Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Germinated Tartary Buckwheat Samples
2.3. Preparation of TBP
2.4. Determination of Free Sulfhydryl Content
2.5. Determination of Fourier Transform Infrared Spectroscopic
2.6. Determination of Ultraviolet Spectra
2.7. Determination of Hydration Properties
2.8. Determination of Emulsifying Properties
2.9. Determination of Foaming Properties
2.10. Determination of In Vitro Protein Digestibility
2.11. Determination of Antioxidant Activities
2.12. Statistical Analysis
3. Results and Discussion
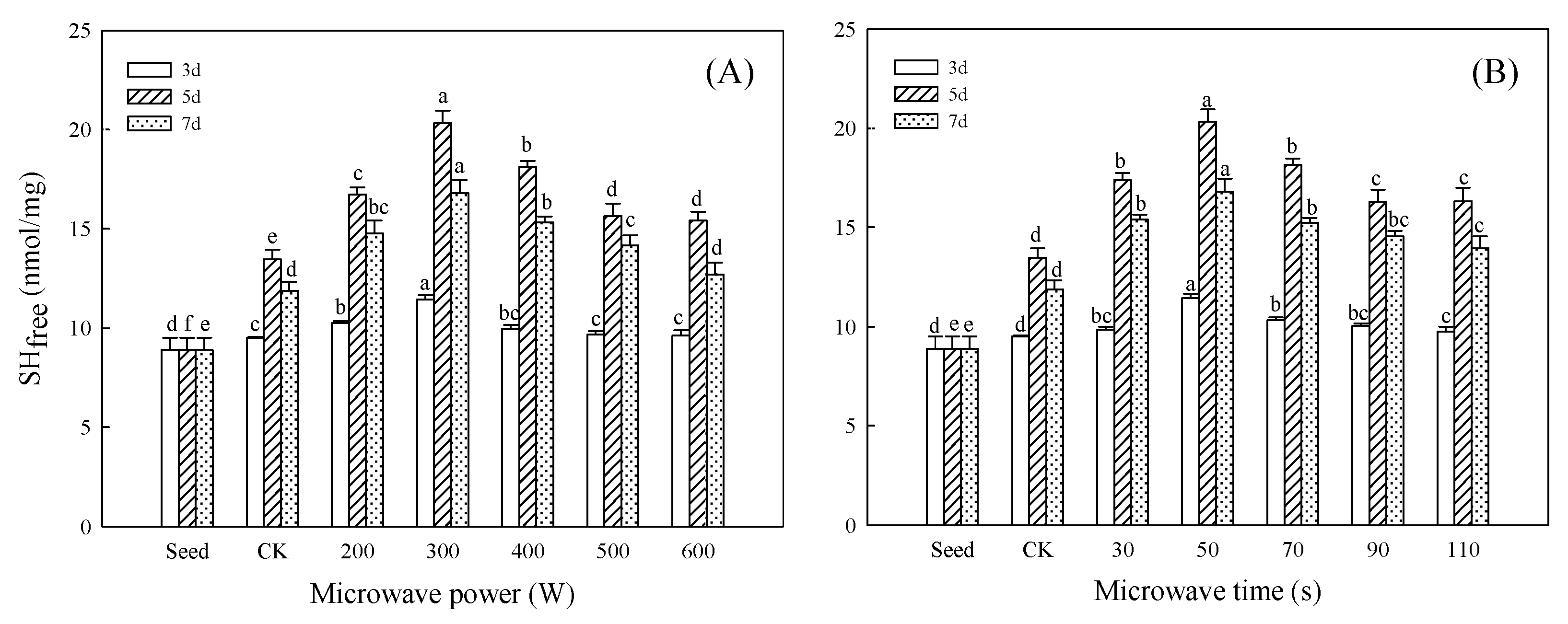
3.1. Effect of Microwave Treatment on SHfree Content of Germinated TBP
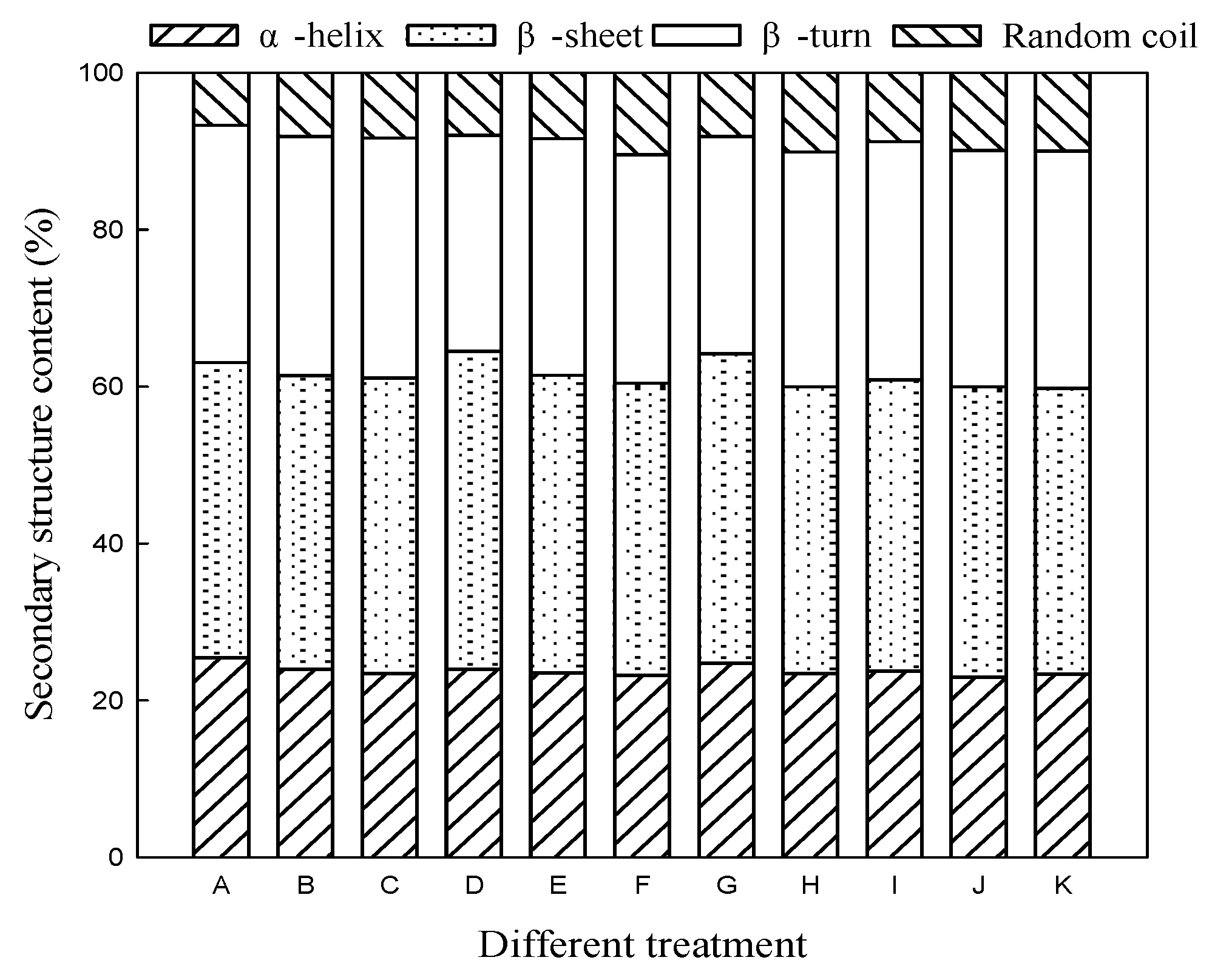
3.2. Effect of Microwave Treatment on Secondary Structure of Germinated TBP
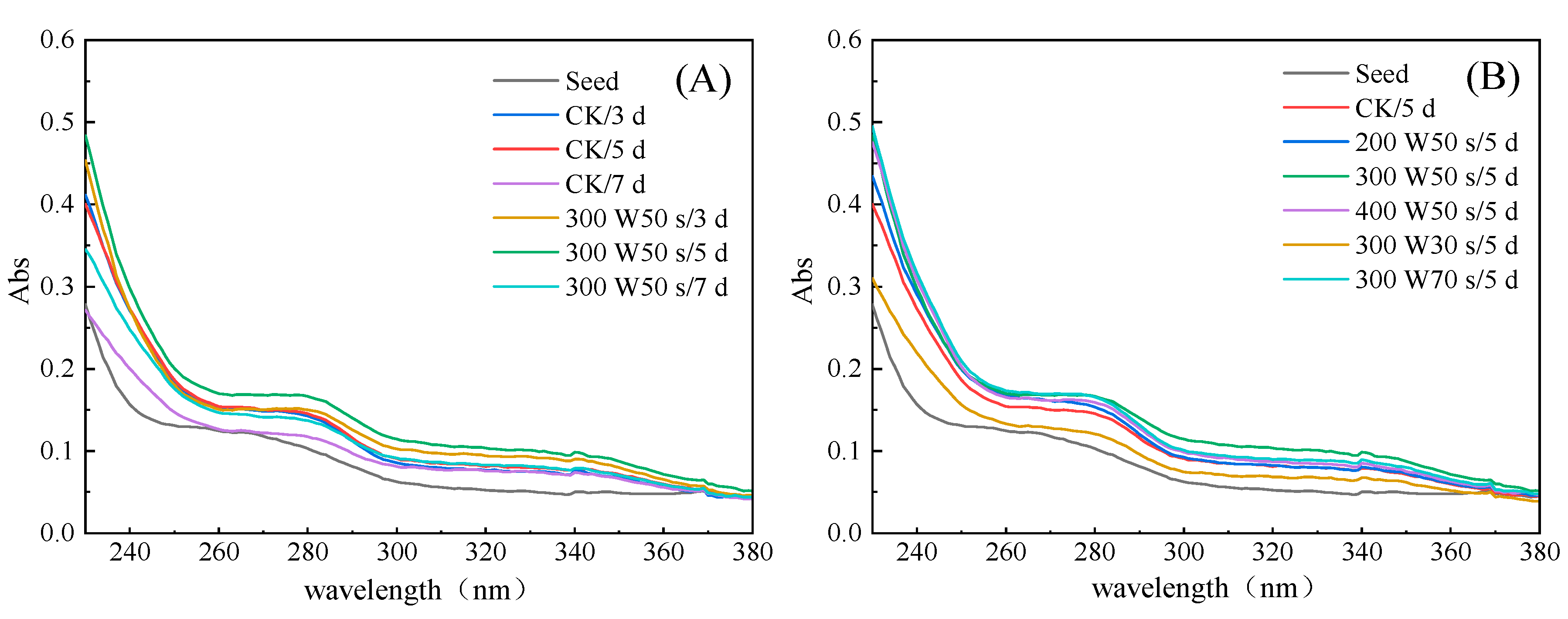
3.3. Effect of Microwave Treatment on UV Spectrum of Germinated TBP
3.4. Effect of Microwave Treatment on Hydration Properties of Germinated TBP
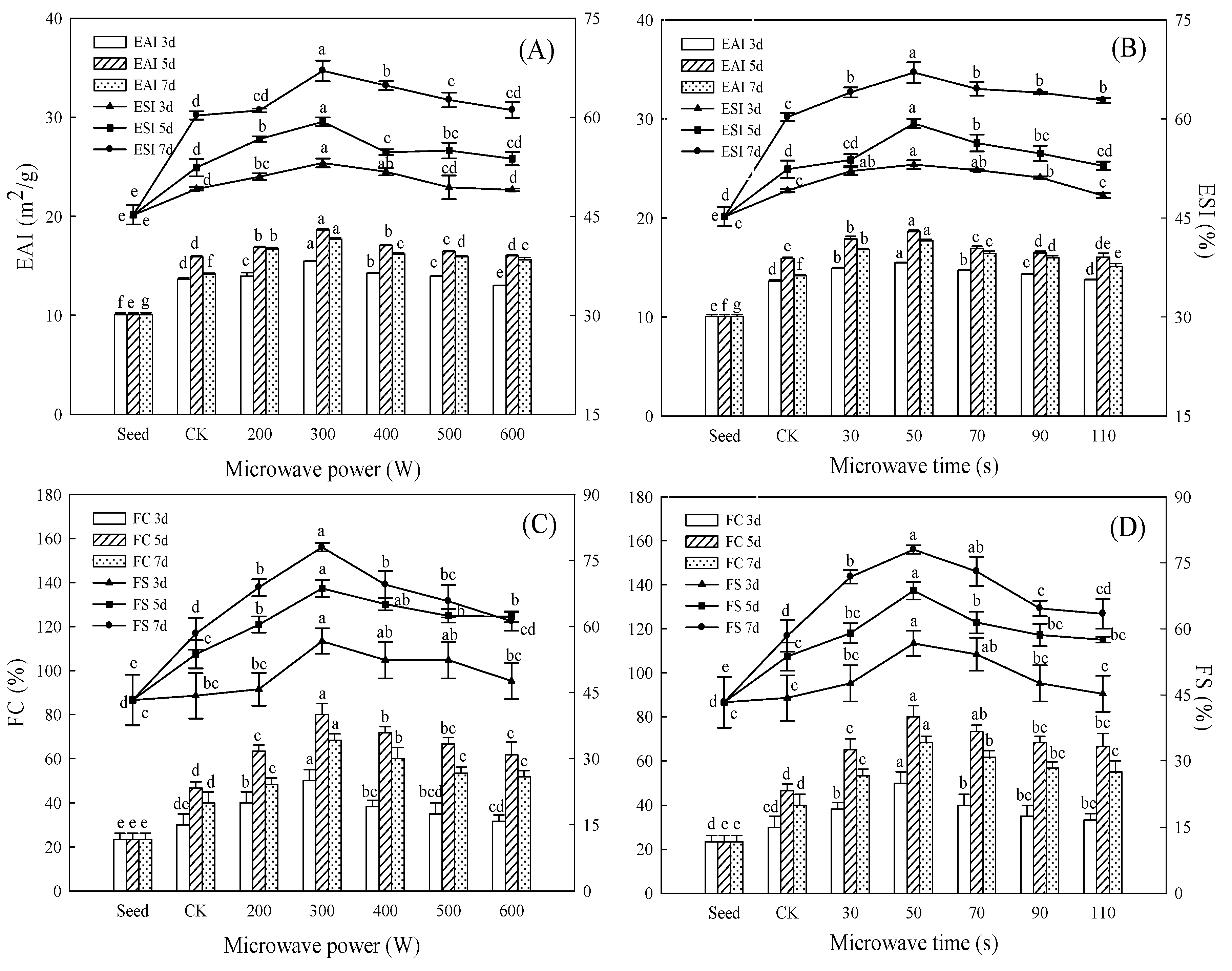
3.5. Effect of Microwave Treatment on Emulsifying Properties of Germinated TBP
3.6. Effect of Microwave Treatment on Foaming Properties of Germinated TBP
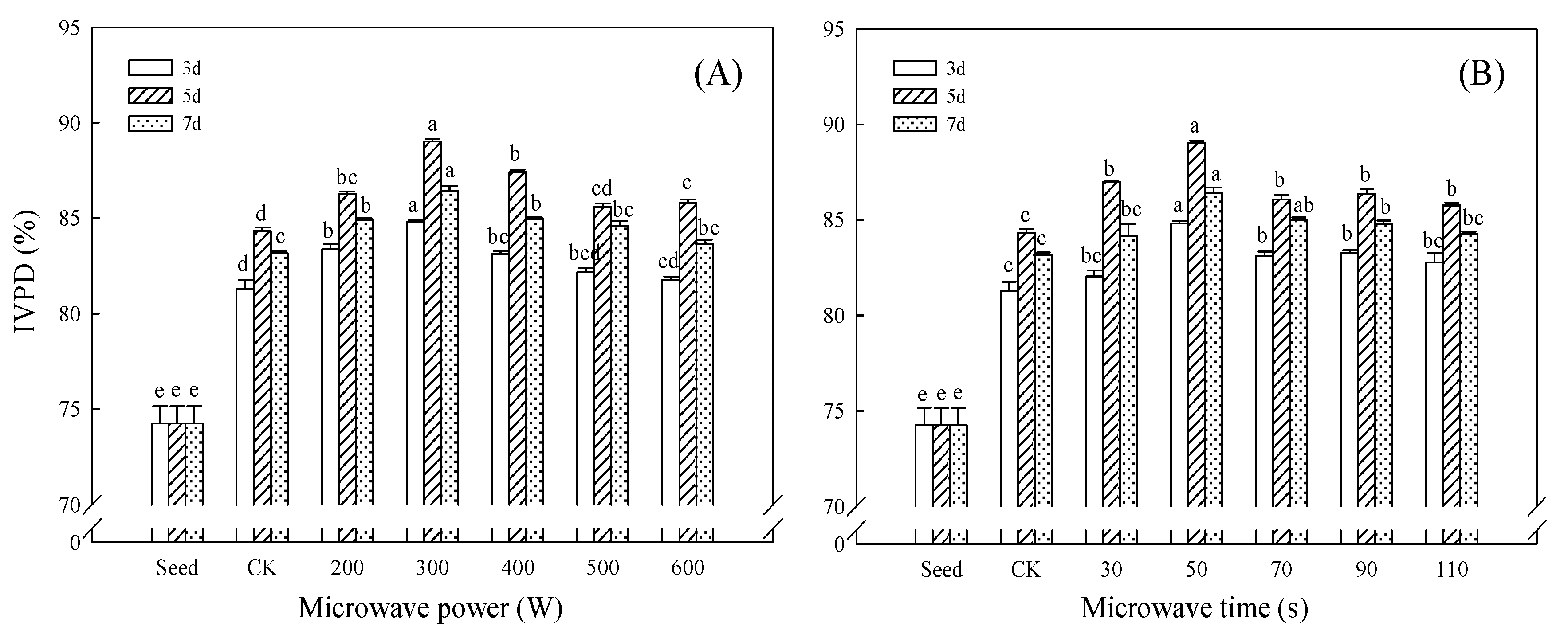
3.7. Effect of Microwave Treatment on IVPD of Germinated TBP
3.8. Effect of Microwave Treatment on Antioxidant Activities of Germinated TBP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glavač, N.K.; Stojilkovski, K.; Kreft, S.; Park, C.H.; Kreft, I. Determination of Fagopyrins, Rutin, and Quercetin in Tartary Buckwheat Products. LWT—Food Sci. Technol. 2017, 79, 423–427. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat Proteins and Peptides: Biological Functions and Food Applications. Trends Food Sci. Technol. 2021, 110, 155–167. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Du, L.N.; Li, Y.L.; Zhou, X.L.; Chen, Z.D. Effects of High Hydrostatic Pressure and Heat Treatment on Functional Properties of Buckwheat Protein. Food Sci. 2021, 42, 77–83. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Jiang, Y.; Shi, R.H.; Chen, Z.D.; Li, Z.J.; Wei, Y.; Zhou, X.L. Structural and Antioxidant Analysis of Tartary Buckwheat (Fagopyrum Tartaricum Gaertn.) 13S Globulin. J. Sci. Food Agric. 2020, 100, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Helal, N.A.S. Studies of Different Germination Media and Package on Squash and Snake Cucumber Sprouts. Sciences 2016, 6, 374–378. [Google Scholar]
- Singh, A.; Sharma, S.; Singh, B. Effect of Germination Time and Temperature on the Functionality and Protein Solubility of Sorghum Flour. J. Cereal Sci. 2017, 76, 131–139. [Google Scholar] [CrossRef]
- Edgar, V.; Antón, G.; Mario, V.; Roberto, M.; Iván, S.; Wilman, C. Antioxidant Purple Corn Protein Concentrate from Germinated Andean Purple Corn Seeds. Agronomy 2020, 10, 1282. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Singh, B. Characterization of in Vitro Antioxidant Activity, Bioactive Components, and Nutrient Digestibility in Pigeon Pea (Cajanus cajan) as Influenced by Germination Time and Temperature. J. Food Biochem. 2019, 43, e12706. [Google Scholar] [CrossRef]
- Wang, S.M.; Wang, J.F.; Guo, Y.B. Microwave Irradiation Enhances the Germination Rate of Tartary Buckwheat and Content of Some Compounds in Its Sprouts. Pol. J. Food Nutr. Sci. 2018, 68, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.D.; Cao, J.X.; Liu, L.L.; Zhou, X.W.; Barba, F.J. Characterizing Physicochemical, Nutritional and Quality Attributes of Wholegrain Oryza Sativa L. Subjected to High Intensity Ultrasound-Stimulated Pre-Germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Rifna, E.J.; Ramanan, K.R.; Mahendran, R. Emerging Technology Applications for Improving Seed Germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Wang, J.F.; Bian, Z.X.; Wang, S.M.; Zhang, L.X. Effects of Ultrasonic Waves, Microwaves, and Thermal Stress Treatment on the Germination of Tartary Buckwheat Seeds. J. Food Process Eng. 2020, 43, e13494. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.M.; Wang, S.M.; Wang, J.Z.; Peng, W.P. Effects of Microwave Irradiation on the Expression of Key Flavonoid Biosynthetic Enzyme Genes and the Accumulation of Flavonoid Products in Fagopyrum tataricum Sprouts. J. Cereal Sci. 2021, 101, 103275. [Google Scholar] [CrossRef]
- Hassan, S.; Ahmad, N.; Ahmad, T.; Imran, M.; Xu, C.M.; Khan, M.K. Microwave Processing Impact on the Phytochemicals of Sorghum Seeds as Food Ingredient. J. Food Process. Preserv. 2019, 43, e13924. [Google Scholar] [CrossRef]
- Almaiman, S.A.; Albadr, N.A.; Alsulaim, S.; Alhuthayli, H.F.; Osman, M.A.; Hassan, A.B. Effects of Microwave Heat Treatment on Fungal Growth, Functional Properties, Total Phenolic Content, and Antioxidant Activity of Sorghum (Sorghum bicolor L.) Grain. Food Chem. 2021, 348, 128979. [Google Scholar] [CrossRef]
- Deng, Y.; Padilla-Zakour, O.; Zhao, Y.Y.; Tao, S.S. Influences of High Hydrostatic Pressure, Microwave Heating, and Boiling on Chemical Compositions, Antinutritional Factors, Fatty Acids, in Vitro Protein Digestibility, and Microstructure of Buckwheat. Food Bioprocess Technol. 2015, 8, 2235–2245. [Google Scholar] [CrossRef]
- Sun, X.H.; Ohanenye, I.C.; Ahmed, T.; Udenigwe, C.C. Microwave Treatment Increased Protein Digestibility of Pigeon Pea (Cajanus cajan) Flour: Elucidation of Underlying Mechanisms. Food Chem. 2020, 329, 127196. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.M.; Zhu, H.Y.; He, Z.Y.; Liu, J.H.; Hao, W.J.; Jiao, R. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef]
- Hong, G.P.; Avramenko, N.; Stone, A.; Abbott, D.; Classen, H.; Nickerson, M. Extractability and Molecular Modifications of Gliadin and Glutenin Proteins Withdrawn from Different Stages of a Commercial Ethanol Fuel/Distillers Dried Grains with Solubles Process Using a Wheat Feedstock. Cereal Chem. 2012, 89, 276–283. [Google Scholar] [CrossRef]
- Tiwari, R.S.; Venkatachalam, M.; Sharma, G.M.; Su, M.; Roux, K.H.; Sathe, S.K. Effect of Food Matrix on Amandin, Almond (Prunus Dulcis L.) Major Protein, Immunorecognition and Recovery. LWT—Food Sci Technol. 2009, 43, 675–683. [Google Scholar] [CrossRef]
- Amza, T.; Balla, A.; Tounkara, F.; Man, L.; Zhou, H.M. Effect of Hydrolysis Time on Nutritional, Functional and Antioxidant Properties of Protein Hydrolysates Prepared from Gingerbread Plum (Neocarya macrophylla) Seeds. Int. Food Res. J. 2013, 20, 2081–2090. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Wet-Milled Chickpea Coproduct as an Alternative to Obtain Protein Isolates. LWT—Food Sci. Technol. 2019, 115, 108468. [Google Scholar] [CrossRef]
- Fan, D.X.; Li, J.J.; Yang, J.Q.; Shen, Q. Effects of heat treatments on the in vitro digestibility of millet protein. J. Chin. Inst. Food Sci. Technol. 2016, 16, 56–61. [Google Scholar] [CrossRef]
- Bian, Z.X.; Wang, J.F.; Ma, H.; Wang, S.M.; Luo, L.; Wang, S.M. Effect of Microwave Radiation on Antioxidant Capacities of Tartary Buckwheat Sprouts. J. Food Sci. Technol. MYS 2020, 57, 3913–3919. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G. Influence of High-Pressure Homogenization on Structural Properties and Enzymatic Hydrolysis of Milk Proteins. LWT—Food Sci. Techonl. 2020, 130, 109657. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Teng, F.; Zhang, S.; Qi, B.K.; Wu, C.L.; Tian, T.; Wang, Z.J.; Li, Y. Application of Ultrasound Treatment for Modulating the Structural, Functional and Rheological Properties of Black Bean Protein Isolates. Int. J. Food Sci. Technol. 2020, 55, 1637–1647. [Google Scholar] [CrossRef]
- Zeng, Q.; Hu, M.; Wang, H.; Zhong, M.M.; Qi, B.K.; Jiang, L.Z. Effect of pH treatment on structure, rheological properties and emulsifying properties of black bean protein isolate. Food Sci. 2020, 41, 15–21. [Google Scholar] [CrossRef]
- Han, C.W.; Ma, M.; Zhang, H.H.; Li, M.; Sun, Q.J. Progressive Study of the Effect of Superfine Green Tea, Soluble Tea, and Tea Polyphenols on the Physico-Chemical and Structural Properties of Wheat Gluten in Noodle System. Food Chem. 2020, 308, 125676. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.R.; Liu, J.N.; Chen, S.; Wang, X.D.; Wang, X.B.; Jiang, L.Z. Relationship between Surface Functional Properties and Flexibility of Soy Protein Isolate-Glucose Conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Huang, L.R.; Ding, X.N.; Li, Y.L.; Ma, H.L. The Aggregation, Structures and Emulsifying Properties of Soybean Protein Isolate Induced by Ultrasound and Acid. Food Chem. 2019, 279, 114–119. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.H.; Wang, S.J. Effect of Radio Frequency Treatment on Functional, Structural and Thermal Behaviors of Protein Isolates in Rice Bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Ren, Y.J.; Xie, W.; Zhou, D.Y.; Tang, S.R.; Kuang, M.; Wang, Y.Q.; Du, S.K. Physicochemical and Functional Properties of Protein Isolate Obtained from Cottonseed Meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zhou, X.F.; Zheng, Y.R.; Wang, D.F.; Deng, Y.; Zhao, Y.Y. Impact of Ultrasonication/Shear Emulsifying/Microwave-Assisted Enzymatic Extraction on Rheological, Structural, and Functional Properties of Akebia Trifoliata (Thunb.) Koidz. Seed Protein Isolates. Food Hydrocoll. 2021, 112, 106355. [Google Scholar] [CrossRef]
- Yu, C.P.; Wu, F.; Cha, Y.; Zou, H.N.; Bao, J.; Xu, R.N.; Du, M. Effects of High-Pressure Homogenization on Functional Properties and Structure of Mussel (Mytilus edulis) Myofibrillar Proteins. Int. J. Biol. Macromol. 2018, 118, 741–746. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S. Bioactive Components and Functional Properties of Biologically Activated Cereal Grains: A Bibliographic Review. Crit. Rev. Food Sci. 2017, 57, 3051–3071. [Google Scholar] [CrossRef]
- Liang, G.J.; Chen, W.P.; Qie, X.J.; Zeng, M.M.; Qin, F.; He, Z.Y.; Chen, J. Modification of Soy Protein Isolates Using Combined Pre-Heat Treatment and Controlled Enzymatic Hydrolysis for Improving Foaming Properties. Food Hydrocoll. 2020, 105, 105764. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Influence of Grain Germination on Functional Properties of Sorghum Flour. Food Chem. 2010, 121, 387–392. [Google Scholar] [CrossRef]
- Su, Q.Y. Effect of microwave treatment on functional properties of quinoa protein. Food Eng. 2021, 2, 33–37. [Google Scholar]
- Qu, W.J.; Zhang, X.X.; Han, X.; Wang, Z.P.; He, R.H.; Ma, H.L. Structure and Functional Characteristics of Rapeseed Protein Isolate-Dextran Conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B.; Kaur, G. In Vitro Nutrient Digestibility and Antioxidative Properties of Flour Prepared from Sorghum Germinated at Different Conditions. J. Food Sci. Technol. MYS 2019, 56, 3077–3089. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by Abts and Dpph Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Fei, Y.; Xu, Q.Z.; Lei, T.W.; Mo, X.C.; Wang, Z.T.; Zhang, L.L.; Mou, X.; Li, H.M. Isolation and Identification of Antioxidant Peptides from Tartary Buckwheat Albumin (Fagopyrum tataricum Gaertn.) and Their Antioxidant Activities. J. Food Sci. 2020, 85, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.X.; Wang, F.Z.; Yuan, L.; Liu, J.Y.; Sun, D.; Li, X.Y. Physicochemical Property, Antioxidant Activity, and Cytoprotective Effect of the Germinated Soybean Proteins. Food Sci. Nutr. 2019, 7, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbizo-Reyes, U.; Martin-González, M.F.S.; Garcia-Bravo, J.; Vigil, A.L.M.; Liceaga, A.M. Physicochemical Characteristics of Chia Seed (Salvia hispanica) Protein Hydrolysates Produced Using Ultrasonication Followed by Microwave-Assisted Hydrolysis. Food Hydrocoll. 2019, 97, 105187. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Moringa Oleifera Seed Protein Isolate and Enzymatic Hydrolysates. Heliyon 2018, 4, e00877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-J. Changes in the Antioxidant Properties of Rice Bran Protein Isolate Upon Simulated Gastrointestinal Digestion. LWT—Food Sci. Technol. 2020, 126, 109206. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods 2022, 11, 1373. https://doi.org/10.3390/foods11101373
Wang S, Xu X, Wang S, Wang J, Peng W. Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods. 2022; 11(10):1373. https://doi.org/10.3390/foods11101373
Chicago/Turabian StyleWang, Simeng, Xianmeng Xu, Shunmin Wang, Junzhen Wang, and Wenping Peng. 2022. "Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein" Foods 11, no. 10: 1373. https://doi.org/10.3390/foods11101373
APA StyleWang, S., Xu, X., Wang, S., Wang, J., & Peng, W. (2022). Effects of Microwave Treatment on Structure, Functional Properties and Antioxidant Activities of Germinated Tartary Buckwheat Protein. Foods, 11(10), 1373. https://doi.org/10.3390/foods11101373





