Estimation of Carcass Tissue Composition from the Neck and Shoulder Composition in Growing Blackbelly Male Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Animals
2.2. Data Analyses
2.3. Model Validation
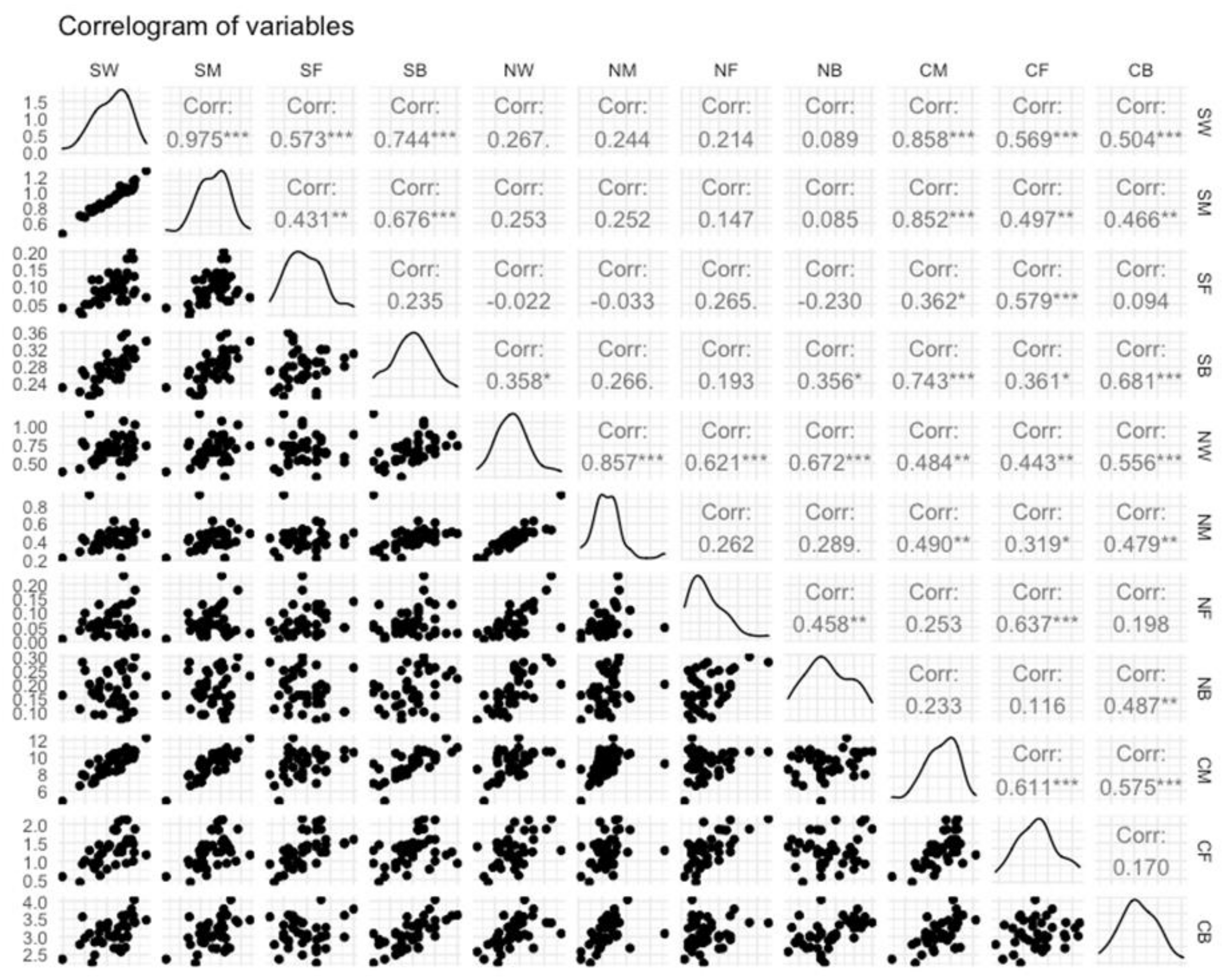
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, M.B.; Neves, M.L.M.W.; Barreto, L.M.G.; Ferreira, M.D.A.; Monnerat, J.P.I.D.S.; Carone, G.M.; Veras, A.S.C. Prediction of carcass composition through measurements in vivo and measurements of the carcass of growing Santa Ines sheep. PLoS ONE 2021, 3, e0247950. [Google Scholar] [CrossRef]
- Rivera-Alegria, F.M.; Ríos-Rincón, F.G.; Macías-Cruz, U.; García-Herrera, R.A.; Herrera-Camacho, J.; Benaouda, M.; Angeles-Hernández, J.C.; Muñoz-Benítez, A.L.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Prediction of carcase characteristics using neck traits from hair-sheep ewes. Ital. J. Anim. Sci. 2022, 21, 106–112. [Google Scholar] [CrossRef]
- Silva, S.R.; Cadavez, V.P. Real-time ultrasound (RTU) imaging methods for quality control of meats. In Computer Vision Technology in the Food and Beverage Industries; Da-Wen, S., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar]
- Civit, D.; Díaz, M.D.; Rodríguez, E.; González, C.A. Características de la canal y efecto de la maduración sobre la calidad de la carne de ovejas de desvieje de raza Corriedale. ITEA-Inf. Tec. Econ. Agrar. 2014, 110, 160–170. [Google Scholar] [CrossRef]
- Gallo, L.; Sturaro, E.; Bittante, G. Body traits, carcass characteristics and price of cull cows as affected by farm type, breed, age and calving to culling interval. Animal 2016, 11, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, L.O. Relationships of retained energy and retained protein that influence the determination of cattle requirements of energy and protein using the California Net Energy System. Transl. Anim. Sci. 2019, 3, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.S.; Chizzotti, M.L.; Busato, K.C.; Rodrigues, R.T.S.; Silva, I.F.; Queiroz, M.A.A. Indirec methods for predicting body composition of Boer crossbreds and indigenous goats form Brazilian semiarid. Trop. Anim. Health Prod. 2015, 47, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, B.; Baygul, O.; Yalcintan, H.; Ozcan, M. Comparison of the decision tree, artificial neural network and multiple regression methods for prediction of carcass tissues composition of goat kids. Meat Sci. 2020, 161, 108011. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Diaz, E.; Mezo-Solis, J.A.; Herrera-Camacho, J.; Cruz-Hernández, A.; Gomez-Vázquez, A.; Tedeschi, L.O.; Lee-Rangel, H.A.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Prediction of carcass traits of hair sheep lambs using body measurements. Animals 2020, 10, 1276. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Clemente, S.; Vázquez-Jiménez, S.; Saravasti, K.; López-Durán, S.; Arcos-Alvarez, D.N.; Arbez-Abnal, T.A.; Piñeiro-Vázquez, A.T.; Muñoz-Benítez, A.L.; Vasgas-Bello-Pérez, E.; Chay-Canul, A.J. Using the 9th–11th rib section to predict carcase tissue composition in Blackbelly sheep. Ital. J. Anim. Sci. 2022, 21, 161–167. [Google Scholar] [CrossRef]
- Argüello, A.; Capote, J.; Ginés, R.; López, J.L. Prediction of kid carcass composition by use of joint dissection. Livest. Prod. Sci. 2001, 67, 293–295. [Google Scholar] [CrossRef]
- Lauces, M.L.; Calvo, C.; Fernández, B.; Fernández, A.; Viana, J.L.; Sánchez, L. Predicting equations for tisular composition in carcass of gallega breed lambs. Arch. Zootec. 2008, 57, 3–14. [Google Scholar]
- Santos, V.A.C.; Silvestre, A.M.; Azevedo, J.M.T.; Silva, S.R. Estimation of carcase composition of goat kids from joint dissection and conformation measurements. Ital. J. Anim. Sci. 2017, 16, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A. Barbados Blackbelly: The Caribbean ovine genetic resource. Trop. Anim. Health Prod. 2017, 2, 239–250. [Google Scholar] [CrossRef]
- Chay-Canul, A.J.; Pineda-Rodríguez, J.J.; Olivares-Perez, J.; Rios-Rincon, F.G.; García-Herrera, R.A.; Piñeiro-Vázquez, A.T.; Casanova-Lugo, F. Prediction of carcass characteristics of discarded Pelibuey ewes by ultrasound measurements. Revista Mexicana de Ciencias Pecuarias 2019, 10, 473–481. [Google Scholar] [CrossRef]
- Yildirim, A.; Ulutas, Z.; Ocak, N.; Sirin, E.; Aksoy, Y. A study on gastrointestinal tract characteristics of ram lambs at the same weights from six Turkish sheep breeds. S. Afr. J. Anim. Sci. 2014, 44, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Skapetas, B.; Sinapis, E.; Hatziminaouglou, J.; Karalazos, A.; Katanos, J. Effect of age at slaughter on carcass characteristics and carcass composition in lambs of mountain Greek breeds. Czech J. Anim. Sci. 2006, 51, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Chay-Canul, A.J.; Magaña-Monforte, J.G.; Chizzotti, M.L.; Piñeiro-Vázquez, A.T.; Canul-Solis, J.R.; Ayala-Burgos, A.J.; Ku-Vera, J.C.; Tedeschi, L.O. Energy requirements of hair sheep in the tropical regions of Latin America. Review. Revista Mexicana de Ciencias Pecuarias 2016, 7, 105–125. [Google Scholar] [CrossRef] [Green Version]
- AFRC Technical Committee on Responses to Nutrients. Energy and Protein Requirements of Ruminants; CAB International: Wallingford, UK, 1993; p. 159. [Google Scholar]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Longitudinal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research. Northwestern University, Evanston, IL. Version ¼2.0.8. 2020. Available online: https://CRAN.R-project.org/package=psych (accessed on 6 January 2022).
- Schoerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’. R Package Version 2.1.2. 2020. Available online: https://CRAN.R-project.org/package=GGally (accessed on 6 January 2022).
- Long, J.A. Jtools: Analysis and Presentation of Social Scientific Data_. R Package Version 2.1.0. 2020. Available online: https://cran.rproject.org/package=jtools (accessed on 6 January 2022).
- Kuhn, M. Caret: Classification and Regression Training. R package Version. 6.0–84. 2019. Available online: https://CRAN.R-project.org/package=caret (accessed on 6 January 2022).
- Costa, M.R.; Pereira, G.F.; Silva, E.S.; Paulino, A.M.A.; Mizubuti, V.R.; Pimentel, I.Y.; Pinto, P.G.; Rocha-Junior, J.N. Body composition and net energy and protein requirements of Morada Nova lambs. Small Rumin. Res. 2013, 114, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Partida, P.J.A.; Braña, V.D.; Martínez, R.L. Productive performance and carcass characteristics in Pelibuey sheep and crossbreds (Pelibuey*Suffolk—Dorset). Técnica Pecuaria en México 2009, 47, 313–322. [Google Scholar]
- Tshabalala, P.A.; Strydom, P.E.; Webb, E.C. Meat quality of designated South African indigenous goat and sheep breeds. Meat Sci. 2003, 65, 563–570. [Google Scholar] [CrossRef]
- Kecici, P.D.; Öztürk, N.; Yalcitan, H.; Kocak, Ö.; Yilmaz, A.; Ekíz, B. Prediction of carcass composition of lambs by joint dissection and carcass traits. Turk. J. Vet. Anim. Sci. 2020, 44, 1125–1135. [Google Scholar] [CrossRef]
- Ali, M.; Eyduran, E.; Tariq, M.M.; Tirink, C.; Abbas, F.; Bajwa, M.A.; Jan, S. Comparison of artificial neural network and decision tree algorithms used for predicting live weight at post weaning period from some biometrical characteristics in Harnai sheep. Pak. J. Zool. 2015, 47, 1579–1585. [Google Scholar]
- García-Osorio, I.; Oliva-Hernández, J.; Hinojosa-Cuéllar, J.A. Tissue composition of the carcass of Blackbelly x Pelibuey suckling lambs. ERA 2016, 3, 203–213. [Google Scholar]
- Miguélez, E.; Zumalacárregui, J.M.; Osorio, M.T.; Beteta, O.; Mateo, J. Carcass characteristics of suckling lambs protected by the PGI “Lechazo de Castilla y León” European quality label: Effect of breed, sex and carcass weight. Meat Sci. 2006, 73, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kempster, A.J.; Avis, R.D.; Cuthbertson, A.; Harrington, G. Prediction of the lean content of lamb carcasses of different breed types. J. Agric. Sci. 1976, 86, 23–34. [Google Scholar] [CrossRef]
| Item | Description | Mean | SD | Min | Max | Skew | Kurtois |
|---|---|---|---|---|---|---|---|
| SW | Shoulder weight (kg) | 1.30 | 0.20 | 0.74 | 1.70 | −0.50 | −0.22 |
| SM | Shoulder muscle (kg) | 0.92 | 0.15 | 0.46 | 1.29 | −0.37 | 0.39 |
| SF | Shoulder fat (kg) | 0.09 | 0.04 | 0.02 | 0.20 | 0.45 | −0.23 |
| SB | Shoulder bone (kg) | 0.27 | 0.03 | 0.21 | 0.36 | 0.05 | −0.50 |
| NW | Neck weight (kg) | 0.68 | 0.17 | 0.32 | 1.17 | 0.51 | 0.32 |
| NM | Neck muscle (kg) | 0.43 | 0.12 | 0.22 | 0.92 | 1.35 | 4.32 |
| NF | Neck fat (kg) | 0.06 | 0.04 | 0.00 | 0.23 | 1.23 | 1.42 |
| NB | Neck bone (kg) | 0.17 | 0.06 | 0.07 | 0.30 | 0.15 | −1.07 |
| CM | Carcass muscle (kg) | 9.28 | 1.52 | 4.83 | 12.26 | −0.59 | 0.32 |
| CF | Carcass fat (kg) | 1.27 | 0.42 | 0.43 | 2.15 | 0.19 | −0.58 |
| CB | Carcass bone (kg) | 3.06 | 0.41 | 2.18 | 4.04 | 0.09 | −0.39 |
| ID | Model | Adj. R2 | MSPE | AIC | BIC |
|---|---|---|---|---|---|
| 1 | = 0.29(0.69) + 5.61(0.51) × W + 3.63(0.87) × NM | 0.81 | 0.37 | 82.67 | 89.42 |
| 2 | = −0.36(0.76) + 5.62(0.83) × SM + 10.49(3.62) × SB + 3.26(0.83) × NM | 0.83 | 0.33 | 79.27 | 87.72 |
| 3 | = −0.40(0.76) + 5.33(0.91) × SM + 2.16(2.67) × SF + 10.68(3.65) × SB + 3.36(0.85) × NM | 0.82 | 0.32 | 80.53 | 90.66 |
| Carcass fat (CF) | |||||
| 4 | = −0.05(0.24) + 0.75(0.29) × SM + 3.31(1.15) × SF + 4.52(0.91) × NF | 0.62 | 0.061 | 11.38 | 19.83 |
| 5 | = −0.17(0.25) + 0.62(0.30) × SM + 3.68(1.16) × SF + 0.51(0.37) × NM + 4.15(0.93) × NF | 0.62 | 0.057 | 11.20 | 21.33 |
| 6 | = −0.06(0.27) + 3.09(0.31) × SM + 3.09(1.29) × SF + 0.55(0.37) × NW + 4.17(1.22) × NF − 1.41(0.94) × NB | 0.63 | 0.055 | 11.95 | 23.77 |
| Carcass bone (CB) | |||||
| 7 | = 0.91(0.32) + 5.98(1.22) × SB + 0.78(0.25) × NW | 0.55 | 0.063 | 11.04 | 17.81 |
| 8 | = 0.84(0.32) + 5.82(1.19) × SB + 1.08(0.31) × NW − 1.74(1.09) × NF | 0.57 | 0.059 | 10.34 | 18.79 |
| 9 | = 0.87(0.32) + 5.67(1.21) × SB + 1.66(0.77) × NW − 0.73(0.90) × NM − 2.56(1.50) × NF | 0.56 | 0.057 | 11.61 | 21.73 |
| Model | SW | SM | SF | SB | NW | NM | NF | NB |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.06 | 1.06 | ||||||
| 2 | 1.86 | 1.88 | 1.09 | |||||
| 3 | 2.21 | 1.27 | 1.88 | 1.11 | ||||
| 4 | 1.23 | 1.29 | 1.08 | |||||
| 5 | 1.35 | 1.37 | 1.19 | 1.17 | ||||
| 6 | 1.41 | 1.68 | 2.56 | 2.03 | 2.08 | |||
| 7 | 1.15 | 1.15 | ||||||
| 8 | 1.15 | 1.80 | 1.63 | |||||
| 9 | 1.18 | 11.14 | 7.01 | 3.0 |
| ID | Predictors | RMSPE | r2 | MAE | RMSPE (SD) | R2 (SD) | MAE (SD) |
|---|---|---|---|---|---|---|---|
| Carcass muscle (CM) | |||||||
| 1 | SW, NM | 0.67 | 0.82 | 0.61 | 0.27 | 0.17 | 0.23 |
| 2 | SM, SB, NM | 0.64 | 0.89 | 0.56 | 0.23 | 0.09 | 0.21 |
| 3 | SM, SF, SB, NM | 0.68 | 0.85 | 0.61 | 0.26 | 0.15 | 0.24 |
| Carcass fat (CF) | |||||||
| 4 | SM, SF, NF | 0.28 | 0.51 | 0.24 | 0.10 | 0.30 | 0.069 |
| 5 | SM, SF, NM, NF | 0.29 | 0.55 | 0.25 | 0.10 | 0.29 | 0.061 |
| 6 | SM, SF, NW, NF, NM | 0.27 | 0.62 | 0.22 | 0.08 | 0.28 | 0.043 |
| Carcass bone (CB) | |||||||
| 7 | SB, NW | 0.32 | 0.54 | 0.25 | 0.19 | 0.37 | 0.15 |
| 8 | SB, NW, NF | 0.31 | 0.50 | 0.24 | 0.14 | 0.36 | 0.11 |
| 9 | SB, NW, NM, NF | 0.32 | 0.52 | 0.25 | 0.12 | 0.37 | 0.11 |
| Comparison | Df 1 | p-Value 2 |
|---|---|---|
| Carcass muscle (CM) | ||
| Model 1 vs. model 2 | 1 | 0.02 |
| Model 1 vs. model 3 | 2 | 0.07 |
| Model 2 vs. model 3 | 1 | 0.42 |
| Carcass fat (CF) | ||
| Model 4 vs. model 5 | 1 | 0.16 |
| Model 4 vs. model 6 | 2 | 0.23 |
| Model 5 vs. model 6 | 1 | 0.31 |
| Carcass bone (CB) | ||
| Model 7 vs. model 8 | 1 | 0.12 |
| Model 7 vs. model 9 | 2 | 0.22 |
| Model 8 vs. model 9 | 1 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastelum-Delgado, M.A.; Aguilar-Quiñonez, J.A.; Arce-Recinos, C.; García-Herrera, R.A.; Macías-Cruz, U.; Lee-Rangel, H.A.; Cruz-Tamayo, A.A.; Ángeles-Hernández, J.C.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Estimation of Carcass Tissue Composition from the Neck and Shoulder Composition in Growing Blackbelly Male Lambs. Foods 2022, 11, 1396. https://doi.org/10.3390/foods11101396
Gastelum-Delgado MA, Aguilar-Quiñonez JA, Arce-Recinos C, García-Herrera RA, Macías-Cruz U, Lee-Rangel HA, Cruz-Tamayo AA, Ángeles-Hernández JC, Vargas-Bello-Pérez E, Chay-Canul AJ. Estimation of Carcass Tissue Composition from the Neck and Shoulder Composition in Growing Blackbelly Male Lambs. Foods. 2022; 11(10):1396. https://doi.org/10.3390/foods11101396
Chicago/Turabian StyleGastelum-Delgado, Miguel A., José Antonio Aguilar-Quiñonez, Carlos Arce-Recinos, Ricardo A. García-Herrera, Ulises Macías-Cruz, Héctor A. Lee-Rangel, Alvar A. Cruz-Tamayo, Juan C. Ángeles-Hernández, Einar Vargas-Bello-Pérez, and Alfonso J. Chay-Canul. 2022. "Estimation of Carcass Tissue Composition from the Neck and Shoulder Composition in Growing Blackbelly Male Lambs" Foods 11, no. 10: 1396. https://doi.org/10.3390/foods11101396







