Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Grape Clones
2.3. Wine Fermentation
2.4. Oak Barrel Aging
2.5. Physicochemical Paremeters
2.6. Headspace Solid-Phase Extraction (HS-SPME)
2.7. Liquid–Liquid Extraction
2.8. GC-MS Conditions
2.9. Odor Activity Values (OAVs) and Aroma Series
2.10. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compound Composition
3.1.1. Alcohols
3.1.2. Esters
3.1.3. Fatty Acids
3.1.4. Terpenes and Norisoprenoids
3.1.5. Volatile Phenols
3.1.6. Furanic Compounds, Phenolic Aldehydes, and Oak Lactones
3.1.7. Other Compounds
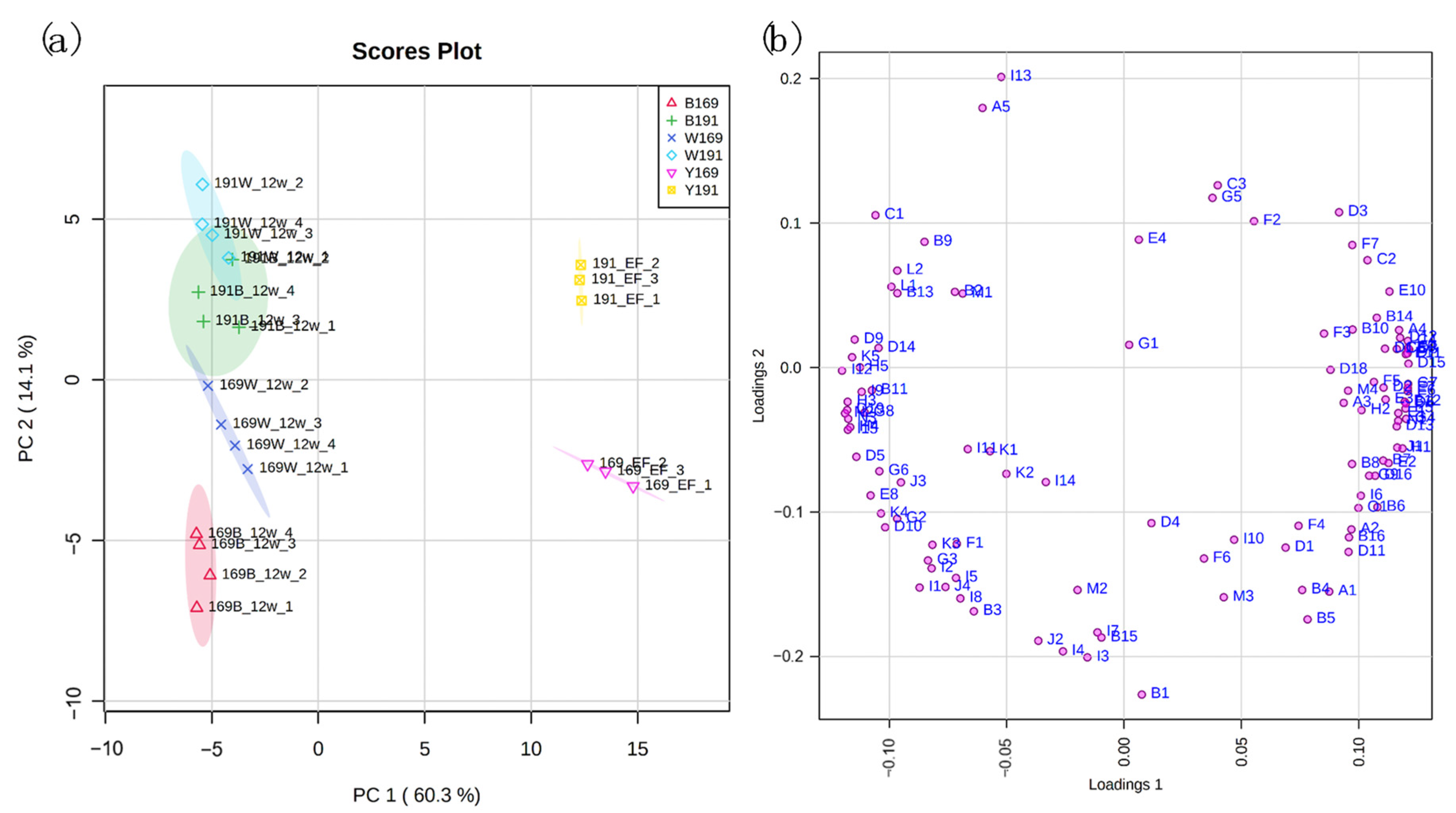
3.2. Principal Component Analysis (PCA)
3.3. Aroma Profile Analysis
| Volatile | Aroma Threshold | Aroma Descriptor | Aroma Series a | OAV (Before Aging) | OAV (12-Month Aging) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (μg/L) | Clone 191 | Clone 169 | Clone 191 (Wolin) | Clone 169 (Wolin) | Clone 191 (Bordeaux) | Clone 169 (Bordeaux) | |||
| C6 alcohols | |||||||||
| 1-Hexanol | 1100 | Herbaceous, grass, woody | 3 [6] | 2.12 ± 0.01 b | 2.31 ± 0.04 a | 1.87 ± 0.13 c | 2.05 ± 0.08 b | 1.88 ± 0.04 c | 2.08 ± 0.06 b |
| Higher alcohols | |||||||||
| Isobutanol | 75,000 | Alcohol, solvent, green, bitter | 3,7 [6] | 0.53 ± 0.01 c | 0.56 ± 0.00 b | 0.59 ± 0.01 a | 0.55 ± 0.01 b | 0.59 ± 0.01 a | 0.57 ± 0.02 b |
| Isopentanol | 60,000 | Solvent, alcohol, nail polish | 6,7 [6] | 3.44 ± 0.02 bc | 3.89 ± 0.01 a | 3.35 ± 0.11 c | 3.49 ± 0.04 b | 3.36 ± 0.02 c | 3.51 ± 0.05 b |
| 3-Methyl-1-pentanol | 500 | Pungent, solvent, green | 3,7 [6] | 0.25 ± 0.00 b | 0.32 ± 0.01 a | 0.21 ± 0.01 c | 0.26 ± 0.01 b | 0.21 ± 0.01 c | 0.26 ± 0.01 b |
| 1-Octen-3-ol | 20 | Mushroom | 9 [6] | 0.37 ± 0.01 b | 0.31 ± 0.01 c | 0.42 ± 0.04 a | 0.38 ± 0.01 ab | 0.40 ± 0.03 ab | 0.39 ± 0.01 ab |
| 2-Phenylethanol | 14,000 | Roses, honey | 2 [6] | 2.31 ± 0.09 b | 2.75 ± 0.03 a | 1.35 ± 0.11 d | 1.78 ± 0.28 c | 1.72 ± 0.34 cd | 1.96 ± 0.33 bc |
| Acetate esters | |||||||||
| Ethyl acetate | 12,270 | Pineapple, varnish, balsamic | 1,7 [56] | 8.61 ± 0.09 d | 6.82 ± 0.13e | 16.61 ± 0.55 b | 13.05 ± 0.18 c | 17.25 ± 0.54 a | 12.74 ± 0.15 c |
| Isoamyl acetate | 160 | Banana | 1 [6] | 2.80 ± 0.03 a | 2.89 ± 0.15 a | 2.29 ± 0.10 c | 2.20 ± 0.08 cd | 2.53 ± 0.11 b | 2.04 ± 0.14 d |
| Ethyl esters | |||||||||
| Ethyl butanoate | 400 | Banana, pineapple, strawberry | 1 [6] | 0.54 ± 0.01 bc | 0.59 ± 0.02 a | 0.52 ± 0.02 c | 0.55 ± 0.01 b | 0.54 ± 0.03 bc | 0.54 ± 0.01 bc |
| Ethyl hexanoate | 80 | Banana, green apple | 1 [6] | 5.14 ± 0.06 a | 4.91 ± 0.31 a | 3.99 ± 0.25 b | 4.15 ± 0.18 b | 4.19 ± 0.22 b | 4.20 ± 0.08 b |
| Ethyl lactate | 100,000 | Strawberry, raspberry, buttery | 1,6 [41] | 0.22 ± 0.00e | 0.53 ± 0.02 d | 1.97 ± 0.15 bc | 2.20 ± 0.10 ab | 1.72 ± 0.26 c | 2.27 ± 0.25 a |
| Ethyl octanoate | 580 | Sweet, floral, fruity, banana, pear | 1,2 [6] | 0.96 ± 0.00 b | 1.04 ± 0.07 a | 0.62 ± 0.05 c | 0.67 ± 0.03 c | 0.67 ± 0.02 c | 0.65 ± 0.01 c |
| Ethyl decanoate | 200 | Fruity, fatty | 1,6 [6] | 1.68 ± 0.00 a | 1.64 ± 0.13 a | 0.84 ± 0.03 b | 0.82 ± 0.02 b | 0.83 ± 0.01 b | 0.80 ± 0.01 b |
| Ethyl dihydrocinnamate | 2 | Sweet, caramel | 4 [57] | 0.36 ± 0.02 b | 0.51 ± 0.08 a | 0.16 ± 0.03 d | 0.24 ± 0.04 c | 0.20 ± 0.07 cd | 0.23 ± 0.02 cd |
| Diethyl succinate | 100,000 | Over-ripe, lavender | 1,2 [41] | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.13 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.02 a | 0.15 ± 0.02 a |
| Other esters | |||||||||
| Isoamyl octanoate | 125 | Sweet, fruity, cheese, cream | 1,4,6 [6] | 0.29 ± 0.01 b | 0.34 ± 0.04 a | 0.13 ± 0.01 c | 0.14 ± 0.01 c | 0.13 ± 0.00 c | 0.13 ± 0.00 c |
| Fatty acids | |||||||||
| Propanoic acid | 8100 | Pungent, rancid, soy | 6 [6] | 0.09 ± 0.01 c | 0.12 ± 0.00 b | 0.12 ± 0.01 b | 0.12 ± 0.01 b | 0.14 ± 0.02 ab | 0.16 ± 0.02 a |
| Isobutyric acid | 2300 | Rancid, butter, cheese | 6 [6] | 0.35 ± 0.01 a | 0.30 ± 0.05 abc | 0.31 ± 0.03 ab | 0.24 ± 0.02 c | 0.29 ± 0.05 abc | 0.26 ± 0.04 bc |
| Isovaleric acid | 3000 | Acid, rancid | 6 [6] | 0.29 ± 0.03 b | 0.34 ± 0.00 a | 0.27 ± 0.02 bc | 0.22 ± 0.02 c | 0.27 ± 0.05 bc | 0.23 ± 0.02 c |
| Hexanoic acid | 420 | Cheese, fatty | 6 [6] | 2.44 ± 0.10 b | 2.85 ± 0.02 a | 2.03 ± 0.19 b | 2.35 ± 0.25 b | 2.27 ± 0.34 b | 2.28 ± 0.33 b |
| Octanoic acid | 500 | Rancid, cheese, fatty acid | 6 [6] | 0.89 ± 0.10 a | 0.83 ± 0.01 a | 0.56 ± 0.05 b | 0.65 ± 0.06 b | 0.62 ± 0.08 b | 0.58 ± 0.08 b |
| Decanoic acid | 1000 | Fatty, rancid | 6 [6] | 0.29 ± 0.05 a | 0.20 ± 0.01 b | 0.16 ± 0.02 c | 0.16 ± 0.01 c | 0.15 ± 0.01 cd | 0.12 ± 0.03 d |
| Terpenes | |||||||||
| cis-Rose oxide | 0.2 | Lychee | 2 [6] | 0.32 ± 0.01 a | 0.26 ± 0.01 a | 0.27 ± 0.05 a | 0.31 ± 0.02 a | 0.30 ± 0.01 a | 0.28 ± 0.05 a |
| Geraniol | 20 | Citric, geranium | 2 [6] | 2.20 ± 0.03 a | 2.28 ± 0.09 a | 2.00 ± 0.05 b | 2.07 ± 0.08 b | 2.02 ± 0.03 b | 2.05 ± 0.08 b |
| Norisoprenoids | |||||||||
| β-Damascenone | 140 | Sweet, exotic flowers, stewed apple | 1,2,4 [6] | 13.25 ± 0.26 b | 14.88 ± 1.09 a | 6.02 ± 0.21 d | 7.70 ± 0.41 c | 6.42 ± 0.36 d | 7.70 ± 0.82 c |
| TDN | 2 | Kerosene, petrol | 7 [58] | 0.24 ± 0.01 c | 0.28 ± 0.04 c | 0.81 ± 0.08 a | 0.75 ± 0.07 ab | 0.67 ± 0.12 b | 0.75 ± 0.05 ab |
| Volatile phenols | |||||||||
| Guaiacol | 10 | Smoky, hospital | 7,8 [56] | 2.45 ± 0.18 d | 2.61 ± 0.07 d | 2.98 ± 0.13 c | 3.43 ± 0.20 b | 3.36 ± 0.36 b | 4.31 ± 0.26 a |
| 4-Methylguaiacol | 65 | Smudging, toasty | 8 [59] | 0.13 ± 0.01 c | 0.14 ± 0.01 c | 0.20 ± 0.01 b | 0.21 ± 0.01 b | 0.22 ± 0.04 b | 0.34 ± 0.03 a |
| o-Cresol | 31 | Tarry, smoke | 8,9 [60] | 0.11 ± 0.00 bc | 0.11 ± 0.01 bc | 0.10 ± 0.02 c | 0.12 ± 0.01 b | 0.10 ± 0.01 c | 0.15 ± 0.01 a |
| p-Cresol | 10 | Tarry, smoke | 8,9 [10] | 4.46 ± 0.33 a | 4.47 ± 0.19 a | 3.49 ± 0.08 c | 3.87 ± 0.25 b | 3.54 ± 0.30 bc | 3.89 ± 0.22 b |
| Eugenol | 6 | Cinnamon, clove, honey | 4,5 [56] | 1.01 ± 0.01 b | 0.84 ± 0.05 b | 4.73 ± 0.26 a | 4.74 ± 0.25 a | 4.78 ± 1.26 a | 4.93 ± 1.66 a |
| 4-Vinylguaiacol | 40 | Spices, curry | 5 [56] | 1.77 ± 0.10 d | 1.73 ± 0.10 d | 2.19 ± 0.03 bc | 3.23 ± 0.21 a | 2.01 ± 0.05 c | 2.23 ± 0.15 b |
| cis-Isoeugenol | 6 | Floral | 2 [61] | nd b | nd b | 0.25 ± 0.02 a | 0.23 ± 0.02 a | 0.24 ± 0.01 a | 0.24 ± 0.02 a |
| 4-Vinylphenol | 180 | Almond shell | 8 [60] | 10.16 ± 0.78 cd | 10.85 ± 0.44 bc | 11.35 ± 0.39 bc | 18.15 ± 1.02 a | 9.30 ± 0.32 d | 11.62 ± 1.24 b |
| Phenolic aldehydes | |||||||||
| Vanillin | 200 | Vanillin | 4 [60] | 0.35 ± 0.00 a | 0.36 ± 0.01 a | 0.18 ± 0.01 d | 0.20 ± 0.01 c | 0.16 ± 0.01e | 0.22 ± 0.01 b |
| Oak lactones | |||||||||
| trans-Whiskey lactone | 67 | Coconut, burn woody, vanilla | 4 [61] | nd c | nd c | 2.33 ± 0.42 a | 2.30 ± 0.53 a | 1.58 ± 0.19 b | 1.20 ± 0.36 b |
| cis-Whiskey lactone | 790 | Coconut, burn woody, vanilla | 4 [61] | nd d | nd d | 0.34 ± 0.05 a | 0.33 ± 0.05 a | 0.22 ± 0.06 b | 0.15 ± 0.05 c |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coutinho, P.; Coelho, E.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Relationships between the varietal volatile composition of the musts and white wine aroma quality. A four year feasibility study. LWT—Food Sci. Technol. 2010, 43, 1508–1516. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of colour and aroma in white wines vinifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of cabernet sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Loscos, N.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010, 120, 205–216. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in volatile composition of madeira wines during their oxidative ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition: A review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Belancic, A.; Agosin, E. Methoxypyrazines in grapes and wines of Vitis vinifera cv. Carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Mercado-Martín, G.I.; Wolpert, J.A.; Smith, R.J. Viticultural evaluation of eleven clones and two field selections of pinot noir grown for production of sparkling wine in los carneros, california. Am. J. Enol. Vitic. 2006, 57, 371–376. [Google Scholar]
- Zamuz, S.; Martínez, M.C.; Vilanova, M. Primary study of enological variability of wines from different clones of Vitis vinifera l cv. Albariño grown in misión biológica de galicia (csic). J. Food Compos. Anal. 2007, 20, 591–595. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Roby, J.-P.; Alonso-Villaverde, V.; Gindro, K. Impact of clonal variability in vitis vinifera cabernet franc on grape composition, wine quality, leaf blade stilbene content, and downy mildew resistance. J. Agric. Food Chem. 2013, 61, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Plaza, E.; Gil-Muñoz, R.; Martínez-Cutillas, A. Multivariate classification of wines from seven clones of monastrell grapes. J. Sci. Food Agric. 2000, 80, 497–501. [Google Scholar] [CrossRef]
- Revilla, E.; GarcÍA-Beneytez, E.; Cabello, F. Anthocyanin fingerprint of clones of tempranillo grapes and wines made with them. Aust. J. Grape Wine Res. 2009, 15, 70–78. [Google Scholar] [CrossRef]
- Burin, V.M.; Costa, L.L.F.; Rosier, J.P.; Bordignon-Luiz, M.T. Cabernet sauvignon wines from two different clones, characterization and evolution during bottle ageing. LWT—Food Sci. Technol. 2011, 44, 1931–1938. [Google Scholar] [CrossRef]
- Pantelić, M.; Zagorac, D.D.; Natić, M.; Gašić, U.; Jović, S.; Vujović, D.; Djordjević, J.P. Impact of clonal variability on phenolics and radical scavenging activity of grapes and wines: A study on the recently developed merlot and cabernet franc clones (Vitis vinifera L.). PLoS ONE 2016, 11, e0163823. [Google Scholar] [CrossRef] [Green Version]
- Kotseridis, Y.; Beloqui, A.A.; Bertrand, A.; Doazan, J. An analytical method for studying the volatile compounds of merlot noir clone wines. Am. J. Enol. Vitic. 1998, 49, 44–48. [Google Scholar]
- Gómez-Plaza, E.; Gil-Muñoz, R.; Carreño-Espín, J.; Fernández-López, J.A.; Martínez-Cutillas, A. Investigation on the aroma of wines from seven clones of monastrell grapes. Eur. Food Res. Technol. 1999, 209, 257–260. [Google Scholar] [CrossRef]
- Šuklje, K.; Antalick, G.; Buica, A.; Langlois, J.; Coetzee, Z.A.; Gouot, J.; Schmidtke, L.M.; Deloire, A. Clonal differences and impact of defoliation on sauvignon blanc (Vitis vinifera L.) wines: A chemical and sensory investigation. J. Sci. Food Agric. 2016, 96, 915–926. [Google Scholar] [CrossRef]
- Ziegler, M.; Wegmann-Herr, P.; Schmarr, H.-G.; Gök, R.; Winterhalter, P.; Fischer, U. Impact of rootstock, clonal selection, and berry size of Vitis vinifera sp. Riesling on the formation of tdn, vitispiranes, and other volatile compounds. J. Agric. Food Chem. 2020, 68, 3834–3849. [Google Scholar] [CrossRef]
- Stanimirović, B.; Djordjević, J.P.; Pejin, B.; Maletić, R.; Vujović, D.; Raičević, P.; Tešić, Ž. Impact of clonal selection on cabernet franc grape and wine elemental profiles. Sci. Hortic. 2018, 237, 74–80. [Google Scholar] [CrossRef]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Application of a modified method for 3-mercaptohexan-1-ol determination to investigate the relationship between free thiol and related conjugates in grape juice and wine. J. Agric. Food Chem. 2011, 59, 4649–4658. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Tondini, F.A.; Jeffery, D.W. Chiral polyfunctional thiols and their conjugated precursors upon winemaking with five vitis vinifera sauvignon blanc clones. J. Agric. Food Chem. 2018, 66, 4674–4682. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Alañón, M.E.; Calvo, E.; Cejudo, M.J.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Volatile compounds as markers of ageing in tempranillo red wines from la mancha d.O. Stored in oak wood barrels. J. Chromatogr. A 2011, 1218, 4910–4917. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-Huerta, C.; Herrera, P.; González-Sanjosé, M.L. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal. Chim. Acta 2004, 513, 341–350. [Google Scholar] [CrossRef]
- Jarauta, I.; Cacho, J.; Ferreira, V. Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: An analytical study. J. Agric. Food Chem. 2005, 53, 4166–4177. [Google Scholar] [CrossRef]
- Cadahía, E.; de Simón, B.F.; Sanz, M.; Poveda, P.; Colio, J. Chemical and chromatic characteristics of tempranillo, cabernet sauvignon and merlot wines from do navarra aged in spanish and french oak barrels. Food Chem. 2009, 115, 639–649. [Google Scholar] [CrossRef]
- Bosso, A.; Petrozziello, M.; Santini, D.; Motta, S.; Guaita, M.; Marulli, C. Effect of grain type and toasting conditions of barrels on the concentration of the volatile substances released by the wood and on the sensory characteristics of montepulciano d’abruzzo. J. Food Sci. 2008, 73, S373–S382. [Google Scholar] [CrossRef]
- CerdÁN, T.G.; GoÑI, D.T.; Azpilicueta, C.A. Changes in the concentration of volatile oak compounds and esters in red wine stored for 18 months in re-used french oak barrels. Aust. J. Grape Wine Res. 2002, 8, 140–145. [Google Scholar] [CrossRef]
- Bautista-OrtÍN, A.B.; Lencina, A.G.; Cano-LÓPez, M.; Pardo-MÍNguez, F.; LÓPez-Roca, J.M.; GÓMez-Plaza, E. The use of oak chips during the ageing of a red wine in stainless steel tanks or used barrels: Effect of the contact time and size of the oak chips on aroma compounds. Aust. J. Grape Wine Res. 2008, 14, 63–70. [Google Scholar] [CrossRef]
- Cheng, G.; He, Y.-N.; Yue, T.-X.; Wang, J.; Zhang, Z.-W. Effects of climatic conditions and soil properties on cabernet sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Cheng, G.; Duan, L.-L.; Jiang, R.; Pan, Q.-H.; Duan, C.-Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in cabernet sauvignon grapes and wines of the north foot of mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef]
- Lan, Y.B.; Qian, X.; Yang, Z.J.; Xiang, X.F.; Yang, W.X.; Liu, T.; Zhu, B.Q.; Pan, Q.H.; Duan, C.Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ‘beibinghong’ grapes in northeastern china. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Aroma profile of garnacha tintorera-based sweet wines by chromatographic and sensorial analyses. Food Chem. 2012, 134, 2313–2325. [Google Scholar] [CrossRef]
- Rothe, M.; Thomas, B. Aromastoffe des brotes. Z. Für Lebensm.-Unters. Und Forsch. 1963, 119, 302–310. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.A.; Medina, M. Aroma series as fingerprints for biological ageing in fino sherry-type wines. J. Sci. Food Agric. 2007, 87, 2319–2326. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Gürbüz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of aroma volatiles in commercial merlot and cabernet sauvignon wines using gas chromatography−olfactometry and gas chromatography−mass spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef]
- Ramey, D.D.; Ough, C.S. Volatile ester hydrolysis or formation during storage of model solutions and wines. J. Agric. Food Chem. 1980, 28, 928–934. [Google Scholar] [CrossRef]
- Ramirez Ramirez, G.; Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A.; Chassagne, D. Aroma compound sorption by oak wood in a model wine. J. Agric. Food Chem. 2001, 49, 3893–3897. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, G.; Chassagne, D.; Feuillat, M.; Voilley, A.; Charpentier, C. Effect of wine constituents on aroma compound sorption by oak wood in a model system. Am. J. Enol. Vitic. 2004, 55, 22–26. [Google Scholar]
- Ferreira, V.; Escudero, A.; Fernández, P.; Cacho, J.F. Changes in the profile of volatile compounds in wines stored under oxygen and their relationship with the browning process. Z. Für Lebensm. Und-Forsch. A 1997, 205, 392–396. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Gómez-Plaza, E. Differences in major volatile compounds of red wines according to storage length and storage conditions. J. Food Compos. Anal. 2003, 16, 697–705. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; García-Carpintero, E.G.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of verdejo white wines from the la mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. In Advances in Applied Microbiology; Laskin, A.I., Bennett, J.W., Gadd, G.M., Eds.; Academic Press: San Diego, CA, USA, 2005; Volume 57, pp. 131–175. [Google Scholar]
- López, E.F.; Gómez, E.F. Comparison of solvents for determination of monoterpenes in wine using liquid-liquid extraction. Chromatographia 2000, 52, 798–802. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Vilela, A.; Cosme, F.; Nunes, F.M. 4-ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Crit. Rev. Food Sci. Nutr. 2019, 59, 1367–1391. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Mozaz, S.R.; Azpilicueta, C.A. Volatile composition of aged wine in used barrels of french oak and of american oak. Food Res. Int. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Jalocha, J. Volatile compounds in a spanish red wine aged in barrels made of spanish, french, and american oak wood. J. Agric. Food Chem. 2003, 51, 7671–7678. [Google Scholar] [CrossRef] [PubMed]
- De Simón, B.F.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (tdn) and concentrations in young riesling and non-riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography−olfactometry and chemical quantitative study of the aroma of six premium quality spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]


| Wine Samples | Reducing Sugar (g/L) | pH | Volatile Acidity (g/L) | Total Acidity (g/L) | Ethanol (%, vol) |
|---|---|---|---|---|---|
| Clone 191 | 3.30 ± 0.14 a | 3.81 ± 0.02 a | 0.47 ± 0.02 b | 5.7 ± 0.10 b | 14.9 ± 0.64 a |
| Clone 169 | 3.50 ± 0.21 a | 3.88 ± 0.02 b | 0.36 ± 0.01 a | 5.3 ± 0.13 a | 14.0 ± 0.14 a |
| Volatile | Before Aging (μg/L) | Y169/Y191 a | 12 Month Aging (μg/L) | p Value b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clone 191 | Clone 169 | Clone 191 (Wolin) | Clone 169 (Wolin) | Clone 191 (Bordeaux) | Clone 169 (Bordeaux) | Clone | Barrel | Clone × Barrel | ||
| C6 alcohols | ||||||||||
| 1-Hexanol (mg/L) | 2.34 ± 0.01 b* | 2.54 ± 0.04 a | 1.09 ** | 2.06 ± 0.15 c | 2.26 ± 0.09 b | 2.07 ± 0.04 c | 2.29 ± 0.07 b | <0.01 | 0.37 | 0.73 |
| (E)-3-Hexen-1-ol | 50.13 ± 1.17 b | 58.67 ± 0.18 a | 1.17 | 43.01 ± 4.76 cd | 45.90 ± 0.77 c | 41.04 ± 2.26 d | 47.05 ± 2.26 bc | 0.01 | 0.69 | 0.18 |
| (Z)-3-Hexen-1-ol | 66.27 ± 1.87 b | 73.47 ± 1.44 a | 1.11 | 58.50 ± 3.96 c | 61.32 ± 4.10 bc | 60.10 ± 5.73 bc | 57.60 ± 5.55 c | 0.16 | <0.01 | 0.64 |
| (E)-2-Hexen-1-ol | 34.65 ± 2.55 a | 31.59 ± 2.10 a | 0.91 | 17.53 ± 2.14 b | 17.37 ± 0.04 b | 17.88 ± 1.14 b | 15.17 ± 1.17 b | 0.98 | 0.07 | 0.64 |
| (Z)-2-Hexen-1-ol | 27.85 ± 1.37 b | 21.13 ± 1.44 d | 0.76 | 30.84 ± 2.03 a | 27.46 ± 0.87 b | 29.00 ± 2.08 ab | 24.91 ± 1.01 c | <0.01 | 0.02 | 0.86 |
| Higher alcohols | ||||||||||
| 1-Butanol (mg/L) | 1.77 ± 0.01 d | 2.57 ± 0.04 a | 1.45 | 1.86 ± 0.05 c | 2.40 ± 0.01 b | 1.87 ± 0.01 c | 2.44 ± 0.06 b | <0.01 | 0.49 | 0.53 |
| Isobutanol (mg/L) | 39.61 ± 0.36 c | 41.74 ± 0.12 b | 1.05 | 44.14 ± 1.06 a | 41.30 ± 0.57 b | 44.06 ± 0.43 a | 42.47 ± 1.63 b | 0.41 | 0.51 | 0.45 |
| 1-Pentanol | 93.50 ± 4.67 c | 136.85 ± 0.74 b | 1.46 | 128.71 ± 10.30 b | 155.35 ± 10.32 a | 123.76 ± 5.38 b | 156.22 ± 7.37 a | <0.01 | 0.09 | 0.06 |
| Isopentanol (mg/L) | 206.14 ± 1.17 bc | 233.48 ± 0.58 a | 1.13 | 201.10 ± 6.54 c | 209.57 ± 2.19 b | 201.31 ± 1.33 c | 210.80 ± 3.14 b | <0.01 | 0.65 | 0.75 |
| 3-Methyl-1-pentanol | 123.61 ± 0.39 c | 159.57 ± 0.50 a | 1.29 | 105.26 ± 1.62 d | 129.28 ± 2.10 b | 105.51 ± 0.84 d | 128.49 ± 1.30 b | <0.01 | 0.44 | 0.59 |
| 4-Methyl-1-pentanol | 55.88 ± 0.56 b | 63.53 ± 2.98 a | 1.14 | 46.30 ± 6.73 cd | 50.21 ± 4.36 c | 45.54 ± 2.35 d | 50.07 ± 2.68 c | <0.01 | 0.85 | 0.71 |
| 1-Octanol | 8.19 ± 0.24 a | 8.33 ± 0.93 a | 1.02 | 2.69 ± 0.03 b | 2.61 ± 0.26 b | 2.49 ± 0.05 c | 2.56 ± 0.42 bc | 0.02 | 0.22 | 0.38 |
| 3-Octanol | 0.85 ± 0.01 a | 0.90 ± 0.05 a | 1.05 | 0.68 ± 0.06 b | 0.82 ± 0.04 a | 0.70 ± 0.04 b | 0.71 ± 0.02 b | 0.04 | 0.14 | 0.07 |
| 1-Octen-3-ol | 7.35 ± 0.11 b | 6.11 ± 0.16 c | 0.83 | 8.81 ± 0.54 a | 7.61 ± 0.31 b | 8.07 ± 0.54 b | 7.89 ± 0.24 b | 0.1 | 0.88 | 0.27 |
| 2-Ethyl-1-hexanol | 10.93 ± 1.32 a | 9.36 ± 0.21 a | 0.86 | 5.42 ± 0.37 c | 4.92 ± 0.36 c | 7.33 ± 0.65 b | 6.00 ± 0.68 bc | 0.49 | 0.92 | 0.93 |
| 2-Nonanol | 0.70 ± 0.08 b | 0.70 ± 0.03 b | 1.01 | 0.97 ± 0.07 a | 1.05 ± 0.08 a | 0.98 ± 0.05 a | 0.96 ± 0.06 a | 0.33 | 0.22 | 0.23 |
| 1-Decanol | 2.48 ± 0.05 a | 2.38 ± 0.22 a | 0.96 | 1.50 ± 0.03 b | 1.66 ± 0.06 b | 1.54 ± 0.02 b | 1.64 ± 0.03 b | <0.01 | 0.28 | 0.02 |
| 1-Dodecanol | 3.23 ± 0.11 c | 3.18 ± 0.04 c | 0.98 | 5.99 ± 0.58 a | 6.61 ± 0.55 a | 6.34 ± 0.82 a | 4.70 ± 0.72 b | 0.53 | 0.15 | 0.31 |
| (Z)-6-Nonen-1-ol | 5.91 ± 0.89 a | 3.62 ± 0.30 b | 0.61 | 1.64 ± 0.05 c | 1.69 ± 0.12 c | 1.72 ± 0.17 c | 1.80 ± 0.05 c | 0.37 | 0.18 | 0.81 |
| Benzyl alcohol | 775.25 ± 14.49 bc | 807.57 ± 0.52 ab | 1.04 | 641.25 ± 45.49 c | 862.46 ± 84.30 ab | 877.46 ± 120.87 ab | 940.59 ± 64.14 a | <0.01 | 0.02 | 25.92 |
| 2-Phenylethanol (mg/L) | 32.39 ± 1.30 b | 38.56 ± 0.36 a | 1.19 | 18.89 ± 1.37 d | 24.87 ± 2.96 c | 24.12 ± 1.28 cd | 27.40 ± 5.16 bc | 0.1 | 0.15 | 0.57 |
| Acetate esters | ||||||||||
| Ethyl acetate (mg/L) | 64.58 ± 0.64 d | 51.18 ± 0.98e | 0.79 | 124.56 ± 4.12 b | 97.85 ± 1.34 c | 129.38 ± 4.05 a | 95.56 ± 1.13 c | <0.01 | 0.23 | 0.02 |
| Isoamyl acetate | 447.38 ± 4.30 a | 461.89 ± 23.65 a | 1.03 | 366.51 ± 16.00 c | 351.62 ± 12.16 cd | 404.63 ± 17.32 b | 327.14 ± 22.17 d | 0.01 | 0.5 | 0.03 |
| Hexyl acetate | 2.57 ± 0.01 a | 2.06 ± 0.11 b | 0.80 | 2.11 ± 0.13 b | 1.57 ± 0.14 c | 2.50 ± 0.13 a | 1.92 ± 0.16 b | <0.01 | 0.01 | 0.83 |
| 2-Ethyl-1-hexyl acetate | 9.41 ± 0.20 a | 8.66 ± 0.76 a | 0.92 | 1.15 ± 0.08 b | 0.92 ± 0.05 b | 1.44 ± 0.07 b | 0.90 ± 0.20 b | 0.01 | 0.34 | 0.26 |
| Phenethyl acetate | 13.30 ± 0.05 a | 12.45 ± 0.47 b | 0.94 | 9.21 ± 0.36 d | 9.31 ± 0.35 d | 10.02 ± 0.48 c | 9.50 ± 0.22 cd | 0.15 | 0.01 | 0.05 |
| Ethyl esters | ||||||||||
| Ethyl butanoate | 216.24 ± 2.23 b | 237.81 ± 6.26 a | 1.10 | 206.30 ± 8.48 c | 219.98 ± 3.88 b | 215.66 ± 10.23 b | 216.84 ± 4.32 b | 0.03 | 0.22 | 0.04 |
| Ethyl hexanoate | 411.35 ± 4.45 a | 392.91 ± 24.72 a | 0.96 | 319.30 ± 19.68 b | 331.86 ± 14.81 b | 335.59 ± 17.95 b | 335.97 ± 6.07 b | 0.03 | <0.01 | 0.04 |
| Ethyl 2-hexenoate | 8.72 ± 0.03 a | 6.72 ± 0.48 b | 0.77 | 6.13 ± 0.22 c | 5.55 ± 0.25 d | 6.43 ± 0.32 bc | 5.61 ± 0.06 d | <0.01 | 0.01 | 0.04 |
| Ethyl heptanoate | 1.39 ± 0.01 ab | 1.42 ± 0.16 ab | 1.03 | 1.26 ± 0.11 c | 1.29 ± 0.10 bc | 1.48 ± 0.11 a | 1.50 ± 0.08 a | 0.3 | <0.01 | 0.51 |
| Ethyl lactate (mg/L) | 21.97 ± 0.09 d | 53.02 ± 1.56 c | 2.41 | 197.22 ± 14.55 a | 219.69 ± 10.13 a | 158.48 ± 0.84 b | 218.14 ± 21.52 a | 0.04 | 0.52 | 0.26 |
| Ethyl octanoate | 556.83 ± 1.63 a | 600.48 ± 42.72 a | 1.08 | 358.76 ± 27.73 b | 390.53 ± 19.68 b | 387.09 ± 14.76 b | 377.98 ± 6.14 b | 0.27 | 0.43 | 0.08 |
| Ethyl nonanoate | 2.81 ± 0.01 a | 2.81 ± 0.21 a | 1.00 | 1.66 ± 0.10 b | 1.60 ± 0.06 b | 1.76 ± 0.04 b | 1.66 ± 0.03 b | 0.1 | 0.11 | 0.62 |
| Ethyl decanoate | 336.35 ± 0.40 a | 328.40 ± 25.46 a | 0.98 | 167.63 ± 5.20 b | 163.57 ± 4.32 b | 166.26 ± 2.19 b | 159.43 ± 1.44 b | 0.03 | 0.17 | 0.45 |
| Ethyl 2-hydroxy-4-methylpentanoate | 7.19 ± 0.16 d | 9.09 ± 0.20 d | 1.26 | 29.52 ± 2.92 a | 27.41 ± 1.82 ab | 25.88 ± 0.60 bc | 24.65 ± 1.89 c | 0.13 | 0.14 | 0.8 |
| Ethyl furoate | 20.84 ± 1.44 d | 26.18 ± 5.18 cd | 1.26 | 31.76 ± 1.03 bc | 34.72 ± 1.73 ab | 33.75 ± 1.05 ab | 39.63 ± 3.03 a | 0.03 | 0.06 | 0.33 |
| Ethyl benzoate | 0.59 ± 0.01 b | 0.65 ± 0.01 a | 1.10 | 0.40 ± 0.04 d | 0.53 ± 0.03 c | 0.41 ± 0.03 d | 0.52 ± 0.01 c | <0.01 | 0.44 | 0.84 |
| Ethyl undecanoate | 0.18 ± 0.00 a | 0.14 ± 0.01 b | 0.78 | 0.04 ± 0.00 c | 0.04 ± 0.01 c | 0.02 ± 0.01 d | 0.03 ± 0.00 cd | 0.47 | 0.04 | 0.25 |
| Ethyl 9-decenoate | 1.67 ± 0.06 b | 2.65 ± 0.31 a | 1.59 | 0.48 ± 0.10 c | 0.56 ± 0.03 c | 0.39 ± 0.07 c | 0.50 ± 0.04 c | 0.03 | 0.06 | 0.43 |
| Ethyl phenylacetate | 1.67 ± 0.04 b | 1.50 ± 0.04 c | 0.90 | 1.85 ± 0.04 a | 1.81 ± 0.07 a | 1.87 ± 0.10 a | 1.87 ± 0.04 a | 0.69 | 0.35 | 0.6 |
| Ethyl dodecanoate | 32.79 ± 1.27 a | 31.60 ± 4.02 a | 0.96 | 16.33 ± 0.29 b | 16.97 ± 0.27 b | 16.23 ± 0.34 b | 16.22 ± 0.29 b | 0.14 | 0.07 | 0.13 |
| Ethyl dihydrocinnamate | 0.57 ± 0.03 ab | 0.82 ± 0.13 a | 1.43 | 0.28 ± 0.02 c | 0.39 ± 0.07 bc | 0.41 ± 0.01 b | 0.37 ± 0.03 bc | 0.18 | 0.74 | 0.57 |
| Ethyl myristate | 0.91 ± 0.08 b | 1.07 ± 0.04 a | 1.18 | 0.53 ± 0.01 c | 0.61 ± 0.05 c | 0.49 ± 0.05 cd | 0.36 ± 0.02 d | 1 | <0.01 | 0.12 |
| Ethyl palmitate | 0.94 ± 0.04 a | 0.94 ± 0.31 a | 1.00 | 0.63 ± 0.04 bc | 0.81 ± 0.08 ab | 0.68 ± 0.11 bc | 0.58 ± 0.03 c | 0.4 | 0.16 | 0.28 |
| Diethyl succinate (mg/L) | 2.17 ± 0.03 b | 3.02 ± 0.06 b | 1.40 | 13.43 ± 0.89 a | 13.83 ± 1.14 a | 13.13 ± 1.01 a | 15.26 ± 1.34 a | 0.47 | 0.4 | 0.82 |
| Other esters | ||||||||||
| Methyl octanoate | 1.96 ± 0.03 a | 2.05 ± 0.26 a | 1.04 | 1.10 ± 0.09 b | 1.24 ± 0.09 b | 1.30 ± 0.08 b | 1.25 ± 0.03 b | 0.16 | 0.03 | 0.04 |
| Isoamyl hexanoate | 5.25 ± 0.01 b | 5.50 ± 0.16 a | 1.05 | 4.91 ± 0.06 c | 5.02 ± 0.07 c | 4.87 ± 0.04 c | 4.96 ± 0.01 c | <0.01 | 0.06 | 0.74 |
| Isobutyl hexanoate | 4.73 ± 1.03 ab | 5.69 ± 0.46 a | 1.20 | 4.17 ± 0.29 b | 4.45 ± 0.33 b | 4.40 ± 0.37 b | 4.26 ± 0.22 b | 0.49 | 0.85 | 0.09 |
| Methyl salicylate | 3.04 ± 0.12 a | 2.42 ± 0.05 c | 0.80 | 2.79 ± 0.15 ab | 2.79 ± 0.10 ab | 2.59 ± 0.06 bc | 2.52 ± 0.21 bc | 0.94 | 0.21 | 0.9 |
| Propyl octanoate | 2.27 ± 0.24 a | 2.54 ± 0.33 a | 1.12 | 0.97 ± 0.08 b | 1.07 ± 0.10 b | 0.87 ± 0.08 b | 1.05 ± 0.09 b | 0.04 | 0.48 | 0.72 |
| Isoamyl octanoate | 36.30 ± 0.66 b | 42.83 ± 5.19 a | 1.18 | 16.34 ± 0.74 c | 17.28 ± 0.67 c | 15.79 ± 0.38 c | 16.03 ± 0.31 c | 0.04 | 0.01 | 0.17 |
| Isobutyl octanoate | 5.70 ± 0.10 b | 6.96 ± 0.68 a | 1.22 | 2.19 ± 0.12 c | 2.17 ± 0.21 c | 1.91 ± 0.15 c | 1.92 ± 0.05 c | 0.58 | 0.09 | 0.63 |
| Isoamyl lactate | 39.19 ± 5.75 c | 64.35 ± 3.56 c | 1.64 | 237.46 ± 21.12 b | 325.03 ± 29.01 a | 192.76 ± 6.96 b | 314.23 ± 27.36 a | <0.01 | 0.17 | 0.46 |
| Methyl decanoate | 4.26 ± 0.01 a | 3.98 ± 0.51 a | 0.93 | 0.92 ± 0.07 b | 0.82 ± 0.06 b | 0.93 ± 0.03 b | 0.83 ± 0.02 b | 0.02 | 0.7 | 0.97 |
| Methyl laurate | 0.18 ± 0.03 a | 0.15 ± 0.04 a | 0.81 | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.04 ± 0.01 b | 0.04 | 0.43 | 0.43 |
| Isopentyl decanoate | 18.97 ± 1.18 a | 18.06 ± 5.32 a | 0.95 | 3.39 ± 0.33 b | 3.56 ± 0.39 b | 2.73 ± 0.19 b | 2.81 ± 0.34 b | 0.35 | 0.01 | 0.62 |
| Fatty acids | ||||||||||
| Propanoic acid | 731.48 ± 105.59 c | 947.47 ± 19.73 bc | 1.30 | 1010.84 ± 96.32 b | 951.40 ± 95.11 bc | 1051.44 ± 87.44 b | 1298.21 ± 126.66 a | 0.51 | 0.03 | 0.2 |
| Isobutyric acid | 795.43 ± 17.93 a | 688.84 ± 111.48 ab | 0.87 | 704.41 ± 63.00 ab | 561.95 ± 36.01 b | 617.54 ± 31.08 b | 632.65 ± 81.36 b | 0.09 | 0.92 | 0.53 |
| Isovaleric acid | 858.84 ± 99.48 b | 1023.07 ± 7.99 a | 1.19 | 798.66 ± 74.96 bc | 686.42 ± 67.29 c | 731.94 ± 30.35 bc | 699.42 ± 64.56 c | 0.05 | 0.69 | 0.74 |
| Hexanoic acid | 1022.90 ± 42.01 ab | 1196.13 ± 6.22 a | 1.17 | 851.48 ± 78.70 b | 941.24 ± 66.55 b | 1011.46 ± 100.62 ab | 957.15 ± 138.99 b | 0.34 | 0.61 | 0.37 |
| Octanoic acid | 445.52 ± 52.02 a | 415.31 ± 4.53 a | 0.93 | 279.56 ± 25.76 b | 324.01 ± 32.14 b | 327.25 ± 24.14 b | 271.18 ± 21.12 b | 0.56 | 0.94 | 0.19 |
| Butanoic acid | 690.70 ± 43.13 c | 1059.52 ± 13.97 a | 1.53 | 766.02 ± 73.38 bc | 781.65 ± 73.55 bc | 726.46 ± 28.13 c | 895.94 ± 88.95 b | 0.64 | 0.39 | 0.81 |
| Decanoic acid | 290.17 ± 47.89 a | 198.78 ± 5.81 b | 0.69 | 158.22 ± 2.28 bc | 160.81 ± 10.56 bc | 146.31 ± 9.71 bc | 126.09 ± 20.13 c | 0.12 | 0.01 | 0.06 |
| Terpenes | ||||||||||
| cis-Rose oxide (ng/L) | 63.39 ± 1.22 a | 52.62 ± 2.44 b | 0.83 | 57.66 ± 5.01 ab | 61.23 ± 5.06 ab | 59.56 ± 2.56 ab | 60.17 ± 8.01 ab | 0.42 | 0.78 | 0.11 |
| Linalool | 1.00 ± 0.04 c | 1.05 ± 0.04 c | 1.06 | 1.15 ± 0.06 b | 1.24 ± 0.03 a | 1.15 ± 0.05 b | 1.23 ± 0.03 ab | 0.02 | 0.7 | 0.7 |
| Citronellyl acetate (ng/L) | 566.46 ± 16.76 c | 548.90 ± 8.95 c | 0.97 | 1181.30 ± 78.68 bc | 1259.14 ± 55.20 bc | 1835.88 ± 522.79 ab | 2016.56 ± 162.96 a | 0.01 | <0.01 | 0.07 |
| Citronellol | 4.56 ± 0.16 a | 5.00 ± 0.74 a | 1.10 | 1.38 ± 0.05 b | 1.87 ± 0.11 b | 1.39 ± 0.02 b | 1.92 ± 0.19 b | 0.02 | 0.87 | 0.79 |
| Nerol | 35.29 ± 3.13 a | 33.31 ± 0.69 a | 0.94 | 33.92 ± 0.73 a | 33.33 ± 0.39 a | 33.65 ± 0.45 a | 33.11 ± 0.45 a | 0.05 | 0.28 | 0.89 |
| α-Terpineol (ng/L) | 645.41 ± 17.09 b | 639.05 ± 6.21 b | 0.99 | 784.05 ± 52.98 a | 870.56 ± 33.39 a | 809.56 ± 46.15 a | 859.30 ± 57.95 a | 0.1 | 0.83 | 0.59 |
| Methyl geranate | 0.35 ± 0.04 a | 0.39 ± 0.01 a | 1.10 | 0.07 ± 0.01 b | 0.08 ± 0.01 b | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.43 | 0.33 | 0.54 |
| Geranylacetone | 589.10 ± 9.23 b | 589.01 ± 192.79 b | 1.00 | 860.48 ± 48.94 a | 897.26 ± 47.11 a | 904.25 ± 62.87 a | 916.84 ± 57.00 a | 0.55 | 0.45 | 0.77 |
| Geraniol | 43.96 ± 0.60 ab | 45.65 ± 1.85 a | 1.04 | 40.10 ± 0.99 c | 41.50 ± 1.67 bc | 40.35 ± 0.55 c | 41.23 ± 1.68 c | 0.2 | 0.88 | 0.63 |
| Norisoprenoids | ||||||||||
| β-Damascenone (ng/L) | 1854.80 ± 36.71 b | 2083.75 ± 153.17 a | 1.12 | 843.12 ± 28.84 d | 1078.59 ± 57.50 c | 899.28 ± 49.80 d | 1129.11 ± 64.70 c | 0.01 | 0.55 | 0.55 |
| Riesling acetal | 0.73 ± 0.04 a | 0.65 ± 0.04 b | 0.89 | 0.55 ± 0.03 c | 0.57 ± 0.03 c | 0.50 ± 0.03 c | 0.57 ± 0.02 c | 0.04 | 0.26 | 0.26 |
| Vitispirane A | 0.58 ± 0.03 d | 0.65 ± 0.08 d | 1.11 | 2.21 ± 0.14 b | 2.43 ± 0.11 a | 1.98 ± 0.08 c | 2.24 ± 0.07 b | 0.01 | 0.02 | 0.7 |
| Vitispirane B | 0.47 ± 0.03 d | 0.52 ± 0.02 d | 1.10 | 1.33 ± 0.10 b | 1.48 ± 0.07 a | 1.21 ± 0.04 c | 1.43 ± 0.03 a | <0.01 | 0.04 | 0.29 |
| TDN | 0.48 ± 0.03 b | 0.56 ± 0.08 b | 1.17 | 1.55 ± 0.12 a | 1.50 ± 0.15 a | 1.25 ± 0.20 a | 1.49 ± 0.10 a | 0.93 | 0.31 | 0.35 |
| Volatile phenols | ||||||||||
| Guaiacol | 23.32 ± 1.66 d | 24.76 ± 0.65 d | 1.06 | 28.34 ± 0.12 c | 32.56 ± 0.64 b | 31.95 ± 4.24 b | 40.92 ± 0.52 a | 0.01 | 0.02 | 0.2 |
| 4-Methylguaiacol | 8.74 ± 0.73 c | 9.19 ± 0.01 c | 1.05 | 12.73 ± 0.41 b | 13.43 ± 0.33 b | 14.15 ± 3.57 b | 22.32 ± 2.46 a | 0.05 | 0.03 | 0.07 |
| o-Cresol | 3.53 ± 0.13 bc | 3.54 ± 0.27 bc | 1.00 | 3.17 ± 0.58 c | 3.86 ± 0.51 b | 3.21 ± 0.05 c | 4.64 ± 0.31 a | 0.02 | 0.2 | 0.34 |
| Phenol | 31.66 ± 1.38 bc | 31.11 ± 0.12 c | 0.98 | 30.24 ± 0.34 c | 34.43 ± 1.02 ab | 29.42 ± 0.30 c | 37.45 ± 2.63 a | <0.01 | 0.34 | 0.13 |
| 4-Ethylguaiacol | 1.44 ± 0.04 bc | 1.36 ± 0.09 c | 0.94 | 1.69 ± 0.05 b | 1.76 ± 0.19 b | 1.78 ± 0.38 b | 2.74 ± 0.26 a | 0.05 | 0.04 | 0.07 |
| p-Cresol | 44.58 ± 3.30 a | 44.71 ± 1.95 a | 1.00 | 34.94 ± 0.93 b | 38.71 ± 0.31 b | 35.39 ± 2.05 b | 38.87 ± 2.08 b | 0.03 | 0.8 | 0.9 |
| m-Cresol | 3.65 ± 0.36 b | 3.20 ± 0.04 bc | 0.88 | 2.68 ± 0.22 c | 3.27 ± 0.30 bc | 3.21 ± 0.24 bc | 4.94 ± 0.16 a | <0.01 | <0.01 | 0.03 |
| 4-Propylguaiacol | 1.02 ± 0.04 c | 0.90 ± 0.03 c | 0.89 | 1.00 ± 0.18 c | 1.46 ± 0.23 ab | 1.24 ± 0.06 bc | 1.76 ± 0.29 a | 0.03 | 0.14 | 0.84 |
| Eugenol | 6.05 ± 0.03 b | 5.06 ± 0.32 b | 0.84 | 28.40 ± 1.86 a | 28.46 ± 0.95 a | 28.70 ± 9.19 a | 29.60 ± 12.12 a | 0.93 | 0.9 | 0.94 |
| 4-Ethylphenol | 14.34 ± 0.08 b | 16.99 ± 0.20 a | 1.19 | 14.57 ± 0.16 b | 15.99 ± 0.64 a | 12.82 ± 0.10 c | 14.68 ± 1.22 b | 0.03 | 0.04 | 0.68 |
| 4-Vinylguaiacol | 70.70 ± 3.98 d | 69.39 ± 3.95 d | 0.98 | 87.79 ± 0.23 bc | 129.23 ± 3.39 a | 80.44 ± 0.56 c | 89.14 ± 4.30 b | <0.01 | <0.01 | <0.01 |
| cis-Isoeugenol | nd b | nd b | 1.48 ± 0.12 a | 1.36 ± 0.16 a | 1.42 ± 0.08 a | 1.47 ± 0.12 a | 0.7 | 0.78 | 0.39 | |
| trans-Isoeugenol | 1.90 ± 0.04 c | 1.69 ± 0.01e | 0.89 | 2.48 ± 0.02 a | 1.82 ± 0.07 cd | 2.21 ± 0.00 b | 1.77 ± 0.07 de | <0.01 | 0.01 | 0.04 |
| 4-Vinylphenol (mg/L) | 1.83 ± 0.14 bc | 1.95 ± 0.08 bc | 1.07 | 2.04 ± 0.06 b | 3.27 ± 0.09 a | 1.67 ± 0.01 c | 2.09 ± 0.23 b | <0.01 | <0.01 | 0.01 |
| Syringol | 205.71 ± 9.27 c | 196.59 ± 5.36 c | 0.96 | 330.17 ± 9.93 b | 342.41 ± 12.67 ab | 347.83 ± 22.01 ab | 373.88 ± 14.15 a | 0.15 | 0.09 | 0.56 |
| Phenolic aldehydes | ||||||||||
| Vanillin | 70.15 ± 0.06 a | 72.26 ± 1.39 a | 1.03 | 35.92 ± 1.03 d | 40.41 ± 1.42 c | 31.95 ± 1.28e | 44.46 ± 0.07 b | <0.01 | 0.72 | <0.01 |
| Acetovanilone | 60.89 ± 2.33 b | 64.23 ± 6.41 b | 1.06 | 56.73 ± 1.15 b | 64.79 ± 1.21 b | 64.60 ± 10.33 b | 97.64 ± 7.46 a | <0.01 | <0.01 | <0.01 |
| Syringaldehyde | 6.62 ± 0.27 d | 6.28 ± 0.39 d | 0.95 | 19.43 ± 2.46 c | 44.45 ± 11.55 b | 56.61 ± 8.89 a | 52.18 ± 2.04 ab | 0.01 | <0.01 | <0.01 |
| Acetosyringone | 21.34 ± 1.15 c | 21.38 ± 1.32 c | 1.00 | 35.43 ± 0.92 bc | 40.24 ± 2.39 b | 45.72 ± 11.78 b | 90.72 ± 7.16 a | <0.01 | <0.01 | <0.01 |
| Furanic compounds | ||||||||||
| Furfural | 5.10 ± 0.15 c | 0.91 ± 0.01 d | 0.18 | 36.57 ± 0.71 b | 25.90 ± 13.26 b | 298.14 ± 36.68 a | 300.74 ± 44.77 a | 0.96 | <0.01 | 0.93 |
| 5-Methyl furfural | nd b | nd b | nd b | nd b | 121.05 ± 0.17 a | 71.11 ± 14.81 a | 0.85 | <0.01 | 0.85 | |
| Acetylfuran | 6.27 ± 0.19 c | 4.65 ± 0.16 d | 0.74 | 18.19 ± 0.53 bc | 20.00 ± 4.99 bc | 34.64 ± 14.52 b | 59.73 ± 5.28 a | <0.01 | <0.01 | <0.01 |
| Maltol | 117.68 ± 7.71 c | 110.93 ± 5.23 c | 0.94 | 216.55 ± 0.28 b | 220.01 ± 14.34 b | 234.36 ± 12.62 b | 331.63 ± 6.36 a | <0.01 | <0.01 | <0.01 |
| Cyclotene | 1.04 ± 0.03 c | 1.09 ± 0.04 c | 1.05 | 4.41 ± 0.07 b | 5.25 ± 0.64 a | 4.66 ± 0.27 ab | 4.06 ± 0.19 b | 0.29 | 0.05 | <0.01 |
| Oak lactones | ||||||||||
| trans-Whiskey lactone | nd c | nd c | 155.81 ± 34.43 a | 154.35 ± 41.43 a | 106.04 ± 14.88 b | 80.29 ± 29.08 b | 0.32 | <0.01 | 0.36 | |
| cis-Whiskey lactone | nd d | nd d | 265.85 ± 52.08 a | 263.26 ± 46.32 a | 177.55 ± 61.37 b | 116.81 ± 46.35 c | 0.16 | <0.01 | 0.2 | |
| Carbonyl compounds | ||||||||||
| Acetoin (mg/L) | 1.38 ± 0.03 d | 2.47 ± 0.18 c | 1.79 | 5.21 ± 0.18 a | 5.12 ± 0.36 a | 3.82 ± 0.38 b | 3.91 ± 0.17 b | 0.14 | <0.01 | 0.62 |
| Benzaldehyde | 18.49 ± 0.85 bc | 18.92 ± 0.04 abc | 1.02 | 18.59 ± 0.37 bc | 19.85 ± 0.15 ab | 17.95 ± 1.78 c | 20.00 ± 0.21 a | 0.02 | 0.63 | 0.45 |
| Benzeneacetaldehyde | 83.52 ± 4.89 ab | 86.90 ± 3.64 a | 1.04 | 74.37 ± 3.43 b | 81.16 ± 5.58 ab | 82.17 ± 3.97 ab | 85.48 ± 5.38 a | 0.15 | 0.1 | 0.57 |
| Decanal | 2.58 ± 0.05 a | 2.48 ± 0.18 a | 0.96 | 1.78 ± 0.07 b | 1.51 ± 0.07 b | 1.79 ± 0.15 b | 1.72 ± 0.22 b | 0.97 | 0.3 | 0.31 |
| Benzenes | ||||||||||
| Styrene | 4.20 ± 0.21 b | 4.44 ± 0.01 a | 1.06 | 3.41 ± 0.04 c | 3.46 ± 0.07 c | 3.58 ± 0.04 c | 3.58 ± 0.09 c | 0.52 | 0.01 | 0.4 |
| Naphthalene | 1.06 ± 0.02 d | 1.04 ± 0.01 d | 0.98 | 1.45 ± 0.01 c | 1.48 ± 0.03 bc | 1.54 ± 0.02 ab | 1.58 ± 0.04 a | 0.19 | 0.02 | 0.89 |
| 1-Methylnaphthalene | 0.05 ± 0.01 c | 0.03 ± 0.01 c | 0.67 | 0.26 ± 0.01 b | 0.30 ± 0.02 ab | 0.32 ± 0.02 ab | 0.35 ± 0.04 a | 0.2 | 0.09 | 0.87 |
| others | ||||||||||
| Methionol | 703.42 ± 16.31 b | 953.59 ± 50.82 a | 1.36 | 448.91 ± 24.81 c | 516.17 ± 39.67 c | 475.17 ± 75.22 c | 605.09 ± 78.41 bc | 0.15 | 0.53 | 0.51 |
| 3-Isobutyl-2-methoxypyrazine | trace | trace | trace | trace | trace | trace | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Jia, F.; Cai, J.; Shi, Y.; Duan, C.; Lan, Y. Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging. Foods 2022, 11, 74. https://doi.org/10.3390/foods11010074
Qian X, Jia F, Cai J, Shi Y, Duan C, Lan Y. Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging. Foods. 2022; 11(1):74. https://doi.org/10.3390/foods11010074
Chicago/Turabian StyleQian, Xu, Fangyuan Jia, Jian Cai, Ying Shi, Changqing Duan, and Yibin Lan. 2022. "Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging" Foods 11, no. 1: 74. https://doi.org/10.3390/foods11010074
APA StyleQian, X., Jia, F., Cai, J., Shi, Y., Duan, C., & Lan, Y. (2022). Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging. Foods, 11(1), 74. https://doi.org/10.3390/foods11010074









