Comparison of Imitation Crab Sticks with Real Snow Crab (Chionoecetes opilio) Leg Meat Based on Physicochemical and Sensory Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Properties
2.2.1. Gel Strength
2.2.2. Texture Profile
2.2.3. Color
2.2.4. Scanning Electron Microscope (SEM)
2.2.5. Inverted Microscope
2.3. Chemical Properties
2.3.1. Proximate Composition
2.3.2. Sugars
2.3.3. Free Amino Acids
2.3.4. Nucleotides
2.3.5. Electronic Tongue
2.3.6. Solid-Phase Microextraction (SPME)–Gas Chromatography (GC)–Mass Spectrometry (MS) Analysis and Identification of Aromatic Compounds Using GC–Olfactometry Testing
2.3.7. Electronic Nose
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
3.2. Proximate Composition
3.3. Colors
3.4. Rheological Properties
3.5. Microstructure
3.6. Taste
3.7. Aroma
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, J.S.; Cho, W.J.; Jeong, E.J.; Cha, Y.J. Change in volatile compounds in red snow crab Chionoecetes japonicus Cooker effluent during concentration. Fish Aquat. Sci. 2006, 39, 437–440. [Google Scholar]
- Jin, S.K.; Kim, I.S.; Choi, Y.J.; Kim, B.G.; Hur, S.J. The development of imitation crab stick containing chicken breast surimi. Lwt-Food Sci. Technol. 2009, 42, 150–156. [Google Scholar] [CrossRef]
- Park, J.W. Surimi seafood: Products, market, and manufacturing. In Surimi and Surimi Seafood; Park, J.W., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 201–236. [Google Scholar]
- Park, J.W.; Beliveau, J.L. Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 245–270. [Google Scholar]
- Hema, K.; Velayutham, P.; Mohan, C.O.; Sukumar, D.; Sundaramoorthy, B.; Athithan, S.; Kumar, K.A. Innovative studies on “Analogue shrimp products” from lizard fish using 3d printing. Ind. J. Anim. Res. 2020, 54, 918–923. [Google Scholar] [CrossRef]
- Vidal-Giraud, B.; Chateau, D. World surimi market. Globefish Res. Program. 2007, 89, 1–128. [Google Scholar]
- Bashir, K.M.I.; Kim, J.S.; An, J.H.; Sohn, J.H.; Choi, J.S. Natural food additives and preservatives for fish-paste products: A review of the past, present, and future states of research. J. Food Qual. 2017, 2017, 9675469. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Lee, J.; Kim, D.S.; Cho, J.; Hong, S.J.; Pan, J.H.; Kim, J.K.; Shin, E.C. Perilla frutescens Britton: A comprehensive study on flavor/taste and chemical properties during the roasting process. Molecules 2019, 24, 1374. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Kim, H.S.; Lee, J.; Pan, J.H.; Kim, Y.J.; Kim, J.K.; Woo, S.; Shin, E.C. Wasabia Koreana Nakai: A preliminary study on nutrients and chemical compounds that may impact sensory properties. Molecules 2018, 23, 2512. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.E.; Andrews, L.S.; Coggins, P.C.; Wes Schilling, M.; Marshall, D.L. Microbial quality, safety, and sensory acceptability of X-ray treated fresh channel catfish fillets. J. Aquat. Food Prod. Technol. 2009, 18, 299–311. [Google Scholar] [CrossRef]
- Konosu, S.; Yamaguchi, K.; Hayashi, T. Studies on flavor components in boiled crabs-I, amino acids and related compounds in the extracts. Bull. Jpn. Soc. Sci. Flsh. 1978, 44, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.B.; Park, J.W.; Kim, B.Y. Surimi-starch interactions based on mixture design and regression models. J. Food Sci. 1997, 62, 555–560. [Google Scholar] [CrossRef]
- Park, J.W.; Lanier, T.C. Processing of surimi and surimi seafood. In Marine and Freshwater Products Handbook.; Martin, R.E., Ed.; Technomic Publishing Company: Lancaster, NH, USA, 2000; pp. 417–445. [Google Scholar]
- Liang, F.; Lin, L.; He, T.; Zhou, X.; Jiang, S.; Lu, J. Effect of transglutaminase on gel properties of surimi and precocious Chinese mitten crab (Eriocheir sinensis) meat. Food Hydrocoll. 2020, 98, 105261. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Pan, D.; Bu, G. Gel properties of surimi from silver carp (Hypophthalmichthys molitrix) as affected by heat treatment and soy protein isolate. Food Hydrocoll. 2008, 22, 1513–1519. [Google Scholar] [CrossRef]
- Kim, B.Y.; Park, J.W.; Yoon, W.B. Rheology and texture properties of surimi gels. In Surimi and Surimi Seafood, 2nd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 491–582. [Google Scholar]
- Huang, J.; Bakry, A.M.; Zeng, S.; Xiong, S.; Yin, T.; You, J.; Huang, Q. Effect of phosphates on gelling characteristics and water mobility of myofibrillar protein from grass carp (Ctenopharyngodon idellus). Food Chem. 2019, 272, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S. Impact of salted duck egg albumen powder on proteolysis and gelling properties of sardine surimi. J. Text. Stud. 2019, 50, 434–442. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S. Compositions, protease inhibitor and gelling property of duck egg albumen as affected by salting. Korean J. Food Sci. Anim. Res. 2018, 38, 14. [Google Scholar]
- Auh, J.H.; Lee, H.G.; Kim, J.W.; Kim, J.C.; Yoon, H.S.; Park, K.H. Highly concentrated branched oligosaccharides as cryoprotectant for surimi. J. Food Sci. 1999, 64, 418–422. [Google Scholar] [CrossRef]
- Wu, S. Effect of pullulan on gel properties of Scomberomorus niphonius surimi. Int. J. Biol. Macromol. 2016, 93, 1118–1120. [Google Scholar] [CrossRef]
- Perry, M.J.; Tait, J.; Hu, J.; White, S.C.; Medler, S. Skeletal muscle fiber types in the ghost crab, Ocypode quadrata: Implications for running performance. J. Exp. Biol. 2009, 212, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Wei, W.; Hu, W.; Zhang, X.Y.; Zhang, F.P.; Sun, S.Q.; Liu, Y.; Xu, C.H. Analysis of protein structure changes and quality regulation of surimi during gelation based on infrared spectroscopy and microscopic imaging. Sci. Rep. 2018, 8, 5566. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, D.S.; Kim, S.I.; Kim, D.S.; Pyeun, J.H. Nitrogenous constituents in the extract of crabs caught in the Korean adjacent sea. Korean J. Soc. Food Sci. 1996, 12, 469–480. [Google Scholar]
- Oh, K.S.; Sung, D.W.; Choi, J.D.; Lee, E.H. Changes in Food Components of Dark, White-Fleshed Fishes by Retort Sterilization Processing-1. Changes in Nitrogenous Extractives and Textures. Fish Aquat. Sci. 1991, 24, 123–129. [Google Scholar]
- Daniels, D.H.; Joe, F.L., Jr.; Diachenko, G.W. Determination of free glutamic acid in a variety of foods by high-performance liquid chromatography. Food Addit. Contam. 1995, 12, 21–29. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, K.T.; Lee, S. Taste profile characterization of white ginseng by electronic tongue analysis. Afr. J. Biotechnol. 2012, 11, 9280–9287. [Google Scholar]
- Xiao, H.; Wang, J. Discrimination of Xihulongjing tea grade using and electronic tongue. Afr. J. Biotechnol. 2009, 8, 6985–6992. [Google Scholar]
- He, W.; Hu, X.; Zhao, L.; Liao, X.; Zhang, Y.; Zhang, M.; Wu, J. Evaluation of Chinese tea by the electronic tongue: Correlation with sensory properties and classification according to geographical origin and grade level. Food Res. Int. 2009, 42, 1462–1467. [Google Scholar] [CrossRef]
- Cha, Y.J.; Cadwallader, K.R. Aroma-active compounds in skipjack tuna sauce. J. Agric. Food Chem. 1998, 46, 1123–1128. [Google Scholar] [CrossRef]
- Cha, Y.J.; Cho, W.J.; Jeong, E.J. Comparison of volatile flavor compounds in meat of the blue crab using V-SDE and SPME methods. J. Korean Soc. Fish. 2006, 39, 441–446. [Google Scholar]
- Hayashi, T.; Ishii, H.; Shinohara, A. Novel model experiment of cooking flavor research on crab leg meat. Food Rev. Int. 1990, 6, 521–536. [Google Scholar] [CrossRef]
- Cha, Y.J.; Cadwallader, K.R.; Baek, H.H. Volatile flavor components in snow crab cooker effluent and effluent concentrate. J. Food Sci. 1993, 58, 525–530. [Google Scholar] [CrossRef]
- Chung, H.Y. Volatile Components in Crabmeats of Charybdis feriatus. J. Agric. Food Chem. 1999, 47, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
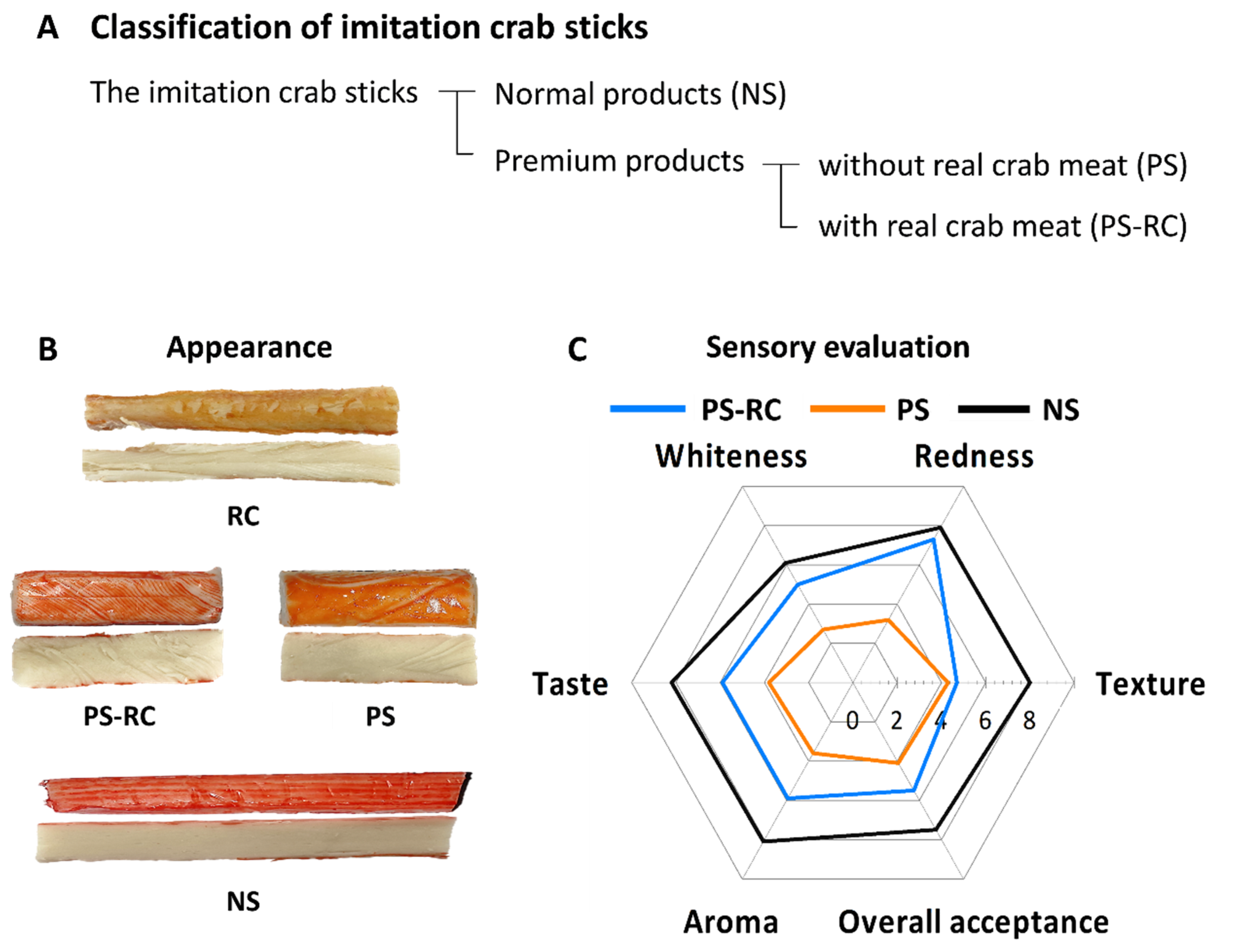





| RC | PS-RC | PS | NS | |
|---|---|---|---|---|
| Proximate Composition (g/100 g) | ||||
| Moisture | 78.9 ± 0.1 d | 73.3 ± 0.2 b | 74.6 ± 0.5 c | 67.4 ± 0.1 a |
| Carbohydrate | 0.5 ± 0.1 a | 12.7 ± 0.1 c | 10.7 ± 0.5 b | 23.3 ± 0.3 d |
| Protein | 17.8 ± 0.3 d | 11.1 ± 0.1 c | 10.7 ± 0.2 b | 6.3 ± 0.1 a |
| Lipid | 1.2 ± 0.2 d | 0.16 ± 0.1 a | 0.9 ± 0.1 c | 0.8 ± 0.2 b |
| Ash | 1.6 ± 0.1 a | 2.8 ± 0.1 c | 3.2 ± 0.1 d | 2.3 ± 0.1 b |
| RC | PS-RC | PS | NS | |
|---|---|---|---|---|
| Outside | ||||
| L* | 67.2 ± 6.8 a | 67.5 ± 3.6 a | 73.7 ± 7.0 a | 63.5 ± 0.7 a |
| a* | 10.8 ± 8.5 a | 24.6 ± 4.5 bc | 17.6 ± 7.0 ab | 31.7 ± 2.6 c |
| b* | 15.4 ± 3.8 a | 21.4 ± 4.6 a | 21.1 ± 5.1 a | 21.2 ± 1.5 a |
| Inside | ||||
| Whiteness | 78.9 ± 0.8 d | 71.1 ± 0.8 c | 66.5 ± 2.1 b | 58.5 ± 0.7 a |
| RC | PS-RC | PS | NS | |
|---|---|---|---|---|
| Total Sugar (g/100 g) | ||||
| Sucrose | ND | 2.5 ± 0.1 | 4.4 ± 0.1 | 2.4 ± 0.1 |
| Maltose | ND | ND | ND | ND |
| Glucose | ND | ND | ND | ND |
| Fructose | ND | ND | ND | ND |
| Lactose | ND | ND | ND | ND |
| Nucleotides (g/100 g) | ||||
| AMP | 24.0 ± 1.4 | ND | ND | ND |
| IMP | ND | 11.9 ± 0.2 | 6.9 ± 0.1 | 1.6 ± 0.1 |
| GMP | ND | 5.1 ± 0.1 | 2.6 ± 0.1 | ND |
| Free Amino Acids | Contents (mg/100 g) | Taste Attribute | Taste Threshold (mg/100 mL) | Taste Activity Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RC | PS-RC | PS | NS | PS | PS-RC | PS | NS | |||
| Aspartic acid | 6.7 ± 0.9 a | 0.6 b | 1.1 ± 0.2 b | 0.5 b | Umami (+) | 100 | 0.1 | 0.01 | 0.01 | 0.01 |
| Glutamic acid | 24.2 ± 1.4 a | 192.3 ± 23.2 ab | 189.1 ± 175.6 ab | 257 ± 47.2 c | Umami (+) | 30 | 0.8 | 6.4 | 6.3 | 8.6 |
| Glycine | 418.5 ± 99.2 a | 408.03 ± 31.2 a | 240.9 ± 13.5 a | 306.8 ± 155.6 a | Sweet (+) | 130 | 3.2 | 3.2 | 1.9 | 2.4 |
| Alanine | 74.9 ± 12.3 a | ND b | 4.1 ± 3.6 b | ND b | Sweet (+) | 60 | 1.2 | ND | 0.1 | ND |
| Threonine | 2.6 ± 0.6 a | 0.6 ± 0.2 b | 0.7 ± 0.1 b | 0.3 b | Sweet (+) | 260 | ND | ND | ND | ND |
| Serine | 5.8 ± 1.6 a | 0.5 ± 0.2 b | 0.7 ± 0.2 b | 0.3 ± 0.1 b | Sweet (+) | 150 | 0.04 | ND | ND | ND |
| Valine | 14.2 ± 1.7 a | 2.4 ± 1.5 b | 1.7 ± 0.2 b | 1.9 ± 1.4 b | Sweet/bitter (−) | 40 | 0.4 | 0.1 | 0.04 | 0.1 |
| Proline | 52.9 ± 9.6 a | ND b | 0.5 ± 0.9 b | ND b | Sweet/bitter (+) | 300 | 0.2 | ND | ND | ND |
| Lysine | 4.6 ± 0.4 a | 2.4 ± 0.4 b | 2.0 ± 0.1 b | 0.7 ± 0.2 c | Sweet/bitter (−) | 50 | 0.1 | 0.1 | 0.04 | 0.01 |
| Isoleucine | 5.2 ± 1.0 a | 0.5 ± 0.1 b | 0.5 ± 0.1 b | 0.3 ± 0.1 b | Bitter (−) | 90 | 0.1 | 0.01 | 0.01- | ND |
| Leucine | 4.5 ± 0.3 a | 1.0 ± 0.1 b | 1.1 ± 0.1 b | 0.7 ± 0.1 c | Bitter (−) | 190 | 0.02 | 0.01 | 0.01 | ND |
| Phenylalanine | 3.1 ± 0.9 a | 0.3 ± 0.5 b | 0.2 ± 0.4 b | 0.2 ± 0.4 b | Bitter (−) | 90 | 0.03 | ND | ND | ND |
| Histidine | 3.8 ± 1.1 a | 0.6 ± 0.3 b | ND b | 0.7 ± 0.1 b | Bitter (−) | 20 | 0.2 | 0.03 | ND | ND |
| Arginine | 498 ± 34.7 a | 1.6 ± 0.6 b | 1.1 ± 0.1 b | 0.8 ± 0.1 b | Bitter/sweet (+) | 50 | 10.0 | 0.03 | 0.02 | 0.02 |
| Methionine | 13.1 ± 7.4 a | 0.9 ± 0.3 b | 0.8 ± 0.1 b | 0.8 ± 0.3 b | Bitter/sweet/sulfurous (−) | 30 | 0.4 | 0.03 | 0.03 | 0.03 |
| Compounds | RT(1) | RI(2) | Sensory Description | RC | PS-RC | PS | NS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MXT-5 | MXT-1701 | MXT-5 | MXT-1701 | MXT-5 | MXT-1701 | MXT-5 | MXT-1701 | MXT-5 | MXT-1701 | MXT-5 | MXT-1701 | ||
| Butane | 14.34 | -(3) | 377 | - | Faint | 0.07 ± 0.00 a | - | 0.12 ± 0.03 a | - | 0.12 ± 0.01 a | - | 0.10 ± 0.02 a | - |
| Ethanol | 17.80 | - | 444 | - | Alcoholic, pungent, sweet | 1.01 ± 0.06 c | - | 90.41 ± 8.61 b | - | 95.81 ± 1.88 b | - | 315.83 ± 23.47 a | - |
| Pentane | 19.34 | - | 474 | - | Alkane, gasoline | 0.59 ± 0.26 c | - | 4.77 ± 0.25 a | - | 2.05 ± 0.39 b | - | 2.82 ± 0.36 b | - |
| 2-Propanol | 20.76 | - | 502 | - | Acetone, alcoholic, pleasant | 2.52 ± 0.13 c | - | 82.83 ± 16.31 a | - | 18.20 ± 4.34 bc | - | 45.13 ± 25.78 ab | - |
| 1-Propanol | 23.60 | - | 556 | - | Alcoholic, fruity, pungent | 0.18 ± 0.04 c | - | 2.12 ± 0.13 a | - | 1.63 ± 0.18 b | - | 1.38 ± 0.07 b | - |
| 2-methylfuran | 26.00 | - | 601 | - | Acetone, burnt, chocolate, gassy | 2.67 ± 0.08 b | - | 4.91 ± 0.28 ab | - | 9.17 ± 3.98 a | - | 1.89 ± 0.28 b | - |
| Trichloroethane | 33.26 | - | 665 | - | Chloroform, ethereal, mild, sweet | 0.12 ± 0.01 c | - | 0.94 ± 0.07 a | - | 0.65 ± 0.13 ab | - | 0.42 ± 0.21 bc | - |
| Pyridine | 44.82 | - | 750 | - | Amine, burnt, fishy, pungent | 0.29 ± 0.05 b | - | 0.62 ± 0.08 a | - | 0.80 ± 0.11 a | - | 0.55 ± 0.19 ab | - |
| 1-Chloropentane | 48.14 | - | 772 | - | Green plant, sweet | 0.09 ± 0.00 d | - | 0.93 ± 0.09 b | - | 1.57 ± 0.18 a | - | 0.36 ± 0.04 c | - |
| 2.4-Octadiene | 55.15 | - | 816 | - | Glue, warm | 0.16 ± 0.06 a | - | 0.64 ± 0.11 a | - | 0.64 ± 0.21 a | - | 0.78 ± 0.67 a | - |
| Methional | 69.87 | - | 903 | - | Baked potato, creamy, tomato, vegetable | 0.16 ± 0.02 a | - | 0.19 ± 0.04 a | - | 0.40 ± 0.01 a | - | 1.10 ± 1.35 a | - |
| 3-methyl-3-sulfanylbutanol-1-ol | 83.44 | - | 983 | - | Broth, chervil, meaty, sweet, vegetable | 0.06 ± 0.01 a | - | 0.07 ± 0.00 a | - | 0.09 ± 0.00 a | - | 0.15 ± 0.08 a | - |
| 2,4-Heptadienal,(E,E)- | 89.33 | - | 1018 | - | Aldehydic, cinnamon, fatty, green | 0.08 ± 0.00 a | - | 0.12 ± 0.04 a | - | 0.07 ± 0.01 a | - | 0.54 ± 0.78 a | - |
| Decanal | 119.10 | 126.88 | 1203 | 1308 | Aldehydic, burnt, citrus, fatty, floral | 0.10 ± 0.03 b | 0.19 ± 0.02 a | 0.18 ± 0.02 b | 0.20 ± 0.04 a | 0.19 ± 0.09 b | 0.21 ± 0.08 a | 0.55 ± 0.12 a | 0.36 ± 0.29 a |
| Alpha-Terpinen-7-al | 131.18 | - | 1284 | - | Fatty, spicy | 0.13 ± 0.01 a | - | 0.13 ± 0.03 a | - | 0.12 ± 0.02 a | - | 0.13 ± 0.02 a | - |
| Tridecane | 133.71 | - | 1302 | - | Alkane, citrus, fruity, hydrocarbon | 0.13 ± 0.04 a | - | 0.14 ± 0.03 a | - | 0.16 ± 0.07 a | - | 0.26 ± 0.23 a | - |
| Alpha-Selinene | 163.42 | - | 1523 | - | Amber, orange, pepper | 0.23 ± 0.04 a | - | 0.24 ± 0.03 a | - | 0.20 ± 0.05 a | - | 0.23 ± 0.02 a | - |
| Atraton | 189.74 | - | 1736 | - | Aldehydic, fatty, spicy | 0.09 ± 0.00 a | - | 0.09 ± 0.02 a | - | 0.09 ± 0.01 a | - | 0.08 ± 0.01 a | - |
| Perfluorononane | - | 14.74 | - | 407 | - | - | 0.10 ± 0.01 b | - | 0.13 ± 0.02 a | - | 0.11 ± 0.00 ab | - | 0.11 ± 0.01 ab |
| Acetaldehyde | - | 17.89 | - | 473 | Aldehydic, ethereal, fresh, fruity | - | 0.15 ± 0.01 d | - | 0.84 ± 0.04 c | - | 1.12 ± 0.08 b | - | 1.91 ± 0.08 a |
| Methyl formate | - | 18.79 | - | 491 | Agreeable, fruity, plum | - | 0.42 ± 0.05 c | - | 3.86 ± 0.03 a | - | 1.25 ± 0.44 b | - | 1.01 ± 0.34 bc |
| Hexane | - | 24.12 | - | 601 | Alkane, ethereal, gasoline | - | 2.71 ± 0.11 b | - | 6.63 ± 0.33 ab | - | 9.82 ± 4.10 a | - | 1.93 ± 0.11 b |
| Methylcyclopentane | - | 27.37 | - | 635 | Gasoline | - | 0.09 ± 0.02 b | - | 4.10 ± 0.30 a | - | 4.36 ± 0.83 a | - | 0.32 ± 0.10 b |
| Cyclohexane | - | 31.82 | - | 681 | Chloroform | - | 0.09 ± 0.01 d | - | 8.14 ± 0.83 a | - | 5.12 ± 0.57 b | - | 2.13 ± 0.12 c |
| Pyridine,2-pentyl- | - | 84.25 | - | 1029 | Fatty, green pepper, mushroom, tallowy | - | 0.14 ± 0.01 a | - | 0.15 ± 0.01 a | - | 0.14 ± 0.02 a | - | 0.94 ± 1.38 a |
| 2-Octanone | - | 95.56 | - | 1100 | Apple, cheese, fatty, fruity | - | 0.08 ± 0.04 a | - | 0.25 ± 0.02 a | - | 0.15 ± 0.02 a | - | 0.23 ± 0.18 a |
| 3-Octanol | - | 97.86 | - | 1114 | Citrus, nutty, mushroom, herbaceous | - | 0.07 ± 0.01 a | - | 0.08 ± 0.01 a | - | 0.07 ± 0.03 a | - | 0.54 ± 0.82 a |
| 5-Methylfurfural | - | 103.64 | - | 1152 | Acidic, almond, burnt sugar, caramelized | - | 0.07 ± 0.01 a | - | 0.07 ± 0.01 a | - | 0.09 ± 0.03 a | - | 0.28 ± 0.32 a |
| 2-Butyloctanol | - | 114.95 | - | 1226 | - | - | 0.14 ± 0.07 a | - | 0.17 ± 0.02 a | - | 0.22 ± 0.05 a | - | 0.35 ± 0.24 a |
| 8-Methyl pentadecane | - | 156.99 | - | 1535 | - | - | 0.25 ± 0.02 a | - | 0.23 ± 0.00 a | - | 0.24 ± 0.02 a | - | 0.36 ± 0.26 a |
| Methyl tetradecanoate | - | 192.17 | - | 1820 | Coconut, cognac, fatty, oily, orris | - | 0.12 ± 0.02 a | - | 0.10 ± 0.01 a | - | 0.10 ± 0.01 a | - | 0.27 ± 0.33 a |
| Tridecyl propanoate | - | 199.15 | - | 1876 | - | - | 1.07 ± 0.14 a | - | 1.13 ± 0.18 a | - | 1.11 ± 0.20 a | - | 1.13 ± 0.08 a |
| Volatile Compounds | RT(1) (min) | RI(2) | Content (μg/100 g) | Odor Intensity | Odor Description | ID(3) | |||
|---|---|---|---|---|---|---|---|---|---|
| RC | PS-RC | PS | NS | ||||||
| Acids (4) | |||||||||
| Oxalic acid isobutyl-nonyl ester | 16.85 | 1078 | 0.12 ± 0.18 | ND | ND | ND(4) | 1 | Crab stick | MS |
| Ethyl octanoate | 20.82 | 1212 | ND | 0.32 ± 0.06 | 0.11 ± 0.16 | 3.68 ± 0.43 | MS/RI | ||
| 2-Ethylhexyl-oxalic acid | 28.57 | 1511 | ND | ND | 0.12 ± 0.17 | ND | MS | ||
| 2,5-Dimethyl benzoic acid | 32.45 | 1678 | ND | 0.06 ± 0.09 | ND | ND | MS | ||
| Hydrocarbons (8) | |||||||||
| Trimethylamine | 3.04 | <800 | 0.21 ± 0.30 | ND | ND | ND | 1 | Fish smell | MS/RI |
| 2,6-Dimethyl-octane | 16.27 | 1061 | 0.23 ± 0.32 | ND | ND | ND | MS | ||
| Trimethyldecane | 16.60 | 1071 | 0.12 ± 0.17 | ND | ND | ND | MS | ||
| Triethyl-phosphate | 18.86 | 1145 | ND | 0.17 ± 0.03 | ND | ND | MS | ||
| 2,3,4-Trimethyl-1-butene | 25.31 | 1379 | 0.29 ± 0.01 | ND | ND | ND | 3 | Real crab | MS |
| Tetradecane | 26.15 | 1412 | ND | ND | ND | 0.13 ± 0.01 | MS/RI | ||
| Eicosane | 28.45 | 1505 | ND | ND | ND | 3.52 ± 4.52 | MS/RI | ||
| Pristane | 33.19 | <1700 | ND | ND | ND | 0.18 ± 0.26 | MS | ||
| Heterocyclic Compound (1) | |||||||||
| Butylated hydroxytoluene | 28.92 | 1526 | 0.68 ± 0.97 | ND | ND | ND | MS | ||
| Ketones (2) | |||||||||
| 2,6-Dimethyl-4-heptanone | 14.04 | 989 | ND | 0.34 ± 0.02 | ND | ND | MS | ||
| Benzophenone | 31.7 | 1645 | ND | ND | 0.30 ± 0.01 | ND | MS | ||
| Total | 1.66 ± 1.33 | 0.83 ± 0.08 | 0.60 ± 0.23 | 7.52 ± 4.34 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.; Shin, E.-C.; Kim, S.; Park, J.; Jeong, C.; Boo, C.-G.; Yu, D.; Sim, J.-H.; Ji, C.-I.; Nam, T.-J.; et al. Comparison of Imitation Crab Sticks with Real Snow Crab (Chionoecetes opilio) Leg Meat Based on Physicochemical and Sensory Characteristics. Foods 2022, 11, 1381. https://doi.org/10.3390/foods11101381
Mun S, Shin E-C, Kim S, Park J, Jeong C, Boo C-G, Yu D, Sim J-H, Ji C-I, Nam T-J, et al. Comparison of Imitation Crab Sticks with Real Snow Crab (Chionoecetes opilio) Leg Meat Based on Physicochemical and Sensory Characteristics. Foods. 2022; 11(10):1381. https://doi.org/10.3390/foods11101381
Chicago/Turabian StyleMun, Sohyun, Eui-Cheol Shin, Seonghui Kim, Joodong Park, Chungeun Jeong, Chang-Guk Boo, Daeung Yu, Jin-Ha Sim, Cheong-Il Ji, Taek-Jeong Nam, and et al. 2022. "Comparison of Imitation Crab Sticks with Real Snow Crab (Chionoecetes opilio) Leg Meat Based on Physicochemical and Sensory Characteristics" Foods 11, no. 10: 1381. https://doi.org/10.3390/foods11101381
APA StyleMun, S., Shin, E.-C., Kim, S., Park, J., Jeong, C., Boo, C.-G., Yu, D., Sim, J.-H., Ji, C.-I., Nam, T.-J., & Cho, S. (2022). Comparison of Imitation Crab Sticks with Real Snow Crab (Chionoecetes opilio) Leg Meat Based on Physicochemical and Sensory Characteristics. Foods, 11(10), 1381. https://doi.org/10.3390/foods11101381








