Lactiplantibacillus plantarum 1201 Inhibits Intestinal Infection of Salmonella enterica subsp. enterica Serovar Typhimurium Strain ATCC 13311 in Mice with High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactiplantibacillus Strains and Culture Conditions
2.2. Animals and Experimental Design
2.3. Determination of Infection in Mice
2.4. Hematoxylin-Eosin Staining and Histopathological Damage Scores
2.5. Analysis of Inflammatory Levels in Intestine of the Mice
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) Detection of TLR4/NF-κB Inflammation Pathway in Serum of the Mice
2.7. S. Typhimurium Inhibition Assay
2.8. Statistical Analysis
3. Results
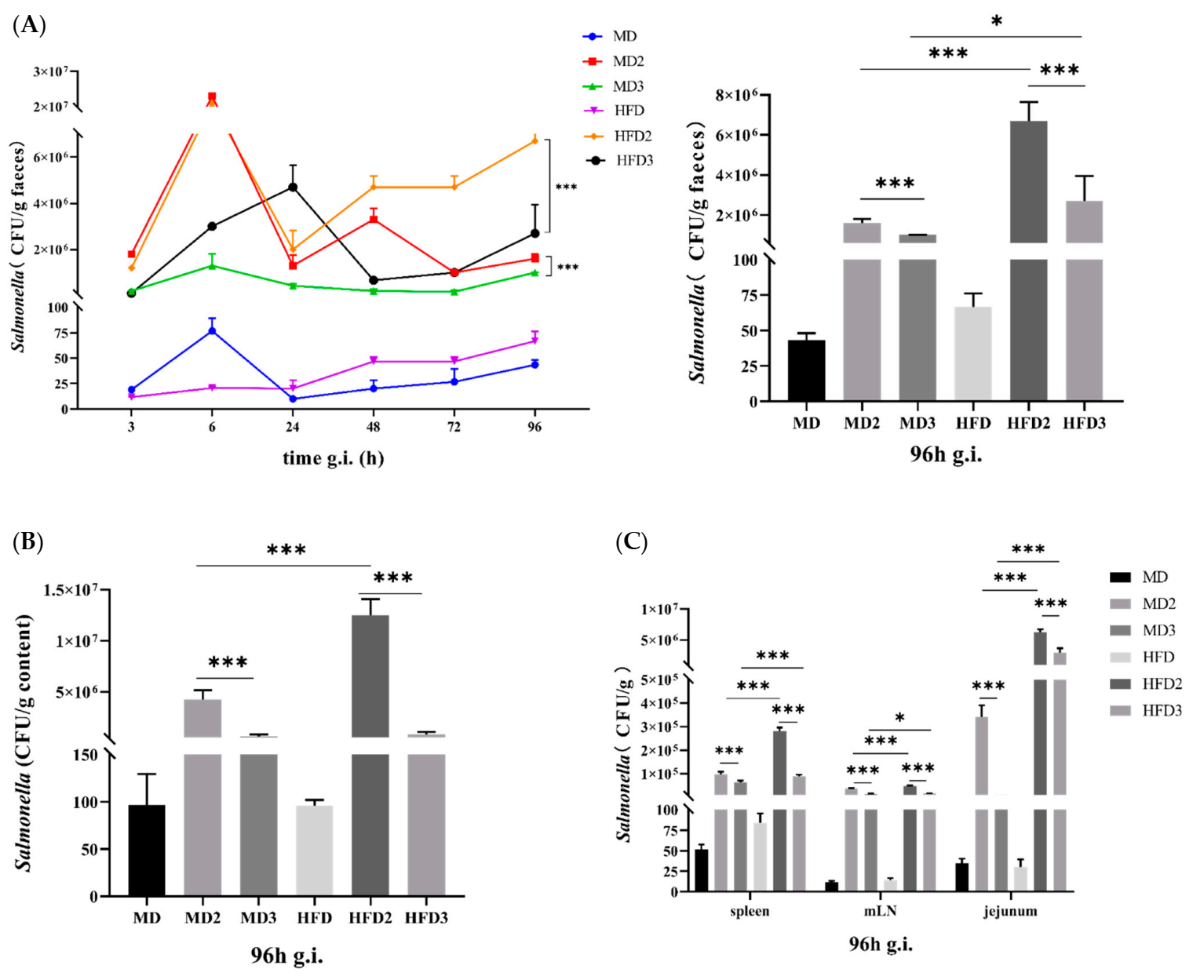
3.1. High-Fat Diet (HFD) Promotes S. Typhimurium ATCC 13311 Colonization while L. plantarum 1201 Inhibits It
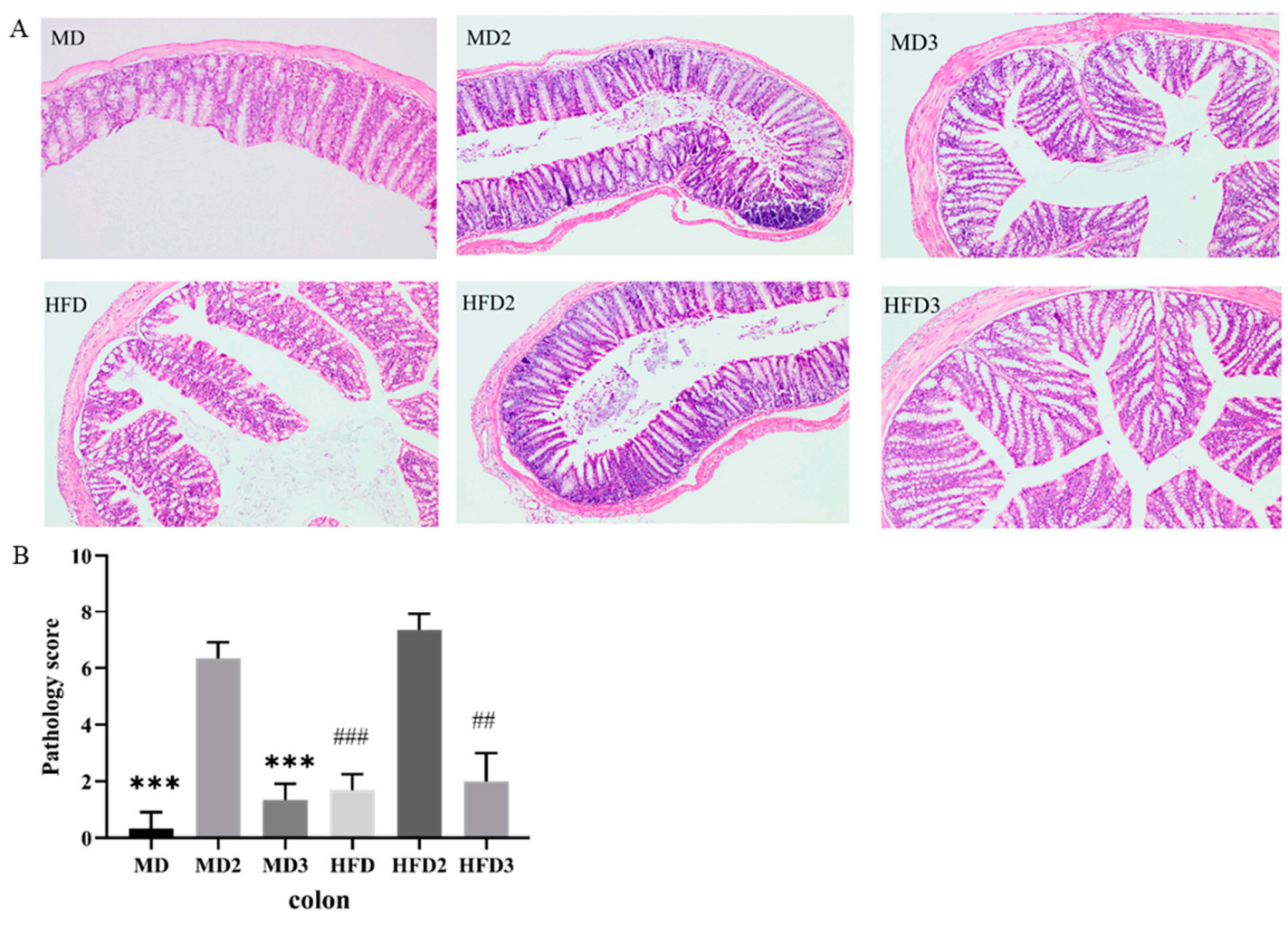
3.2. L. plantarum 1201 Alleviates the Intestinal Injury Induced by S. Typhimurium ATCC 13311
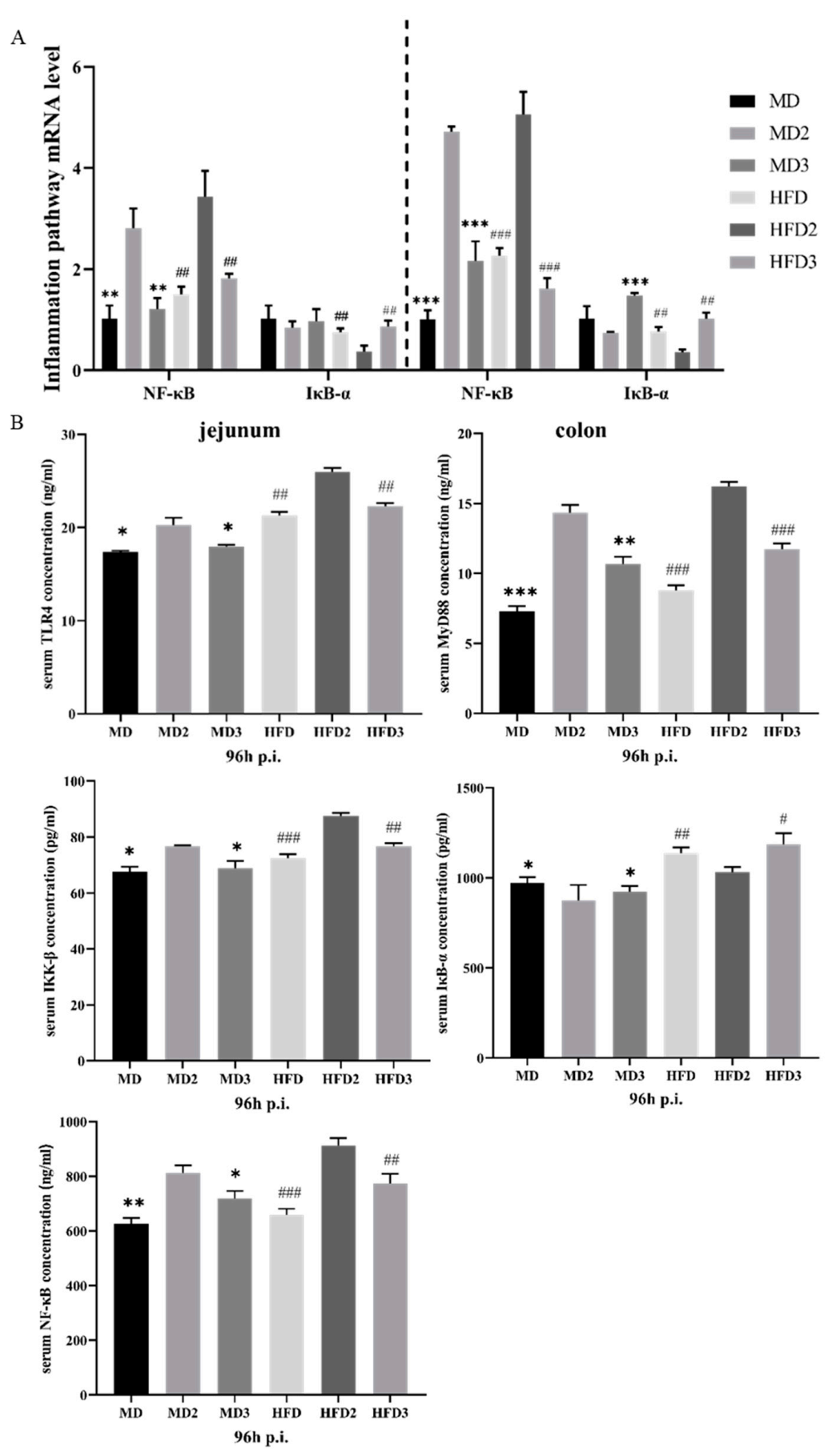
3.3. L. plantarum 1201 Relieves Intestinal Inflammation through the TLR4/NF-κB Inflammatory Pathway
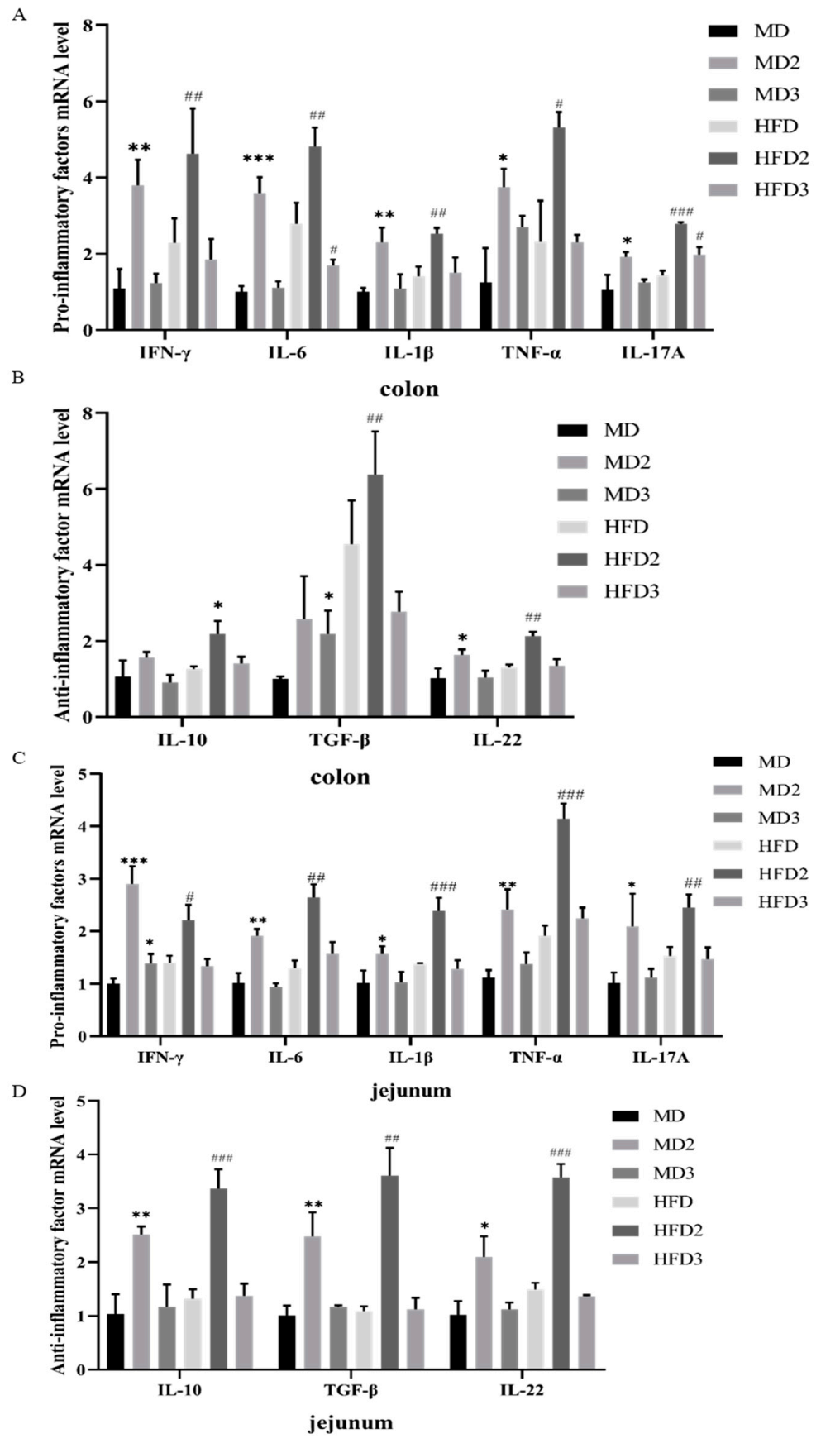
3.4. L. plantarum 1201 Restores the Secretion of Intestinal Inflammatory Factors Caused by S. Typhimurium ATCC 13311 Colonization
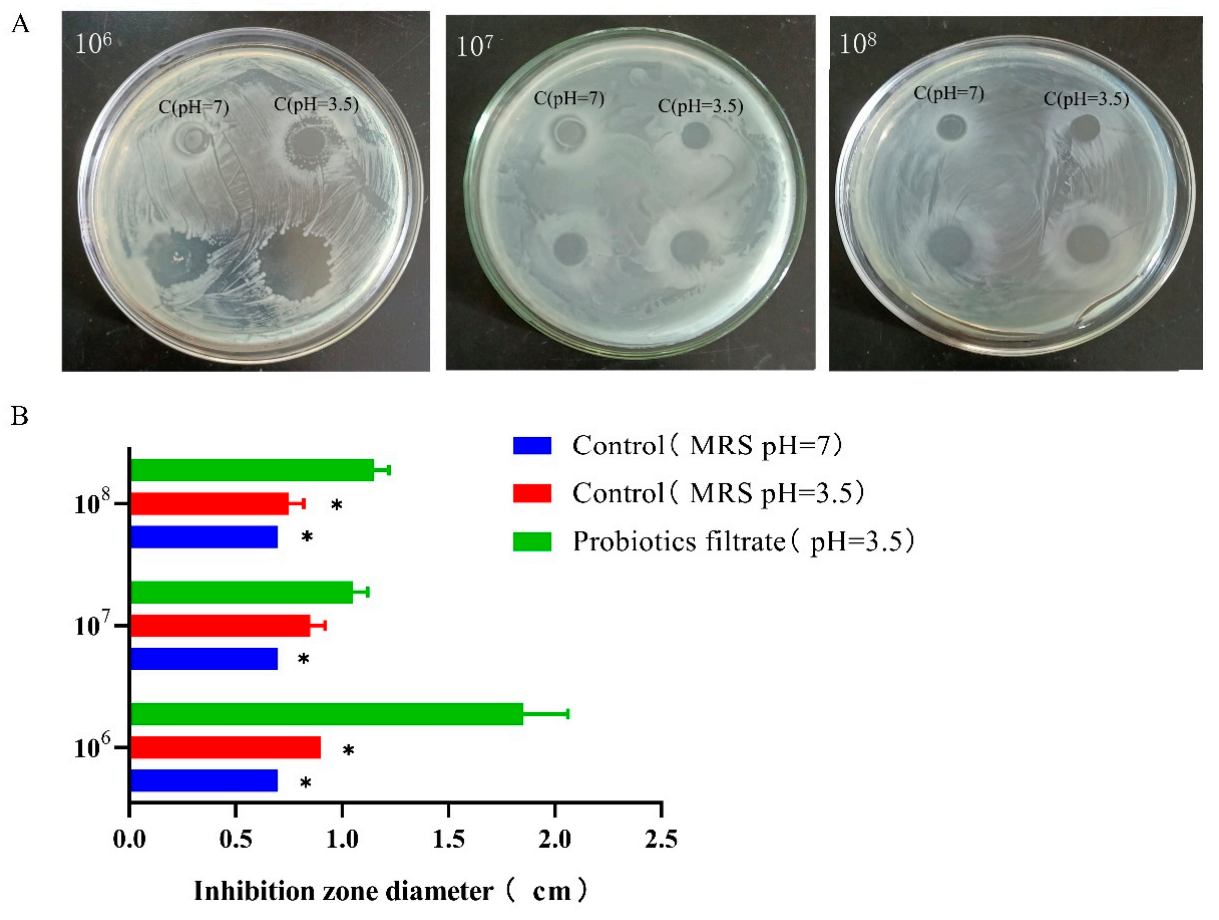
3.5. L. plantarum 1201 Inhibits S. Typhimurium ATCC 13311 Growth Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.; Fedorka-Cray, P.; Dargatz, D.; Ferris, K.; Green, A. Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J. Food Prot. 2001, 64, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Malorny, B.; Hoorfar, J. Toward standardization of diagnostic PCR testing of fecal samples: Lessons from the detection of Salmonellae in pigs. J. Clin. Microbiol. 2005, 43, 3033–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carli, K.; Unal, C.; Caner, V.; Eyigor, A. Detection of salmonellae in chicken feces by a combination of tetrathionate broth enrichment, capillary PCR, and capillary gel electrophoresis. J. Clin. Microbiol. 2001, 39, 1871–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, R.; Kenney, P.; Keswani, J.; Ritz, C. Isolation and characterisation of Salmonella in a turkey production facility. Br. Poult. Sci. 2003, 44, 192–202. [Google Scholar] [CrossRef]
- Thorns, C. Bacterial food-borne zoonoses. Rev. Sci. Tech. 2000, 19, 226–239. [Google Scholar] [CrossRef]
- Jain, S.; Bidol, S.; Austin, J.; Berl, E.; Elson, F.; Williams, M.; Deasy, M.; Moll, M.; Rea, V.; Vojdani, J.; et al. Multistate outbreak of Salmonella Typhimurium and saintpaul infections associated with unpasteurized orange Juice—United States, 2005. Clin. Infect. Dis. 2009, 48, 1065–1071. [Google Scholar] [CrossRef] [Green Version]
- Sivapalasingam, S.; Friedman, C.; Cohen, L.; Tauxe, R. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 2004, 67, 2342–2353. [Google Scholar] [CrossRef]
- Vojdani, J.; Beuchat, L.; Tauxe, R. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food Prot. 2008, 71, 356. [Google Scholar] [CrossRef]
- Matamouros, S.; Miller, S.S. Typhimurium strategies to resist killing by cationic antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 3021–3025. [Google Scholar] [CrossRef] [Green Version]
- Poppe, C.; Smart, N.; Khakhria, R.; Johnson, W.; Spika, J.; Prescott, J. Salmonella typhimurium DT104: A virulent and drug-resistant pathogen. Can. Vet. J. La Rev. Vet. Can. 1998, 39, 559–565. [Google Scholar]
- Wang, Y.; Zou, Y.; Wang, J.; Ma, H.; Wang, S. The protective effects of 2′-Fucosyllactose against E. Coli O157 infection are mediated by the regulation of gut microbiota and the inhibition of pathogen adhesion. Nutrients 2020, 12, 1284. [Google Scholar] [CrossRef]
- Bernet-Camard, M.; Liévin, V.; Brassart, D.; Neeser, J.; Servin, A.; Hudault, S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 1997, 63, 2747–2753. [Google Scholar] [CrossRef] [Green Version]
- Soledad, B.; Juan, E.; Fernando, V.; Covadonga, B. Adherence of human vaginal Lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar]
- Coconnier, M.-H.; Liévin, V.; Lorrot, M.; Servin, A. Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica serovar Typhimurium infecting human enterocyte-like Caco-2/TC-7 cells. Appl. Environ. Microbiol. 2000, 66, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lai, F.; Chen, Z. Research progress on the mechanism of action and antibacterial properties of probiotics. Agric. Prod. Process. 2011, 9, 65–68. [Google Scholar]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 2020, 11, 61. [Google Scholar] [CrossRef]
- Beltran, S.; Munoz, C.; Elola, L.; Quintana, J.; Segovia, C.; Trombert, A. The expression of heterologous MAM-7 in Lactobacillus rhamnosus reduces its intrinsic capacity to inhibit colonization of pathogen Vibrio parahaemolyticus in vitro. Biol. Res. 2016, 49, 2. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, K.; Nakazato, A.; Ueno, S.; Seto, Y.; Kakuda, T.; Takai, S.; Yamamoto, Y.; Mukai, T. Cell surface-associated aggregation-promoting factor from Lactobacillus gasseri SBT2055 facilitates host colonization and competitive exclusion of Campylobacter jejuni. Mol. Microbiol. 2015, 98, 712–726. [Google Scholar] [CrossRef]
- Hai, D.; Lu, Z.; Huang, X.; Lv, F.; Bie, X. In vitro screening of chicken-derived Lactobacillus strains that effectively inhibit Salmonella colonization and adhesion. Foods 2021, 10, 569. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Yang, R.; Zhao, X. Condition optimization and application of FM4-64 and Hoechst dyes in live bacterial membranes and nucleoid marker positioning. Acta Microbiol. Sin. 2015, 55, 1068–1073. [Google Scholar]
- Ruiz, J.; Núñez, M.; Lorente, I.; Pérez, J.; Simarro, E.; Gómez, J. Performance of six culture media for isolation of Salmonella species from stool samples. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 1996, 15, 922–926. [Google Scholar] [CrossRef]
- Zappa, M.; Doblas, S.; Cazals-Hatem, D.; Milliat, F.; Lavigne, J.; Daniel, F.; Jallane, A.; Garteiser, P.; Vilgrain, V.; Ogier-Denis, E.; et al. Quantitative MRI in murine radiation-induced rectocolitis: Comparison with histopathological inflammation score. NMR Biomed. 2018, 31, e3897. [Google Scholar] [CrossRef]
- Sahami, S.; Wildenberg, M.; Koens, L.; Doherty, G.; Martin, S.; D’Haens, G.; Cullen, G.; Bemelman, W.; Winter, D.; Buskens, C. Appendectomy for therapy-refractory ulcerative colitis results in pathological improvement of colonic inflammation: Short-term results of the PASSION study. J. Crohns Colitis 2019, 13, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Wotzka, S.; Kreuzer, M.; Maier, L.; Arnoldini, M.; Nguyen, B.; Brachmann, A.; Berthold, D.; Zund, M.; Hausmann, A.; Bakkeren, E.; et al. Escherichia coli limits Salmonella Typhimurium infections after diet shifts and fat-mediated microbiota perturbation in mice. Nat. Microbiol. 2019, 4, 2164–2174. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Zhang, Y.; Song, Y.; Ma, X.; Shi, Y.; Li, X.L. plantarum, L. fermentum, and B. breve beads modified the intestinal microbiota and alleviated the inflammatory response in high-fat diet-fed mice. Probiotics Antimicrob. Proteins 2020, 12, 535–544. [Google Scholar] [CrossRef]
- Ramírez-Orozco, R.; Franco, R.; Pérez, V.; Ramírez, E.; Hernández, L.; López, B. Diet-induced obese mice exhibit altered immune responses to early Salmonella Typhimurium oral infection. J. Microbiol. Biotechnol. 2018, 56, 673–682. [Google Scholar] [CrossRef]
- Lee, J.; Cevallos, S.; Byndloss, M.; Tiffany, C.; Olsan, E.; Butler, B.; Young, B.; Rogers, A.; Nguyen, H.; Kim, K.; et al. High-fat diet and antibiotics cooperatively impair mitochondrial bioenergetics to trigger dysbiosis that exacerbates pre-inflammatory bowel disease. Cell Host Microbe 2020, 28, 273–284.e6. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Umair, I.; Ahmad, M.; Khan, I.; Brohi, S.; Shah, A.; Shinwari, K.; Zhao, D.; Xu, X.; Zhou, G.; et al. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct. 2019, 10, 6903–6914. [Google Scholar] [CrossRef]
- Kawarizadeh, A.; Pourmontaseri, M.; Farzaneh, M.; Hosseinzadeh, S.; Ghaemi, M.; Tabatabaei, M.; Pourmontaseri, Z.; Pirnia, M. Interleukin-8 gene expression and apoptosis induced by Salmonella Typhimurium in the presence of Bacillus probiotics in the epithelial cell. J. Appl. Microbiol. 2020, 131, 449–459. [Google Scholar] [CrossRef]
- Bayoumi, M.; Griffiths, M. Probiotics down-regulate genes in Salmonella enterica serovar typhimurium pathogenicity islands 1 and 2. J. Food Prot. 2010, 73, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Tabashsum, Z.; Peng, M.; Bernhardt, C.; Patel, P.; Carrion, M.; Rahaman, S.; Biswas, D. Limiting the pathogenesis of Salmonella Typhimurium with berry phenolic extracts and linoleic acid overproducing Lactobacillus casei. J. Microbiol. Biotechnol. 2020, 58, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Liang, H.; Yu, B.; Hsieh, C.; Hwang, C.; Chen, M.; Tsen, H. The relative efficacy of different strain combinations of lactic acid bacteria in the reduction of populations of Salmonella enterica Typhimurium in the livers and spleens of mice. FEMS Immunol. Med. Microbiol. 2011, 63, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Ofek, I.; Sharon, N. Adhesins as lectins: Specificity and role in infection. Curr. Top. Microbiol. Immunol. 1990, 151, 91–113. [Google Scholar]
- Darwin, K.; Miller, V. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 1999, 12, 405–428. [Google Scholar] [CrossRef] [Green Version]
- Mohan, V. The role of probiotics in the inhibition of Campylobacter jejuni colonization and virulence attenuation. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1503–1513. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Song, X.; Xia, Y.; Lai, P.; Ai, L. Lactobacillus casei LC2W can inhibit the colonization of Escherichia coli O157:H7 in vivo and reduce the severity of colitis. Food Funct. 2019, 10, 5843–5852. [Google Scholar] [CrossRef]
- Mills, J.; Rao, K.; Young, V. Probiotics for prevention of Clostridium difficile infection. Curr. Opin. Gastroenterol. 2018, 34, 3–10. [Google Scholar] [CrossRef]
- Lim, K.; Staudt, L. Toll-Like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, M.; Cao, J.; Yang, P.; Zheng, X.; Dong, B.; Lu, J. Role of TLR4 in process of colonic inflammation recovery induced by LPS. Chin. J. Pathophysiol. 2017, 33, 336–343. [Google Scholar]
- Zhu, H.; Bian, C.; Yuan, J.; Chu, W.; Xiang, X.; Chen, F.; Wang, C.; Feng, H.; Lin, J. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflamm. 2014, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Zambrano, S.; Tiérrez, A.; Bianchi, M.; Agresti, A.; García, D. Single-cell analyses reveal an attenuated NF-κB response in the Salmonella-infected fibroblast. Virulence 2017, 8, 719–740. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Xue, C.; Zhang, L.; Zhang, T.; Wang, C.; Bi, C.; Shan, A. Oleanolic acid enhances tight junctions and ameliorates inflammation in Salmonella typhimurium-induced diarrhea in mice via the TLR4/NF-κB and MAPK pathway. Food Funct. 2020, 11, 1122–1132. [Google Scholar] [CrossRef]
- Van, W.; Fricke, F.; Herhaus, L.; Gupta, J.; Hötte, K.; Pampaloni, F.; Grumati, P.; Kaulich, M.; Sou, Y.; Komatsu, M.; et al. Linear ubiquitination of cytosolic Salmonella Typhimurium activates NF-κB and restricts bacterial proliferation. Nat. Microbiol. 2017, 2, 17066. [Google Scholar]
- Eom, J.; Song, J.; Choi, H. Protective Effects of a Novel Probiotic Strain of Lactobacillus plantarum JSA22 from Traditional Fermented Soybean Food Against Infection by Salmonella enterica Serovar Typhimurium. J. Microbiol. Biotechnol. 2015, 25, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-parallel inhibition of IL-10 and TGF-β1 controls LPS-induced inflammatory response via NF-κB signaling in grass carp monocytes/macrophages. Fish Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, S.; Li, Y.; Fan, M.; Sun, Y.; Li, W.; Shi, Q. TLR4 activation with LPS inhibits BMP9-induced osteogenic differentiation of immortalized mouse embryonic fibroblasts. Basic Clin. Med. 2017, 37, 25–31. [Google Scholar]
- Hong, D.; Angelo, L.; Kurzrock, R. Interleukin-6 and its receptor in cancer: Implications for translational therapeutics. Cancer 2010, 110, 1911–1928. [Google Scholar] [CrossRef]
- Thiemann, S.; Smit, N.; Roy, U.; Lesker, T.; Galvez, E.; Helmecke, J.; Basic, M.; Bleich, A.; Goodman, A.; Kalinke, U.; et al. Enhancement of IFN-γ production by distinct commensals ameliorates Salmonella-Induced Disease. Cell Host Microbe 2017, 21, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Sharipov, F.; Kalempa, D. Developmentally regulated intestinal expression of IFN-γ and Its target genes and the age-specific response to Enteric Salmonella infection. J. Immunol. 2005, 175, 1127–1136. [Google Scholar]
- Raffatellu, M.; Santos, R.; Verhoeven, D.; George, M.; Wilson, R.; Winter, S.; Godinez, I.; Sankaran, S.; Paixao, T.; Gordon, M.; et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 2008, 14, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.; Kim, S.; Kim, E. The molecular mechanism of transforming growth factor-β signaling for intestinal fibrosis: A mini-review. Front. Pharmacol. 2019, 10, 162. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Sina, C.; Franke, A.; Schreiber, S. Towards a molecular risk map-recent advances on the etiology of inflammatory bowel disease. Semin. Immunol. 2009, 21, 334–345. [Google Scholar] [CrossRef]
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.; Hedrick, S.; Tenzer, S.; Neurath, M. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Thiennimitr, P.; Winter, S.; Winter, M.; Xavier, M.; Tolstikov, V.; Huseby, D.; Sterzenbach, T.; Tsolis, R.; Roth, J.; Baumler, A. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 17480–17485. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, Y.; Zuo, Z.; Han, Q.J.; Zhang, J.; Zhang, C. Mutual regulation between intestinal macrophages and CD4+ T cells enhances resistance to Salmonella typhimurium infection. In Proceedings of the 9th National Immunology Academic Conference of the Chinese Society of Immunology Assembly, Jinan, China, 18–21 October 2014. [Google Scholar]
- Raffatellu, M.; George, M.; Akiyama, Y.; Hornsby, M.; Nuccio, S.; Paixao, T.; Butler, B.; Chu, H.; Santos, R.; Berger, T.; et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Keir, M.; Yi, Y.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Peng, L.; Chen, S.; Pu, Y.; Lv, H.; Wei, H.; Wan, C. Lactiplantibacillus plantarum 1201 Inhibits Intestinal Infection of Salmonella enterica subsp. enterica Serovar Typhimurium Strain ATCC 13311 in Mice with High-Fat Diet. Foods 2022, 11, 85. https://doi.org/10.3390/foods11010085
Ren Z, Peng L, Chen S, Pu Y, Lv H, Wei H, Wan C. Lactiplantibacillus plantarum 1201 Inhibits Intestinal Infection of Salmonella enterica subsp. enterica Serovar Typhimurium Strain ATCC 13311 in Mice with High-Fat Diet. Foods. 2022; 11(1):85. https://doi.org/10.3390/foods11010085
Chicago/Turabian StyleRen, Zhongyue, Lingling Peng, Shufang Chen, Yi Pu, Huihui Lv, Hua Wei, and Cuixiang Wan. 2022. "Lactiplantibacillus plantarum 1201 Inhibits Intestinal Infection of Salmonella enterica subsp. enterica Serovar Typhimurium Strain ATCC 13311 in Mice with High-Fat Diet" Foods 11, no. 1: 85. https://doi.org/10.3390/foods11010085
APA StyleRen, Z., Peng, L., Chen, S., Pu, Y., Lv, H., Wei, H., & Wan, C. (2022). Lactiplantibacillus plantarum 1201 Inhibits Intestinal Infection of Salmonella enterica subsp. enterica Serovar Typhimurium Strain ATCC 13311 in Mice with High-Fat Diet. Foods, 11(1), 85. https://doi.org/10.3390/foods11010085





