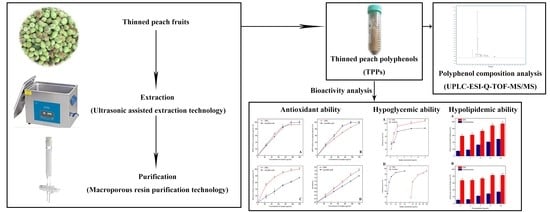
Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Optimization of Conditions for Ultrasonic Assisted Extraction of TPPs
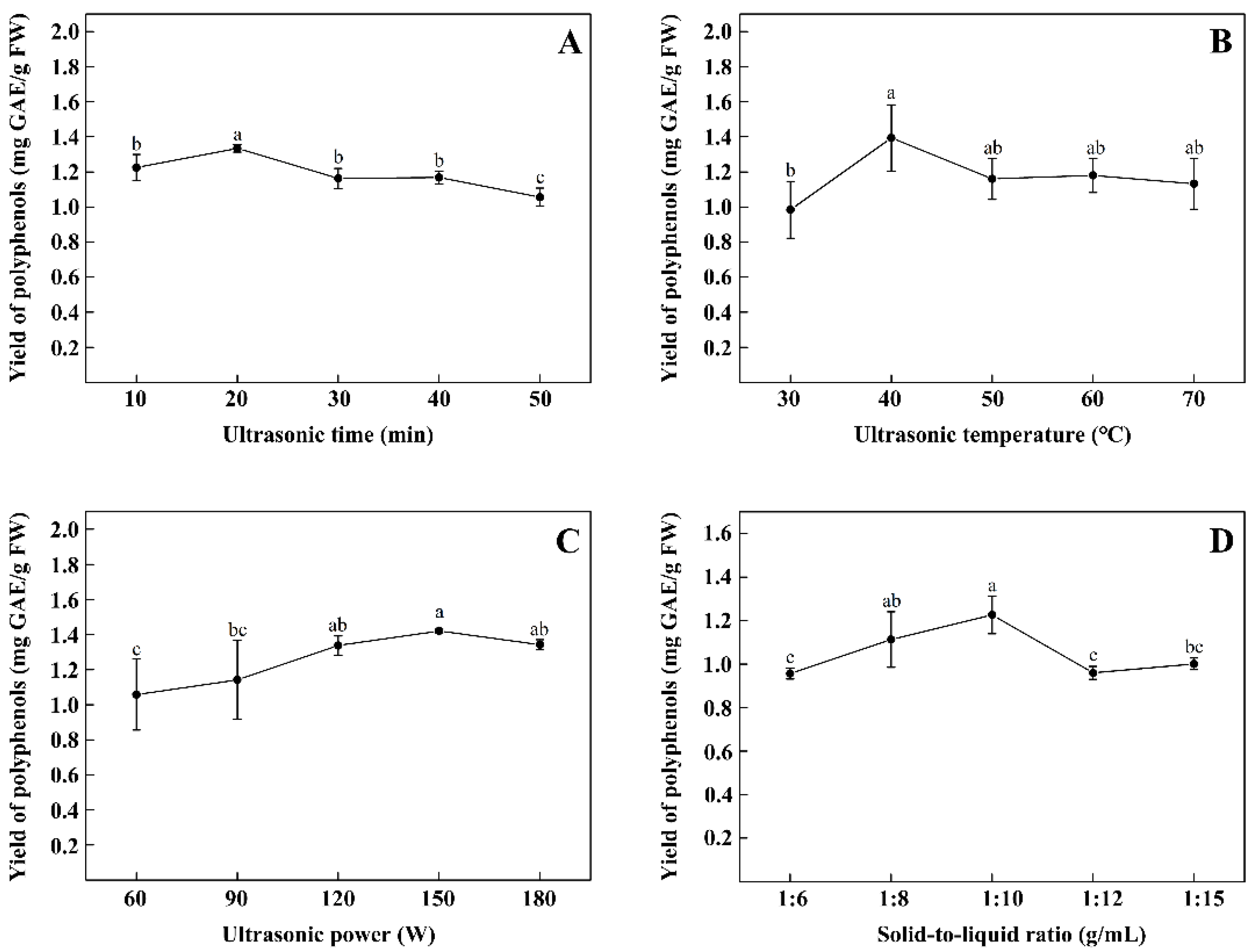
2.2.1. Single Factor Experiments
2.2.2. Response Surface Design Experiments
2.3. Purification of TPPs by Macroporous Adsorption Resin
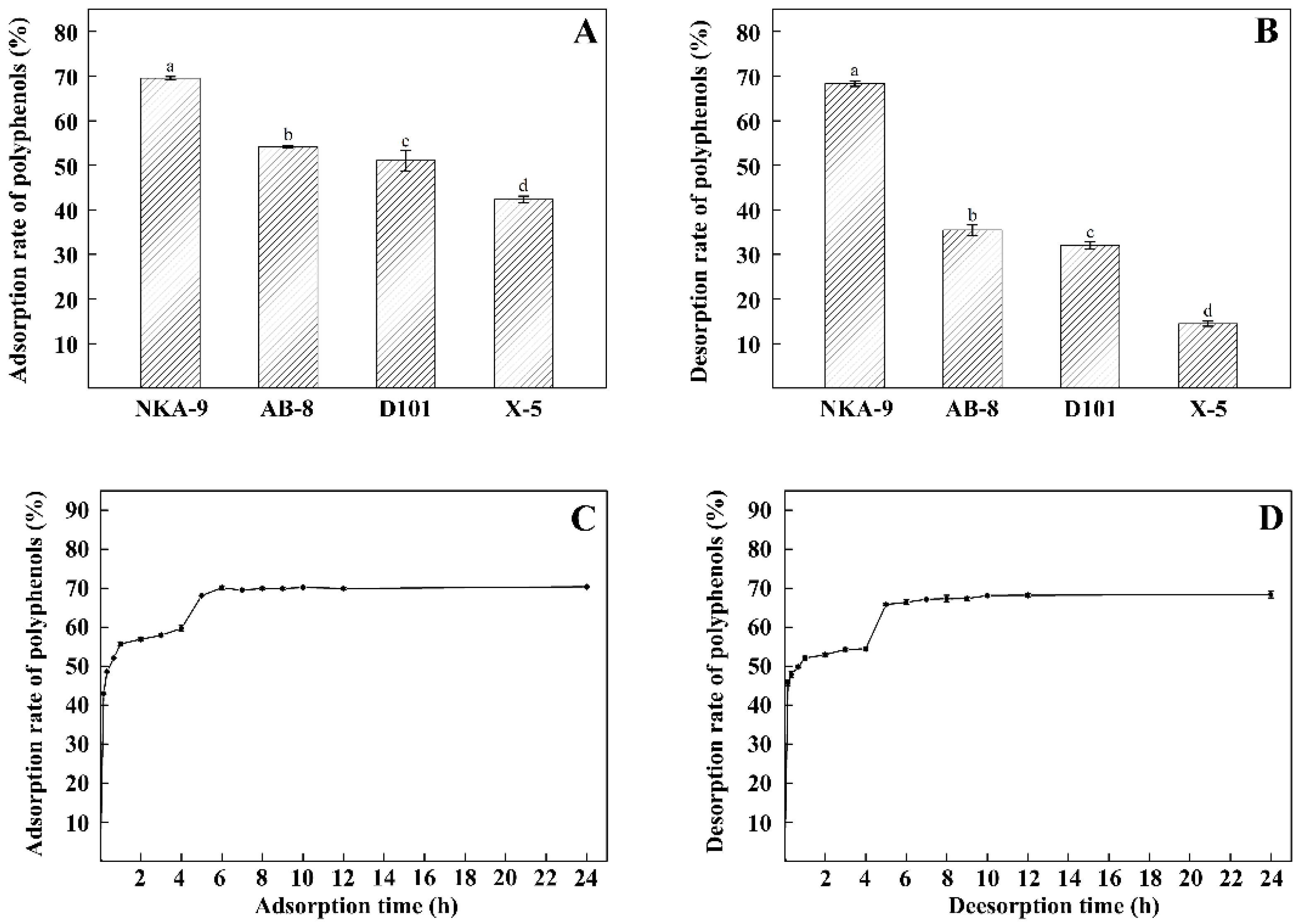
2.3.1. Screening of Macroporous Resins
2.3.2. Static Adsorption and Desorption Kinetic Curves for NKA-9 Resin
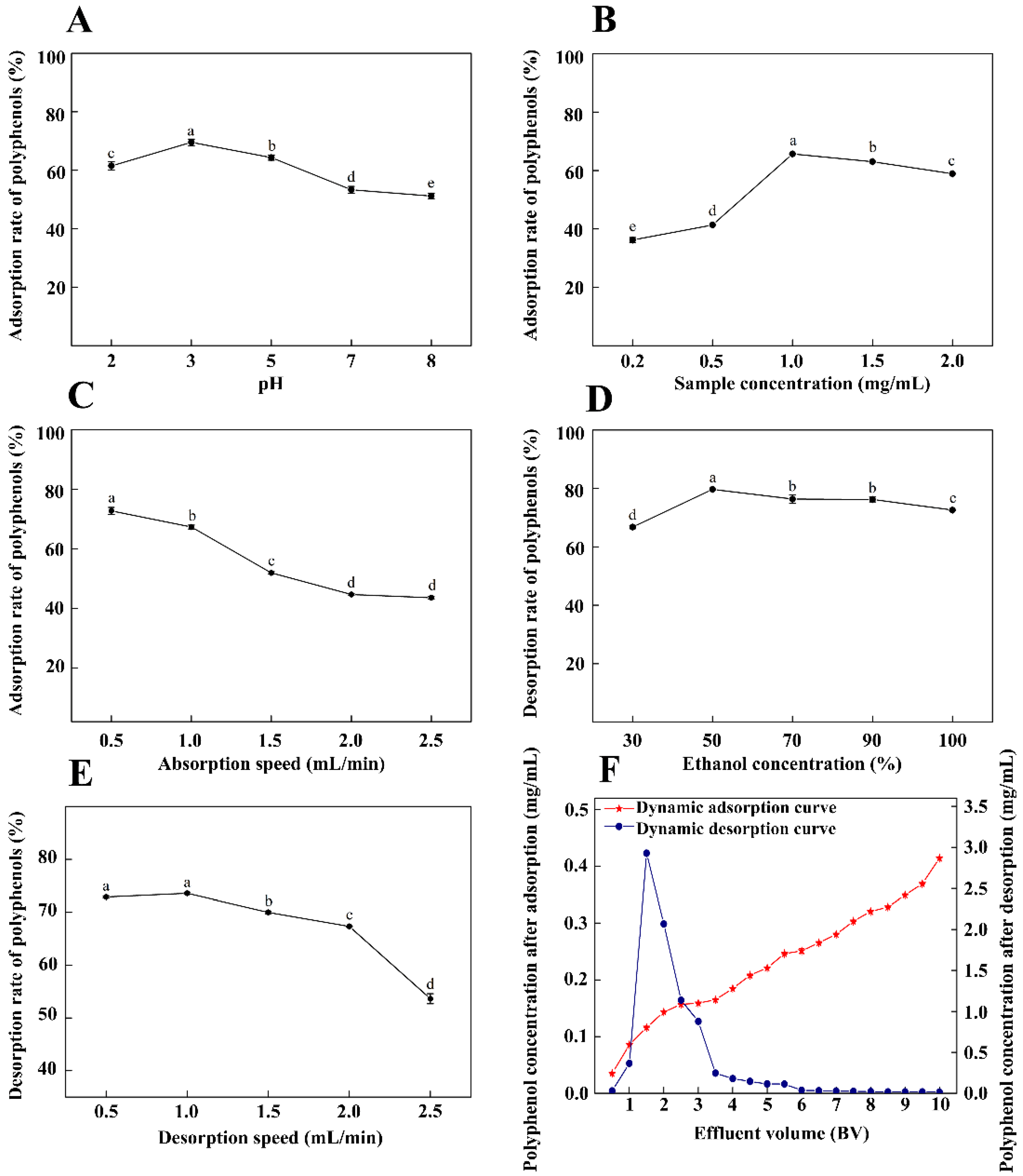
2.3.3. Dynamic Adsorption and Desorption Curves under Optimal Conditions
2.4. Identification and Quantification of TPPs by the UPLC-ESI-QTOF-MS/MS
2.5. Antioxidant Capacity of TPPs
2.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Capacity
2.5.2. 2,2′-Azino-di-3-ethylbenzthiazoline Sulfonic Acid (ABTS) Radical Scavenging Effect
2.5.3. Hydroxyl Radical Scavenging Capacity
2.5.4. Ferric Reducing Antioxidant Power (FRAP)
2.6. Hypoglycemic and Hypolipidemic Effects of TPPs In Vitro
2.6.1. α-Amylase Inhibition by TPPs
2.6.2. α-Glucosidase Inhibition by TPPs
2.6.3. Determination of Bile Acid Binding Activity of TPPs
2.7. Statistical Analysis
3. Results
3.1. Influence of Single Factor on TPPs Yield
3.2. Optimization of Extraction Conditions Using a Response Surface Model
3.3. Screening of Macroporous Resins, and Static Adsorption and Desorption Kinetic for NKA-9
3.4. Dynamic Adsorption and Desorption Curves under Optimal Conditions
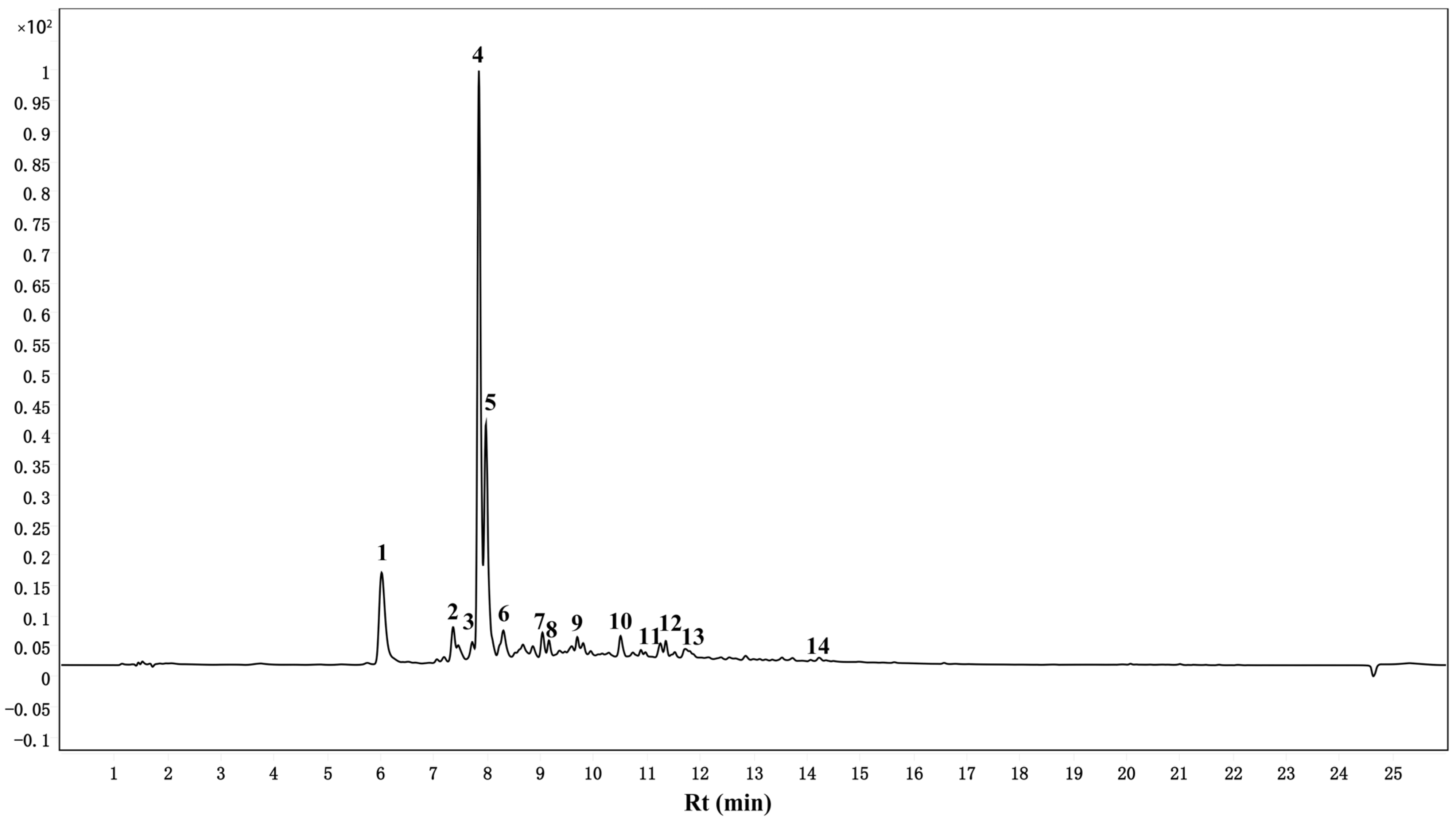
3.5. Identification and Quantification of TPPs by UPLC-ESI-QTOF-MS/MS
3.6. Antioxidant Capacity of TPPs
3.7. Inhibition of α-Amylase and α-Glucosidase by TPPs
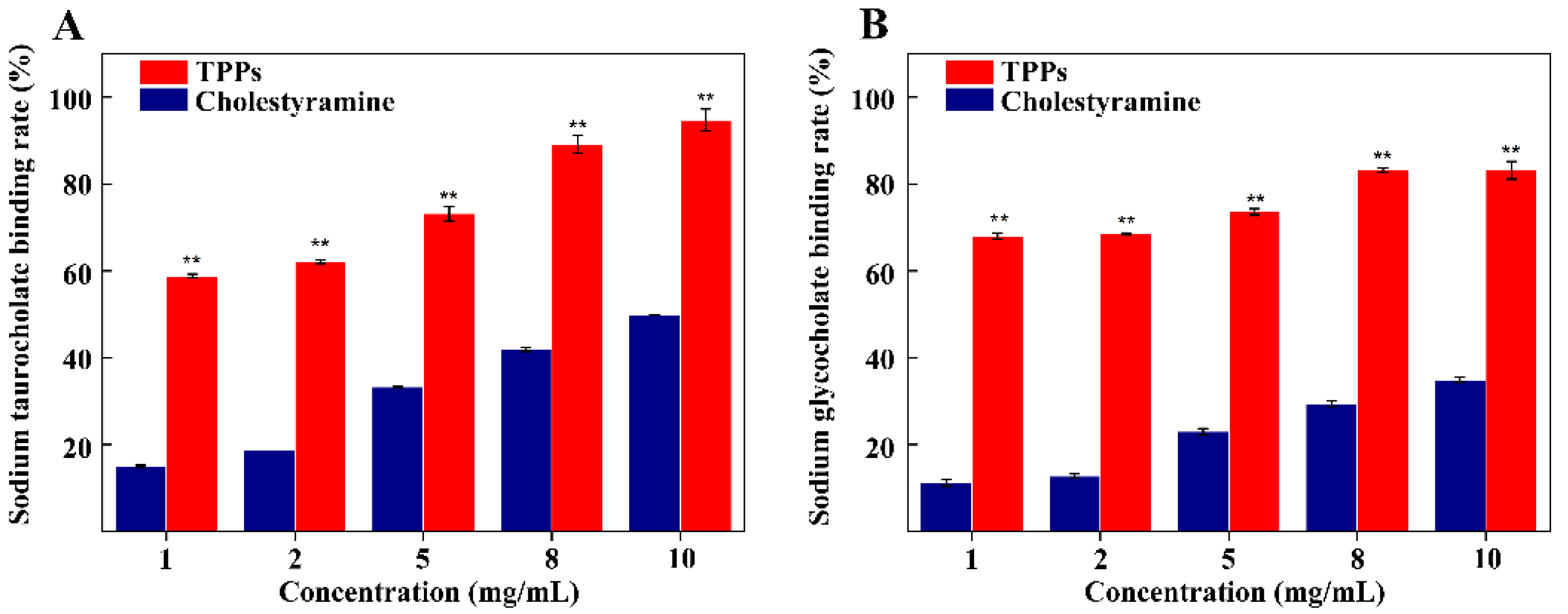
3.8. Binding of Bile Acid by TPPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, C.P.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio(nano)materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Ma, T.; Ge, Q.; Fang, Y.; Sun, X. Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef]
- Guo, C.T.; Bi, J.F.; Li, X.; Lyu, J.; Wu, X.Y.; Xu, Y. Polyphenol metabolic diversity of Chinese peach and nectarine at thinned and ripe stages by UPLC-ESI-Q-TOF-MS combined with multivariate statistical analysis. J. Food Compos. Anal. 2020, 90, 103502. [Google Scholar] [CrossRef]
- Nasser, M.; Cheikh, H.; Hijazi, A.; Merah, O.; Rekaby, A.; Awada, R. Phytochemical profile, antioxidant and antitumor activities of green grape juice. Processes 2020, 8, 507. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Kim, Y.I.; Chung, S.K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Sun, L.Q.; Tao, S.T.; Zhang, S.L. Characterization and quantification of polyphenols and triterpenoids in thinned young fruits of ten pear varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef] [Green Version]
- Karabiyikli, S.; Oncul, N. Inhibitory effect of unripe grape products on foodborne pathogens. J. Food Process. Preserv. 2016, 40, 1459–1465. [Google Scholar] [CrossRef]
- Redondo, D.; Gimeno, D.; Calvo, H.; Venturini, M.E.; Oria, R.; Arias, E. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste Biomass Valorization 2021, 12, 1861–1875. [Google Scholar] [CrossRef]
- Guo, C.T.; Bi, J.F.; Li, X.; Lyu, J.; Zhou, M.; Wu, X.Y. Antioxidant profile of thinned young and ripe fruits of Chinese peach and nectarine varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Yang, J.; Cui, J.Y.; Wu, Y.H.; Han, H.J.; Chen, J.X.; Yao, J.Y.; Liu, Y.P. Comparisons of the active components in four unripe raspberry extracts and their activites. Food Sci. Technol. 2019, 39, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.P.; Li, J.W. A novel process for asparagus polyphenols utilization by ultrasound assisted adsorption and desorption using resins. Ultrason. Sonochem. 2020, 63, 104920. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Zhang, X.Y.; Zhang, Q.; Gao, N.X.; Li, B.; Meng, X.J. Optimation of enrichment and purification of polyphenols from blueberries (Vaccinium spp.) by macroporous resins XAD-7HP. Emir. J. Food Agric. 2017, 29, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.Y.; Cao, K.; Fang, W.C.; Zhu, G.R.; Chen, C.W.; Wang, X.W.; Wang, L.R. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.B.; Cao, J.K.; Ma, L. Evaluation of antioxidant properties of extractable and nonextractable polyphenols in peel and flesh tissue of different peach varieties. J. Food Process. Preserv. 2018, 42, 13624. [Google Scholar] [CrossRef]
- Ge, X.Z.; Jing, L.Z.; Zhao, K.; Su, C.Y.; Zhang, B.; Zhang, Q.; Han, L.H.; Yu, X.Z.; Li, W.H. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X.X. Effects of oolong tea polyphenols, EGCG, and EGCG3 ‘‘ Me on pancreatic alpha-amylase activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.X.; Li, W.F.; Tan, S.; Zheng, Q.R. Phenolic content, antioxidant capacity, and alpha-amylase and alpha-glucosidase inhibitory activities of Dimocarpus longan Lour. Food Sci. Biotechnol. 2020, 29, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Guo, Y.Q.; Li, X.Y.; Gong, K.J.; Liu, K.C. Phenolics and related in vitro functional activities of different varieties of fresh waxy corn: A whole grain. BMC Chem. 2021, 15, 14. [Google Scholar] [CrossRef]
- Cao, J.P.; Jiang, Q.; Lin, J.Y.; Li, X.; Sun, C.D.; Chen, K.S. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Chalot, G. Physicochemical characteristics, vitamin C, and polyphenolic composition of four European commercial blood-flesh peach cultivars (Prunus persica L. Batsch). J. Food Compos. Anal. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Owczarek, A.; Gralak, P.; Ciszewski, P.; Olszewska, M.A. Polyphenolic profile, antioxidant activity, and pro-inflammatory enzymes inhibition of leaves, flowers, bark and fruits of Cotoneaster integerrimus: A comparative study. Phytochem. Lett. 2019, 30, 349–355. [Google Scholar] [CrossRef]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in Lentil. J. Nat. Prod. 2017, 80, 1310–1317. [Google Scholar] [CrossRef]
- Yang, L.C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive characterization of bioactive phenols from new Brazilian superfruits by LC-ESI-QTOF-MS, and their ROS and RNS scavenging effects and anti-inflammatory activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef]
- Yue, T.L.; Shao, D.Y.; Yuan, Y.H.; Wang, Z.L.; Qiang, C.Y. Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. J. Sep. Sci. 2012, 35, 2138–2145. [Google Scholar] [CrossRef]
- Hong, J.J.; Seol, H.G.; Oh, J.Y.; Jeong, E.H.; Chang, Y.H. Effective component contents and antioxidative activities of unripe apple by extraction methods. Korean J. Food Nutr. 2021, 34, 174–180. [Google Scholar]
- Wang, C.K.; Lee, W.H.; Peng, C.H. Contents of phenolics and alkaloids in Areca catechu Linn during maturation. J. Agric. Food Chem. 1997, 45, 1185–1188. [Google Scholar] [CrossRef]
- Shreelakshmi, S.V.; Nazareth, M.S.; Kumar, S.S.; Giridhar, P.; Prashanth, K.V.H.; Shetty, N.P. Physicochemical composition and characterization of bioactive compounds of mulberry (Morus indica L.) Fruit during ontogeny. Plant Foods Hum. Nutr. 2021, 76, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Peng, S.; Peng, M.J.; She, Z.G.; Yang, Q.L.; Huang, T. Adsorption and desorption characteristics of polyphenols from Eucommia ulmoides Oliv. leaves with macroporous resin and its inhibitory effect on alpha-amylase and alpha-glucosidase. Ann. Transl. Med. 2020, 8, 1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Liu, D.J.; Sun, J.J.; Yang, X.B.; Fu, M.H.; Guo, Y.R. Simultaneous separation and purification of chlorogenic acid, epicatechin, hyperoside and phlorizin from thinned young Qinguan apples by successive use of polyethylene and polyamide resins. Food Chem. 2017, 230, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.J.; Guo, Y.R.; Fu, C.C.; Li, J.J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.T.; Bi, J.F.; Li, X.; Lyu, J.; Xu, Y.; Hu, J.X. Investigation on the phenolic composition, related oxidation and antioxidant activity of thinned peach dried by different methods. LWT-Food Sci. Technol. 2021, 147, 111573. [Google Scholar] [CrossRef]
- Sun, L.J.; Chen, W.Q.; Meng, Y.H.; Yang, X.B.; Yuan, L.; Guo, Y.R. Interactions between polyphenols in thinned young apples and porcine pancreatic alpha-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.Q.; Guo, Q.W.; Gao, X.D.; Ma, Q.Q.; Xue, Z.H.; Ferri, N.; Zhang, M.; Chen, H.X. Identification of ellagitannins in the unripe fruit of Rubus Chingii Hu and evaluation of its potential antidiabetic activity. J. Agric. Food Chem. 2019, 67, 7025–7039. [Google Scholar] [CrossRef]
- Mihaylova, D.; Desseva, I.; Popova, A.; Dincheva, I.; Vrancheva, R.; Lante, A.; Krastanov, A. GC-MS metabolic profile and alpha-glucosidase, alpha-amylase, lipase, and acetylcholinesterase-inhibitory activities of eight peach varieties. Molecules 2021, 26, 4183. [Google Scholar] [CrossRef] [PubMed]
- Joymak, W.; Ngamukote, S.; Chantarasinlapin, P.; Adisakwattana, S. Unripe papaya by-product: From food wastes to functional ingredients in pancakes. Foods 2021, 10, 615. [Google Scholar] [CrossRef]



| Number | Ultrasonic Time (min) | Ultrasonic Temperature (°C) | Ultrasonic Power (W) | Solid-to-Liquid (g/mL) | Yield of Polyphenols (mg GAE/g FW) |
|---|---|---|---|---|---|
| 1 | 10 (−1) | 30 (−1) | 150 (0) | 1:10 (0) | 1.331 ± 0.026 |
| 2 | 30 (1) | 30 (−1) | 150 (0) | 1:10 (0) | 1.152 ± 0.008 |
| 3 | 10 (−1) | 50 (1) | 150 (0) | 1:10 (0) | 1.351 ± 0.010 |
| 4 | 30 (1) | 50 (1) | 150 (0) | 1:10 (0) | 1.389 ± 0.015 |
| 5 | 20 (0) | 40 (0) | 120 (−1) | 1:8 (−1) | 1.066 ± 0.027 |
| 6 | 20 (0) | 40 (0) | 180 (1) | 1:8 (−1) | 1.113 ± 0.014 |
| 7 | 20 (0) | 40 (0) | 120 (−1) | 1:12 (1) | 1.370 ± 0.030 |
| 8 | 20 (0) | 40 (0) | 180 (1) | 1:12 (1) | 1.489 ± 0.022 |
| 9 | 10 (−1) | 40 (0) | 150 (0) | 1:8 (−1) | 1.137 ± 0.039 |
| 10 | 30 (1) | 40 (0) | 180 (1) | 1:8 (−1) | 1.077 ± 0.021 |
| 11 | 10 (−1) | 40 (0) | 150 (0) | 1:12 (1) | 1.449 ± 0.009 |
| 12 | 30 (1) | 40 (0) | 150 (0) | 1:12 (1) | 1.489 ± 0.016 |
| 13 | 20 (0) | 30 (−1) | 120 (−1) | 1:10 (0) | 1.181 ± 0.009 |
| 14 | 20 (0) | 50 (1) | 120 (−1) | 1:10 (0) | 1.354 ± 0.060 |
| 15 | 20 (0) | 30 (−1) | 180 (1) | 1:10 (0) | 1.246 ± 0.075 |
| 16 | 20 (0) | 50 (1) | 180 (1) | 1:10 (0) | 1.316 ± 0.036 |
| 17 | 10 (−1) | 40 (0) | 120 (−1) | 1:10 (0) | 1.214 ± 0.029 |
| 18 | 30 (1) | 40 (0) | 120 (−1) | 1:10 (0) | 1.297 ± 0.033 |
| 19 | 10 (−1) | 40 (0) | 180 (1) | 1:10 (0) | 1.287 ± 0.015 |
| 20 | 30 (1) | 40 (0) | 180 (1) | 1:10 (0) | 1.187 ± 0.019 |
| 21 | 20 (0) | 30 (−1) | 150 (0) | 1:8 (−1) | 1.087 ± 0.012 |
| 22 | 20 (0) | 50 (1) | 150 (0) | 1:8 (−1) | 1.232 ± 0.005 |
| 23 | 20 (0) | 30 (−1) | 150 (0) | 1:12 (1) | 1.485 ± 0.040 |
| 24 | 20 (0) | 50 (1) | 150 (0) | 1:12 (1) | 1.547 ± 0.041 |
| 25 | 20 (0) | 40 (0) | 150 (0) | 1:10 (0) | 1.424 ± 0.040 |
| 26 | 20 (0) | 40 (0) | 150 (0) | 1:10 (0) | 1.419 ± 0.043 |
| 27 | 20 (0) | 40 (0) | 150 (0) | 1:10 (0) | 1.408 ± 0.040 |
| 28 | 20 (0) | 40 (0) | 150 (0) | 1:10 (0) | 1.386 ± 0.041 |
| 29 | 20 (0) | 40 (0) | 150 (0) | 1:10 (0) | 1.399 ± 0.013 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.53 | 14 | 0.038 | 53.42 | <0.0001 | ** |
| A (ultrasonic time) | 2.61 × 10−3 | 1 | 2.61 × 10−3 | 3.68 | 0.0757 | |
| B (ultrasonic temperature) | 0.042 | 1 | 0.042 | 58.77 | <0.0001 | ** |
| C (ultrasonic power) | 2.00 × 10−3 | 1 | 2.00 × 10−3 | 2.82 | 0.1152 | |
| D (solid-to-liquid) | 0.37 | 1 | 0.37 | 525.61 | <0.0001 | ** |
| AB | 0.012 | 1 | 0.012 | 16.61 | 0.0011 | ** |
| AC | 8.45 × 10−3 | 1 | 8.45 × 10−3 | 11.89 | 0.0039 | ** |
| AD | 2.51 × 10−3 | 1 | 2.51 × 10−3 | 3.54 | 0.081 | |
| BC | 2.68 × 10−3 | 1 | 2.68 × 10−3 | 3.78 | 0.0723 | |
| BD | 1.69 × 10−3 | 1 | 1.69 × 10−3 | 2.38 | 0.1453 | |
| CD | 1.28 × 10−3 | 1 | 1.28 × 10−3 | 1.8 | 0.2011 | |
| A2 | 0.031 | 1 | 0.031 | 43.34 | <0.0001 | ** |
| B2 | 5.89 × 10−3 | 1 | 5.89 × 10−3 | 8.29 | 0.0121 | * |
| C2 | 0.063 | 1 | 0.063 | 89.3 | <0.0001 | ** |
| D2 | 0.014 | 1 | 0.014 | 19.68 | 0.0006 | ** |
| Residual | 9.95 × 10−3 | 14 | 7.10 × 10−3 | |||
| Lack of Fit | 8.99 × 10−3 | 10 | 8.99 × 10−3 | 3.76 | 0.1069 | |
| Cor Total | 0.54 | 28 | ||||
| R2 | 0.9816 | |||||
| Radj2 | 0.9632 |
| Peak | RT (min) | MW | MS (m/z) | MS/MS (m/z) | Tentative Identification | Molecular Formula |
|---|---|---|---|---|---|---|
| 1 | 6.015 | 354 | 355 | 135 | Neochlorogenic acid [20] | C16H18O9 |
| 2 | 7.355 | 449 | 450 | 287 | Cyanidin-3-glucoside [21] | C21H21O11 |
| 3 | 7.715 | 578 | 579 | 289 | Procyanidin B1 [21] | C30H27O12 |
| 4 | 7.842 | 354 | 355 | 135 | Chlorogenic acid [20] | C16H18O9 |
| 5 | 7.975 | 290 | 291 | 139, 123 | Catechin [22] | C15H14O6 |
| 6 | 8.302 | 866 | 867 | 289, 287 | B-type (epi)catechin trimer [22] | C45H38O18 |
| 7 | 9.035 | 290 | 291 | 139, 123 | Epicatechin [22] | C15H14O6 |
| 8 | 9.155 | 338 | 339 | 119 | Coumaroylquinic acid [20] | C16H18O8 |
| 9 | 9.582 | 866 | 867 | 451, 289 | procyanidin C1 [23] | C45H38O18 |
| 10 | 10.502 | 610 | 611 | 303 | Rutin [24] | C27H30O16 |
| 11 | 10.889 | 464 | 465 | 303 | Hyperoside [24] | C21H20O12 |
| 12 | 11.349 | 448 | 449 | 303 | Quercitrin [24] | C21H20O11 |
| 13 | 11.715 | 478 | 479 | 151 | Isorhamnetin-3-O-glucoside [25] | C22H22O12 |
| 14 | 14.229 | 302 | 303 | 257, 153 | Quercetin [26] | C15H10O7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, K.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhang, X.; Shao, X. Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties. Foods 2022, 11, 99. https://doi.org/10.3390/foods11010099
Dai K, Wei Y, Jiang S, Xu F, Wang H, Zhang X, Shao X. Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties. Foods. 2022; 11(1):99. https://doi.org/10.3390/foods11010099
Chicago/Turabian StyleDai, Kun, Yingying Wei, Shu Jiang, Feng Xu, Hongfei Wang, Xin Zhang, and Xingfeng Shao. 2022. "Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties" Foods 11, no. 1: 99. https://doi.org/10.3390/foods11010099
APA StyleDai, K., Wei, Y., Jiang, S., Xu, F., Wang, H., Zhang, X., & Shao, X. (2022). Lab Scale Extracted Conditions of Polyphenols from Thinned Peach Fruit Have Antioxidant, Hypoglycemic, and Hypolipidemic Properties. Foods, 11(1), 99. https://doi.org/10.3390/foods11010099