Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection and Preparation
2.1.1. Samples from Tunisia
2.1.2. Samples from Europe
2.2. Multielemental Analysis
2.2.1. Reagents and Chemicals
2.2.2. Olive Oil Mineralization
2.3. Soil Extraction
2.4. Multielemental Analysis
2.5. Analytical Quality Control
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. Trace Elements Concentrations in Olive Oils
| Element | Tunisia | Spain (Basque Country) | France | Literature * | |||
|---|---|---|---|---|---|---|---|
| Cd | <LOQ | <LOQ | <LOQ | (0.001–0.15) | |||
| Co | 0.09 | (0.03–0.31) | 0.07 | (0.04–0.10) | 0.07 | (0.04–0.17) | (0.023–11) |
| As | 0.14 | (0.06–0.88) | 0.10 | (0.09–0.14) | 0.08 | (0.05–0.16) | (0.2–26.6) |
| Rb | 0.35 | (0.09–1.85) | 0.55 | (0.28–0.95) | 0.66 | (0.35–1.94) | (0.036–2.6) |
| Pb | 0.88 | (0.57–2.16) | 0.53 | (0.28–0.94) | 0.70 | (0.47–1.35) | (0.18–6.40) |
| Ba | 1.21 | (0.45–5.17) | 1.28 | (0.93–2.30) | 1.61 | (0.92–17.6) | (0.31–12.3) |
| Sr | 2.58 | (1.18–5.04) | 1.73 | (1.21–2.11) | 2.50 | (1.00–3.55) | (1.52–48.9) |
| V | 3.25 | (2.10–5.45) | 1.14 | (0.52–1.56) | 0.77 | (0.12–1.53) | (4.2–5.8) |
| Mn | 5.03 | (3.58–17.3) | 1.57 | (0.96–2.70) | 2.34 | (1.08–3.71) | (4.4–40) |
| Ni | 3.50 | (2.02–11.6) | 2.65 | (1.86–3.44) | 3.88 | (2.22–6.88) | (5.95–173) |
| Cu | 8.74 | (3.62–23.5) | 5.26 | (3.10–11.1) | 6.16 | (4.40–6.55) | (3.35–66.4) |
| Cr | 10.3 | (7.11–16.8) | 8.48 | (3.45–11.2) | 14.4 | (11.9–18.2) | (15.4–437) |
| Zn | 98 | (39–195) | 129 | (100–152) | 106 | (33–138) | (7–290) |
| Fe | 1240 | (169–1310) | 102 | (80.7–117) | 129 | (54.7–190) | (67.5–1610) |
| Mg | 208 | (138–582) | 226 | (91.9–488) | 368 | (160–785) | (223–1200) |
| K | 534 | (128–3740) | 601 | (214–2970) | 527 | (142–1530) | (498–98,000) |
| Ca | 1240 | (610–2280) | 942 | (607–1990) | 1080 | (580–1640) | (76–10,790) |
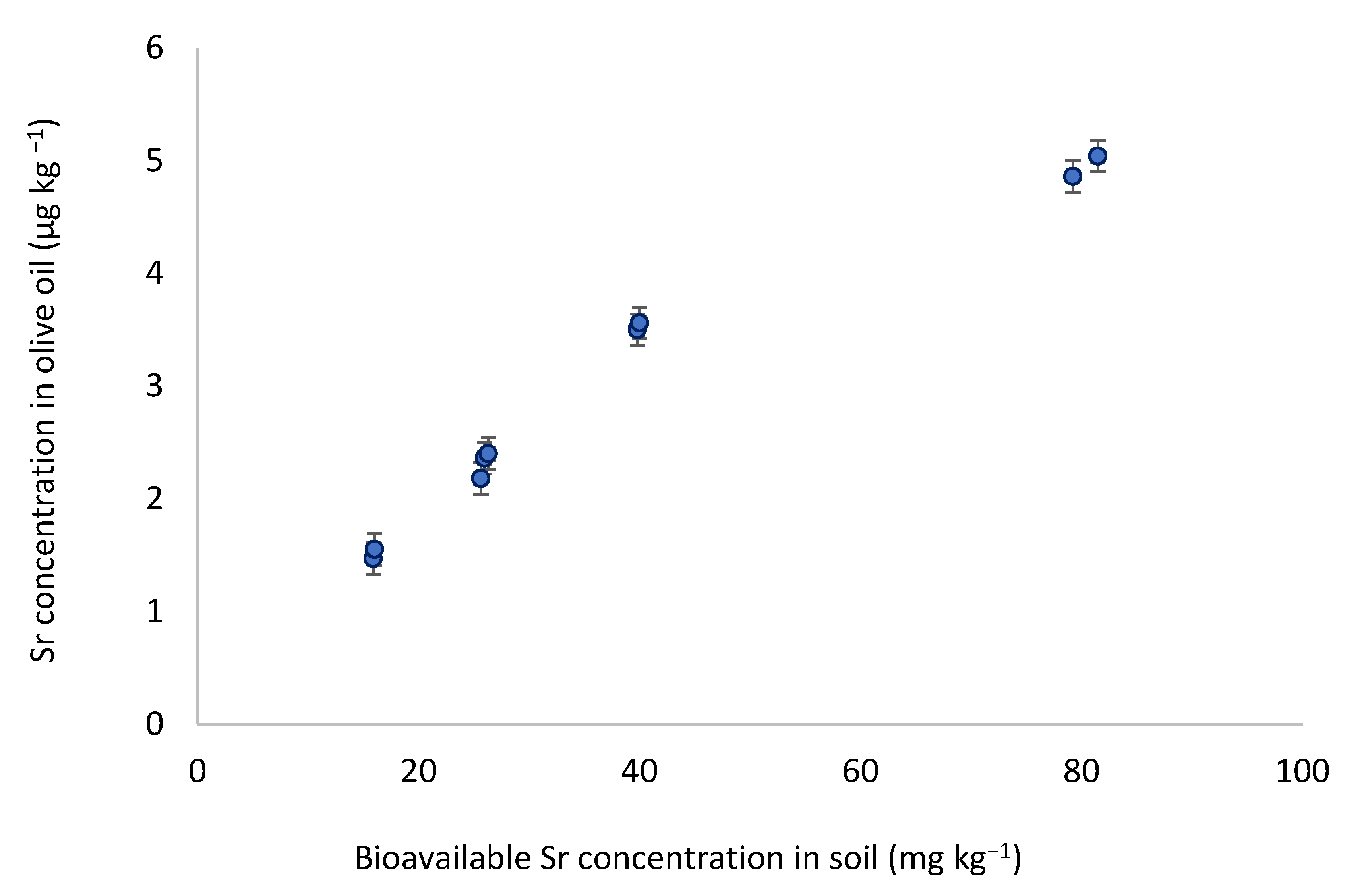
3.2. Relationship between Olive Oil and Soil Elemental Content in Tunisia
3.3. Geographical Classification of Olive Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- lori, L.; Donnini, S.; Calderone, V.; Zinnai, A.; Taglieri, I.; Venturi, F.; Testai, L. The Nutraceutical Value of Olive Oil and Its Bioactive Constituents on the Cardiovascular System. Focusing on Main Strategies to Slow Down Its Quality Decay during Production and Storage. Nutrients 2019, 11, 1962. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J.; Aparicio, R. Handbook of Olive Oil: Analysis and Properties; John, H., Ramon, A., Eds.; Aspen Publishers: Riverwoods, IL, USA, 2000; ISBN 9781475753714. [Google Scholar]
- Larbi, W.; Chymes, A. The impact of the government policies and incentives to promote the export of agricultural products in Tunisia: The case of olive oil. Food Econ.-Acta Agric. Scand. Sect. C 2010, 7, 107–118. [Google Scholar] [CrossRef]
- Keogh, J.G.; Rejeb, A. Applying HACCP in the Tunisian Olive Oil Industry: A Theoretical Background. J. Bus. Manag. Econ. Res. 2019, 3, 1–18. [Google Scholar] [CrossRef]
- Baourakis, G.; Kalaitzis, P.; Mattas, K. Food Chains: Quality, Safety and Efficiency in a Challenging World; Baourakis, G., Konstadinos Mattas, P.K., Eds.; Taylor & Francis: Oxfordshire, UK, 2014; ISBN 9781317995128, 1317995120. [Google Scholar]
- Matthäus, B.; Willenberg, I.; Engert, S.; Steinberg, P. The German National Reference Centre for Authentic Food (NRZ-Authent). OCL-Oilseeds Fats Crop. Lipids 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Yan, J.; Erasmus, S.W.; Aguilera Toro, M.; Huang, H.; van Ruth, S.M. Food fraud: Assessing fraud vulnerability in the extra virgin olive oil supply chain. Food Control 2020, 111, 107081. [Google Scholar] [CrossRef]
- Mal, A.; Le, Y.; Artaud, J.; Dupuy, N. Exploring the Scientific Interest for Olive Oil Origin: A Bibliometric Study from 1991 to 2018. Foods 2020, 9, 556. [Google Scholar]
- Carini, F.; Bengtsson, G. Post-deposition transport of radionuclides in fruit. J. Environ. Radioact. 2001, 52, 215–236. [Google Scholar] [CrossRef]
- Benincasa, C.; Gharsallaoui, M.; Perri, E.; Briccoli Bati, C.; Ayadi, M.; Khlif, M.; Gabsi, S. Quality and Trace Element Profile of Tunisian Olive Oils Obtained from Plants Irrigated with Treated Wastewater. Sci. World J. 2012, 2012, 535781. [Google Scholar] [CrossRef] [Green Version]
- Gouvinhas, I.; Domínguez, R.; Nelson, P.; Teresa, M.; Matos, C.; Barros, A.I.R.N.A. Effect of Agro-Environmental Factors on the Mineral Content of Olive Oils: Categorization of the Three Major Portuguese Cultivars. J. Am. Oil Chem. Soc. 2016, 93, 813–822. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Balcerzak, M.; Vanhaecke, F. Determination of chromium, cadmium and lead in food-packaging materials by axial inductively coupled plasma time-of-flight mass spectrometry. Anal. Chim. Acta 2003, 479, 191–202. [Google Scholar] [CrossRef]
- Zeiner, M.; Steffan, I.; Cindric, I.J. Determination of trace elements in olive oil by ICP-AES and ETA-AAS: A pilot study on the geographical characterization. Microchem. J. 2005, 81, 171–176. [Google Scholar] [CrossRef]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Camin, F.; Larcher, R.; Nicolini, G.; Bontempo, L.; Bertoldi, D.; Perini, M.; Schlicht, C.; Schellenberg, A.; Thomas, F.; Heinrich, K.; et al. Isotopic and Elemental Data for Tracing the Origin of European Olive oils. J. Agric. Food Chem. 2010, 58, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Pošćić, F.; Furdek, M.; Bačić, N.; Mikac, N.; Bertoldi, D.; Camin, F.; Jukić, M.; Žanetić, M.; Rengel, Z.; Perica, S. Removal of pomace residues is critical in quantification of element concentrations in extra virgin olive oil. J. Food Compos. Anal. 2019, 77, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A preliminary study on the authentication and traceability of extra virgin olive oil made from Taggiasca olives by means of trace and ultra-trace elements distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.L.; Marconi, E.; Vitiello, G.; Massimi, L. An optimized method for sample preparation and elemental analysis of extra-virgin olive oil by inductively coupled plasma mass spectrometry. Food Chem. 2021, 360, 130027. [Google Scholar] [CrossRef]
- Lepri, F.G.; Chaves, E.S.; Vieira, M.A. Determination of Trace Elements in Vegetable Oils and Biodiesel by Atomic Spectrometric Techniques—A Review. Appl. Spectrosc. Rev. 2011, 46, 37–41. [Google Scholar] [CrossRef]
- Angioni, A.; Cabitza, M.; Teresa, M.; Caboni, P. Influence of olive cultivars and period of harvest on the contents of Cu, Cd, Pb, and Zn in virgin olive oils. Food Chem. 2006, 99, 525–529. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Kurtulus, Y.B.; Yurtsever, S. Comparison of extraction induced by emulsion breaking, ultrasonic extraction and wet digestion procedures for determination of metals in edible oil samples in Turkey using ICP-OES. Food Chem. 2013, 138, 770–775. [Google Scholar] [CrossRef]
- Brkljača, M.; Giljanović, J.; Prkić, A. Determination of Metals in Olive Oil by Electrothermal Atomic Absorption Spectrometry: Validation and Uncertainty Measurements. Anal. Lett. 2013, 46, 2912–2926. [Google Scholar] [CrossRef]
- de Leonardis, A.; Macciola, V.; de Felice, M. Copper and iron determination in edible vegetable oils by graphite furnace atomic absorption spectrometry after extraction with diluted nitric acid. Int. J. Food Sci. Technol. 2000, 35, 371–375. [Google Scholar] [CrossRef]
- Mohebbi, M.; Heydari, R.; Ramezani, M. Determination of Cu, Cd, Ni, Pb and Zn in Edible Oils Using Reversed-Phase Ultrasonic Assisted Liquid—Liquid Microextraction and Flame Atomic Absorption Spectrometry 1. J. Anal. Chem. 2018, 73, 30–35. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Damak, F.; Asano, M.; Baba, K.; Suda, A.; Araoka, D.; Wali, A.; Isoda, H.; Nakajima, M.; Ksibi, M.; Tamura, K. Environmental Soil Chemistry Laboratory, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Institute of Agro-Environmental Sciences, NARO, 3-1-3 Kannondai Tsukuba, Ibaraki 305- Geological Survey of Japan (GSJ), National Institute of Advanced Indust. Food Chem. 2019, 283, 654–656. [Google Scholar] [CrossRef] [Green Version]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-de Córdova, M.L.; Ruiz-Medina, A. Analysis of the legislated metals in different categories of olive and olive-pomace oils. Food Control 2011, 22, 221–225. [Google Scholar] [CrossRef]
- Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A. Determination of trace element in Italian virgin olive oils and their characterization according to geographical origin by statistical analysis. Anal. Chim. Acta 2007, 585, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Sayago, A.; González-Domínguez, R.; Beltrán, R.; Fernández-Recamales, Á. Combination of complementary data mining methods for geographical characterization of extra virgin olive oils based on mineral composition. Food Chem. 2018, 261, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Camin, F.; Larcher, R.; Perini, M.; Bontempo, L.; Bertoldi, D.; Gagliano, G.; Nicolini, G.; Versini, G. Characterisation of authentic Italian extra-virgin olive oils by stable isotope ratios of C, O and H and mineral composition. Food Chem. 2010, 118, 901–909. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S. Extraction of trace elements by ultrasound-assisted emulsification from edible oils producing detergentless microemulsions. Food Chem. 2015, 188, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, R.; Shekhar, R.; Kumar, S.J. Ultrasound-assisted extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from edible oils with tetramethylammonium hydroxide and EDTA followed by determination using graphite furnace atomic absorption spectrometer. Food Chem. 2019, 294, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.S.; Velarte, R.; Castillo, J.R. On-line emulsions of olive oil samples and ICP-MS multi-elemental determination. J. Anal. At. Spectrom. 2003, 18, 1154–1162. [Google Scholar] [CrossRef]
- Kruszewski, B.; Obiedziński, M.W. Multivariate analysis of essential elements in raw cocoa and processed chocolate mass materials from three different manufacturers. LWT 2018, 98, 113–123. [Google Scholar] [CrossRef]
- Sakač, M.B.; Jovanov, P.T.; Marić, A.Z.; Pezo, L.L.; Kevrešan, Ž.S.; Novaković, A.R.; Nedeljković, N.M. Physicochemical properties and mineral content of honey samples from Vojvodina (Republic of Serbia). Food Chem. 2019, 276, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.; McComb, K.; Ehtesham, E.; van Hale, R.; Barr, D.; Hoogewerff, J.; Frew, R. Chemical profiling of saffron for authentication of origin. Food Control 2019, 106, 106699. [Google Scholar] [CrossRef]
- Gumus, Z.P.; Celenk, V.U.; Tekin, S.; Yurdakul, O.; Ertas, H. Determination of trace elements and stable carbon isotope ratios in virgin olive oils from Western Turkey to authenticate geographical origin with a chemometric approach. Eur. Food Res. Technol. 2017, 243, 1719–1727. [Google Scholar] [CrossRef]
- Wali, A.; Damak, F.; Kawada, K.; Isoda, H.; Tamura, K.; Ksibi, M. The effects of geographic region and cultivar on oxidative stability and elemental analysis of Tunisian extra virgin olive oil. Eur. Food Res. Technol. 2021, 247, 1401–1409. [Google Scholar] [CrossRef]
- Rauret, G.; Lopez-Sanchez, J.F.; Sahuquillo, A.; Barahona, E.; Lachica, M.; Ure, A.M.; Davidson, C.M.; Gomez, A.; Luck, D.; Bacon, J.; et al. Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid. J. Environ. Monit. 2000, 2, 228–233. [Google Scholar] [CrossRef]
- Sahuquillo, A.; López-Sánchez, J.F.; Rubio, R.; Rauret, G.; Thomas, R.P.; Davidson, C.M.; Ure, A.M. Use of a certified reference material for extractable trace metals to assess sources of uncertainty in the BCR three-stage sequential extraction procedure. Anal. Chim. Acta 1999, 382, 317–327. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ure, M.U.; Thomas, R.; Littlejohn, D. Ammonium acetate extracts and their analysis for the speciation of metal ions in soils and sediments. Int. J. Environ. Anal. Chem. 1993, 51, 65–84. [Google Scholar] [CrossRef]
- Mirón, C.; Sánchez, R.; Prats, S.; Todolí, J. Total polyphenol content and metals determination in Spanish virgin olive oils by means of a dispersive liquid-liquid aerosol phase extraction method and ICP-MS. Anal. Chim. Acta 2019, 1094, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-De Córdova, M.L.; Domínguez-Vidal, A.; Ruiz-Medina, A. Investigation by ICP-MS of trace element levels in vegetable edible oils produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Farrell, M.; Ertan, L. Investigation of trace metals in different varieties of olive oils from Northern Cyprus and their variation in accumulation using ICP-MS and multivariate techniques. Environ. Earth Sci. 2019, 78, 578. [Google Scholar] [CrossRef]
- Mendil, D.; Dogan, Ö.; Tüzen, M.; Soylak, M. Investigation of the levels of some element in edible oil samples produced in Turkey by atomic absorption spectrometry. J. Hazard. Mater. 2009, 165, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, N.; Abdallah, F.B.; Mezghani, I.; Boukhris, M. Accumulation du plomb par quelques espèces végétales cultivées au voisinage d’une fonderie de plomb à Sfax Lead accumulation by sorne plant species cultivated in the vicinity of a lead factory in Sfax. Pollut. Atmos. 2003, 178, 285–294. [Google Scholar]
- Llorent-Martínez, E.J.; Fernández-De Córdova, M.L.; Ortega-Barrales, P.; Ruiz-Medina, A. Quantitation of metals during the extraction of virgin olive oil from olives using ICP-MS after microwave-assisted acid digestion. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1823–1830. [Google Scholar] [CrossRef]
- Kara, D.; Fisher, A.; Hill, S. Detergentless ultrasound-assisted extraction of trace elements from edible oils using lipase as an extractant. Talanta 2015, 144, 219–225. [Google Scholar] [CrossRef]
- Cabrera-Vique, C.; Bouzas, P.R.; Oliveras-López, M.J. Determination of trace elements in extra virgin olive oils: A pilot study on the geographical characterisation. Food Chem. 2012, 134, 434–439. [Google Scholar] [CrossRef]
- Lendinez, E.; Lorenzo, M.L.; Cabrera, C.; López, M.C. Chromium in basic foods of the Spanish diet: Seafood, cereals, vegetables, olive oils and dairy products. Sci. Total Environ. 2001, 278, 183–189. [Google Scholar] [CrossRef]
- Giaccio, M.; Vicentini, A. Determination of the geographical origin of wines by means of the mineral content and the stable isotope ratios: A review. J. Commod. Sci. Technol. Qual. 2008, 47, 267–284. [Google Scholar]
- Gad, S.C. Barium. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 368–370. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Erol, B.; Arslan, G.; Gode, F.; Altun, T.; Özcan, M.M. Determination of some inorganic metals in edible vegetable oils by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Grasas Aceites 2008, 59, 239–244. [Google Scholar]
- Guagliardi, I.; Cicchella, D.; de Rosa, R.; Ricca, N.; Buttafuoco, G. Geochemical sources of vanadium in soils: Evidences in a Southern Italy area. J. Geochem. Explor. 2018, 184, 358–364. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.-R.; Hu, W.-F.; Gao, J.; Yang, J.-Y. Vanadium in soil-plant system: Source, fate, toxicity, and bioremediation. J. Hazard. Mater. 2021, 405, 124200. [Google Scholar] [CrossRef]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar] [CrossRef]
- Madejón, P.; Marañón, T.; Murillo, J.M. Biomonitoring of trace elements in the leaves and fruits of wild olive and holm oak trees. Sci. Total Environ. 2006, 355, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Juranovic-Cindric, I.; Škevin, D. Characterization of extra virgin olive oils derived from the Croatian cultivar Oblica. Eur. J. Lipid Sci. Technol. 2010, 112, 1248–1252. [Google Scholar] [CrossRef]
- Nedjimi, B. Measurement of selected trace elements in Olea europaea L. cv. ‘Sigoise’. J. Trace Elem. Med. Biol. 2020, 62, 126595. [Google Scholar] [CrossRef] [PubMed]
- Al-Habahbeh, K.A.; Al-Nawaiseh, M.B.; Al-Sayaydeh, R.S.; Al-Hawadi, J.S.; Albdaiwi, R.N.; Al-Debei, H.S.; Ayad, J.Y. Long-term irrigation with treated municipal wastewater from the wadi-musa region: Soil heavy metal accumulation, uptake and partitioning in olive trees. Horticulturae 2021, 7, 152. [Google Scholar] [CrossRef]
- Ben Mansour-Gueddes, S.; Saidana-Naija, D.; Flamini, G.; Cheraief, I.; Braham, M. Assessment of the Climatic Condition’s Impact on Volatiles, Polyphenols and Mineral Contents in Tunisian Olive Tree (Olea europaea L.). Pol. J. Environ. Stud. 2021, 31, 219–230. [Google Scholar] [CrossRef]
- Ben Mansour-Gueddes, S.; Saidana, D.; Cheraief, I.; Dkhilali, M.; Braham, M. Biochemical, mineral and anatomical characteristics of the olive tree cv. Chetoui growing in several Tunisian areas. Acta Sci. Pol. Hortorum Cultus 2018, 17, 49–70. [Google Scholar] [CrossRef]
- Pohl, W.L. Economic Geology of Metals. In Economic Geology Principles and Practice: Metals, Minerals, Coal and Hydrocarbons—Introduction to Formation and Sustainable Exploitation of Mineral Deposits; Wiley: Hoboken, NJ, USA, 2011; pp. 149–284. [Google Scholar] [CrossRef]

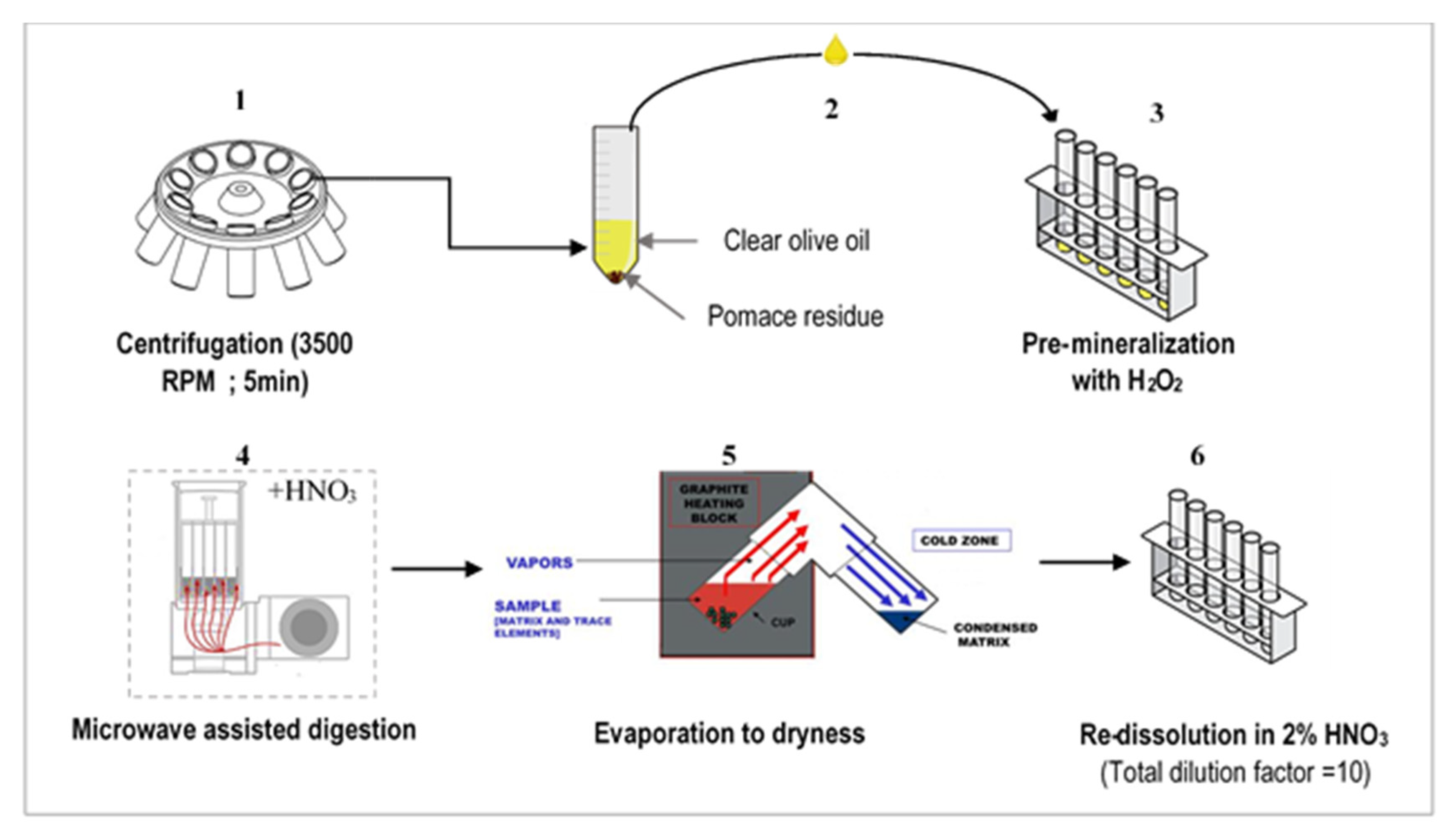


| Geographical Origin | Tunisia | France (South) | Spain (Basque Country) | ||
|---|---|---|---|---|---|
| Samples | EVOO (produced in oil mill) | EVOO (produced in OTIT) | Soil (0–60 cm) | EVOO | EVOO |
| Number of samples | 16 | 9 | 9 | 10 | 7 |
| Country of Origin (Number of Samples) | Geographical Location (Number of Samples) | Sample Code | Cultivar | Bedrock (Mineralization) | Agricultural Practices |
|---|---|---|---|---|---|
| Tunisia (n = 25) | Tataouine (n = 2) | T1 | Zarrazi | limestones and marls | nt |
| T2 | Dhokkari | ||||
| Sousse (n = 2) | T3 | Chemlali | calcareous and gypsum crusts | nt | |
| T3A | Chemlali | ||||
| Mahdia (n = 3) | T4 | Chemlali | conglomerates, sand and clay | Drop irrigation and use of pomace residue as an amendment | |
| T4A | Chemlali | ||||
| T4A’ | Chemlali | ||||
| Sfax (n = 2) | T5 | Chemlali | Recent alluvium | nt | |
| T5A | Chemlali | ||||
| Kasserine (n = 2) | T6 | Arbequina | sandstone and marl | nt | |
| T12 | ns | limestones, dolostones, marls and gypsum (Zn) | |||
| Nabeul (n = 1) | T7 | Zarrazi | ancient limestone and gypsum alluvium | nt | |
| Kairouan (n = 4) | T8 | Chemlali | ancient limestone and gypsum alluvium | nt | |
| T8A | Chemlali | ||||
| T9 | Chemlali | Recent alluvium | Drop irrigation | ||
| T9A | Chemlali | ||||
| Jendouba (n = 2) | T10 | Chetoui | clay-sandstone flysch | nt | |
| T16 | Chetoui | clay-sandstone flysch (Zn and Pb) | |||
| Ariana (n = 3) | T11 | Mixture of different varieties | Recent alluvium | nt | |
| T11A | Chemlali | ||||
| T11A’ | Chemlali | ||||
| Beja (n = 1) | T13 | ns | ancient limestone and gypsum alluvium | nt | |
| Monastir (n = 1) | T14 | Mixture of different varieties | conglomerate, sands and clays | nt | |
| Tozeur (n = 1) | T15 | Mixture of different varieties | conglomerates, sand and clay | nt | |
| Siliana (n = 1) | T17A | ns | Recent alluvium | nt | |
| Spain (Basque country) (n = 7) | Moreda Araba (n = 1) | B1 | A mixture of Arroniz and Arbequina | ns | Use of fertilizer (15-15-15 NPK *; sheep and cattle manure) |
| Añorbe (n = 1) | B2 | A mixture of Arroniz and Arbequina | ns | ns | |
| Mendavia (n = 1) | B3 | Arbequina | ns | ns | |
| Cintruenigo (n = 1) | B4 | Arbequina | ns | ns | |
| Lantziego (n = 1) | B5 | A mixture of Arroniz and Arbequina | ns | Use of fertilizer (15-15-15 NPK; sheep and cattle manure) | |
| Ablitas (n = 1) | B6 | Arroniz | ns | ns | |
| Oion (n = 1) | B7 | A mixture of Arroniz and Arbequina | ns | Use of fertilizer (15-15-15 NPK; sheep and cattle manure) | |
| France (n = 10) | Nyons (n = 2) | FR1 | ns | ns | ns |
| FR3 | ns | ||||
| Baux-de-Provence (n = 1) | FR2 | Mixture of different varieties | ns | ns | |
| Nîmes (n = 2) | FR4 | ns | ns | ns | |
| FR7 | ns | ||||
| Nice (n = 2) | FR5 | Cailletier | ns | ns | |
| FR6 | ns | ||||
| Provence (n = 3) | FR8 | Mixture of different varieties | ns | ns | |
| FR9 | Aglandau | ||||
| FR10 | ns |
| Elements | Measured Concentrations ± SD (mg kg−1) | Certified Concentrations ± SD (mg kg−1) | R (%) | |
|---|---|---|---|---|
| Certified elements (n = 3) | Ca | 421 ± 6 | 411 ± 18 | 102 |
| Cu | 4.26 ± 0.06 | 4.93 ± 0.15 | 86 | |
| Fe | 16.6 ± 0.3 | 16.4 ± 0.8 | 101 | |
| K | 6130 ± 160 | 6070 ± 200 | 101 | |
| Mg | 1696 ± 13 | 1680 ± 70 | 101 | |
| Mn | 15.5 ± 0.2 | 16.0 ± 0.6 | 97 | |
| Zn | 25.1 ± 0.3 | 26.3 ± 1.1 | 96 | |
| Non-certified elements (n = 15) | As | 0.13 ± 0.07 | - | - |
| Ba | 1.43 ± 0.13 | - | - | |
| Cd | 0.051 ± 0.002 | - | - | |
| Co | 0.024 ± 0.002 | - | - | |
| Cr | <LOQ | - | - | |
| Ni | 0.78 ± 0.04 | - | - | |
| Pb | <LOQ | - | - | |
| Rb | 5.66 ± 0.23 | - | - | |
| Sr | 2.95 ± 0.10 | - | - | |
| V | <LOQ | - | - |
| Element | Pearson Correlation Coefficient (r) | Significance (p-Value) |
|---|---|---|
| Sr | 0.85 | 0.006 |
| Ni | −0.8 | 0.04 |
| Mg | 0.78 | 0.02 |
| Mn | 0.63 | 0.01 |
| As | −0.12 | 0.76 |
| Ba | −0.13 | 0.75 |
| Ca | −0.08 | 0.85 |
| Cd | nd | nd |
| Co | −0.42 | 0.29 |
| Cr | 0.03 | 0.93 |
| Cu | −0.25 | 0.54 |
| Fe | 0.02 | 0.94 |
| K | −0.32 | 0.44 |
| Pb | 0.08 | 0.84 |
| Rb | 0.57 | 0.13 |
| V | −0.56 | 0.14 |
| Zn | −0.42 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, E.G.; Epova, E.N.; de Diego, A.; Souissi, R.; Hammami, M.; Abderrazak, H.; F. X. Donard, O. Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods 2022, 11, 82. https://doi.org/10.3390/foods11010082
Nasr EG, Epova EN, de Diego A, Souissi R, Hammami M, Abderrazak H, F. X. Donard O. Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods. 2022; 11(1):82. https://doi.org/10.3390/foods11010082
Chicago/Turabian StyleNasr, Emna G., Ekaterina N. Epova, Alberto de Diego, Radhia Souissi, Mohamed Hammami, Houyem Abderrazak, and Olivier F. X. Donard. 2022. "Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination" Foods 11, no. 1: 82. https://doi.org/10.3390/foods11010082
APA StyleNasr, E. G., Epova, E. N., de Diego, A., Souissi, R., Hammami, M., Abderrazak, H., & F. X. Donard, O. (2022). Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods, 11(1), 82. https://doi.org/10.3390/foods11010082





