64 ± 0.19 f | 22.23 ± 0.17 f |
| NCEO-GA/MD | 93.29 ± 0.08 e | −0.16 ± 0.01 d | 6.90 ± 0.05 b | 6.20 ± 0.04 b |
| NCEO-GA/WPI | 89.83 ± 0.18 b | 0.41 ± 0.04 e | 16.48 ± 0.71 e | 16.01 ± 0.73 e |
| NCEO-MD/WPI | 91.61 ± 0.25 c | −0.75 ± 0.04 b | 16.58 ± 0.57 e | 15.83 ± 0.59 e |
| NCEO-GA/MD/WPI | 93.52 ± 0.07 e | −0.35 ± 0.08 c | 11.39 ± 0.22 d | 10.66 ± 0.21 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Aqeel, S.M.; Cui, H.; Lin, L. Nanoencapsulation of Mandarin Essential Oil: Fabrication, Characterization, and Storage Stability. Foods 2022, 11, 54. https://doi.org/10.3390/foods11010054
Mahdi AA, Al-Maqtari QA, Mohammed JK, Al-Ansi W, Aqeel SM, Cui H, Lin L. Nanoencapsulation of Mandarin Essential Oil: Fabrication, Characterization, and Storage Stability. Foods. 2022; 11(1):54. https://doi.org/10.3390/foods11010054
Chicago/Turabian StyleMahdi, Amer Ali, Qais Ali Al-Maqtari, Jalaleldeen Khaleel Mohammed, Waleed Al-Ansi, Sahibzada Muhammad Aqeel, Haiying Cui, and Lin Lin. 2022. "Nanoencapsulation of Mandarin Essential Oil: Fabrication, Characterization, and Storage Stability" Foods 11, no. 1: 54. https://doi.org/10.3390/foods11010054






