Ribosome Profiling Reveals Genome-Wide Cellular Translational Regulation in Lacticaseibacillus rhamnosus ATCC 53103 under Acid Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Culture Conditions, and Acid Stress
2.2. Total RNA Extraction and Library Construction
2.3. Ribo-Seq
2.4. Analysis of Differentially Expressed Genes
2.5. Analysis of DEGs’ Translational Efficiency, and Correlation with DEGs’ Expression
2.6. Data Analysis
3. Results
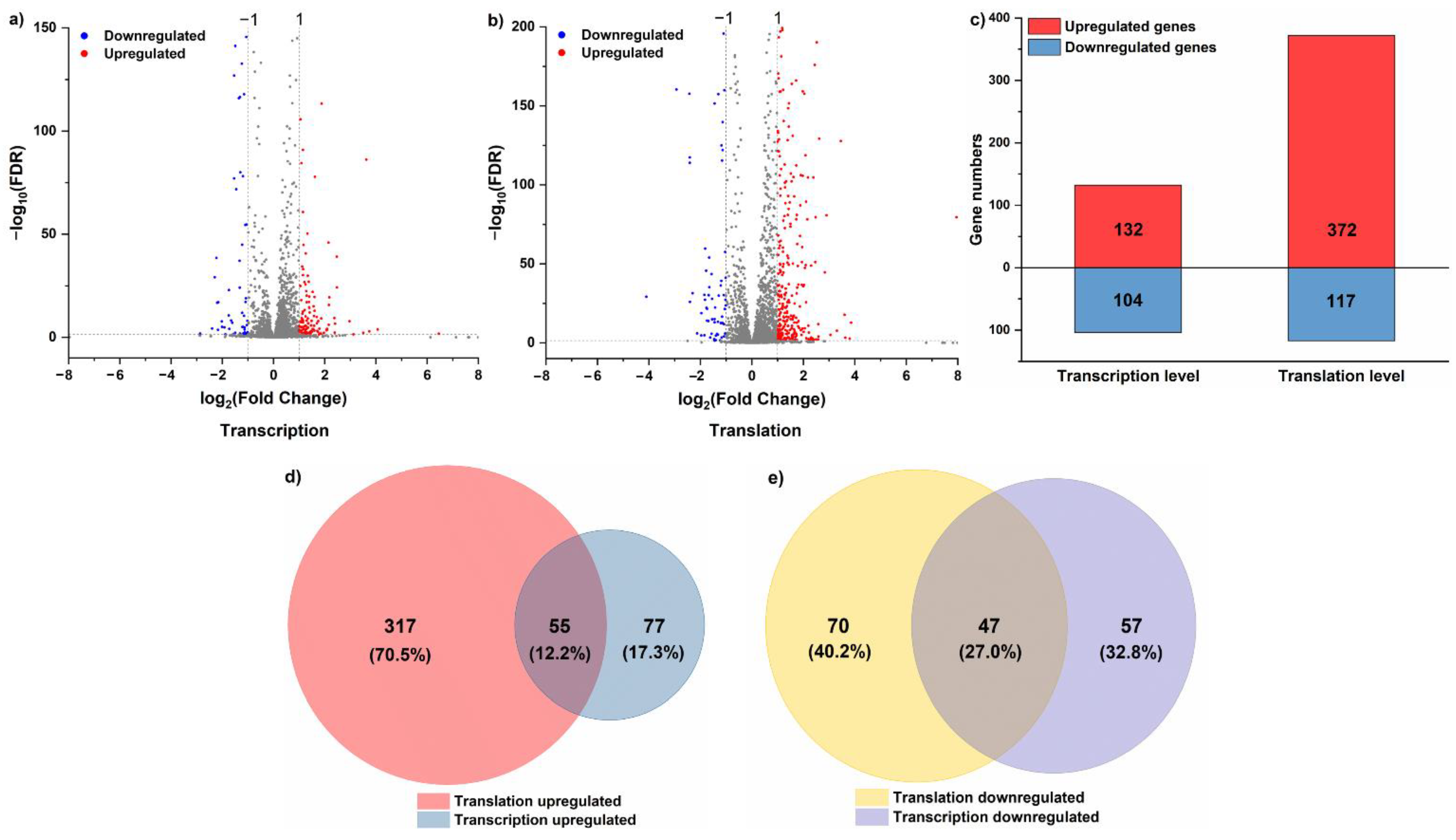
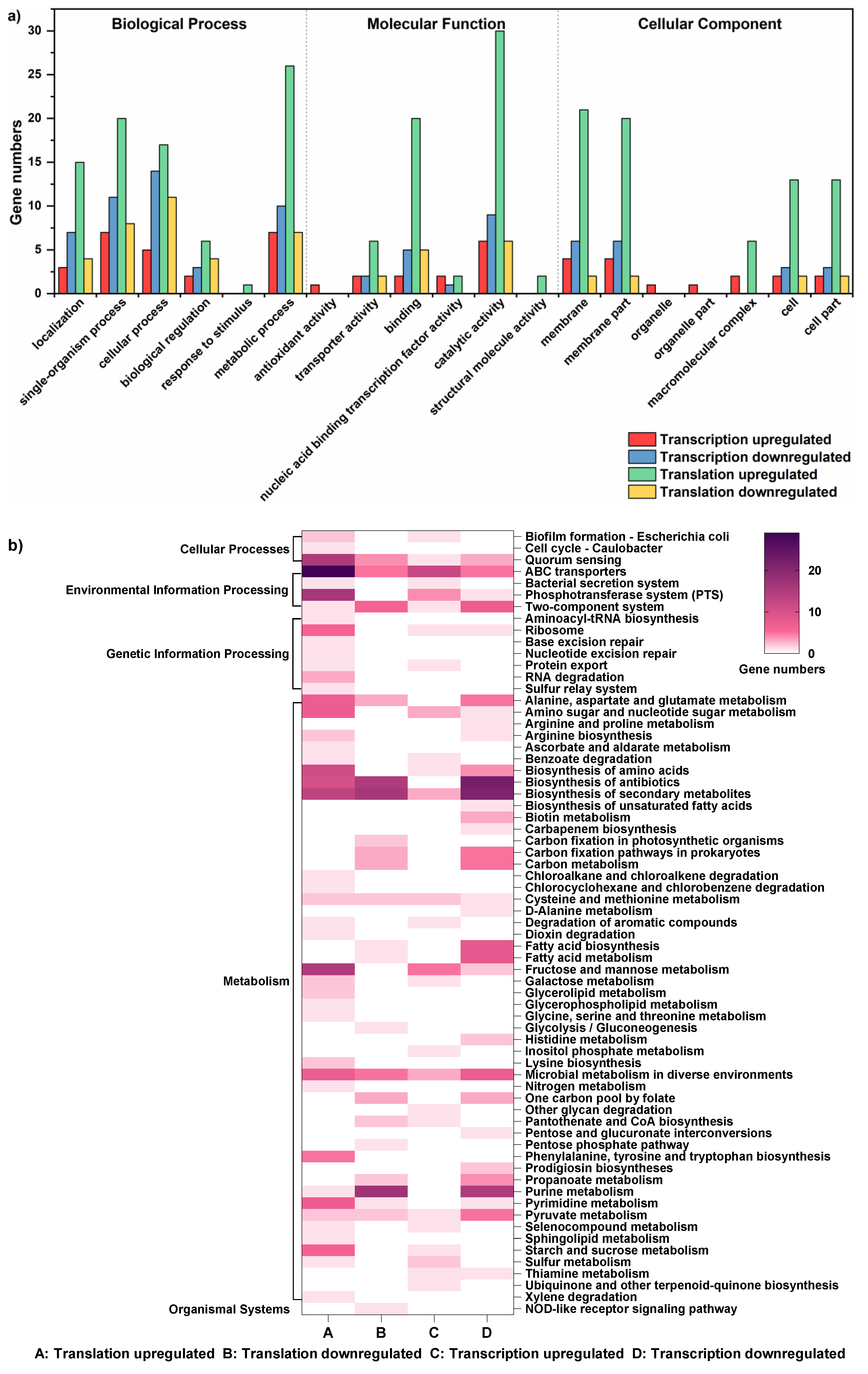
3.1. Effects of Acid Stress on DEGs’ Expression and Function
3.2. Effects of Acid Stress on the Dynamic Profiles of Translation and Transcription
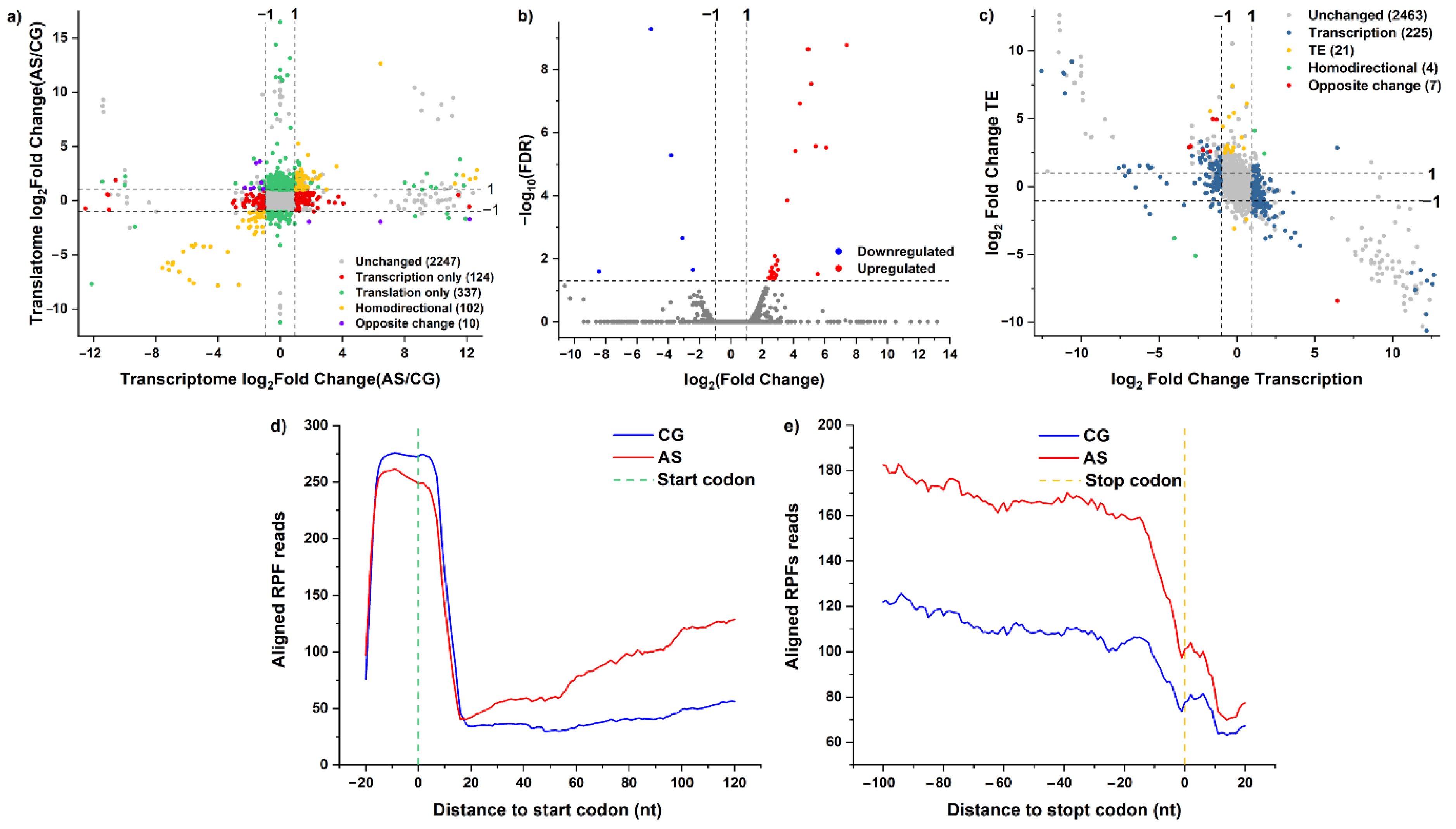
3.3. Effects of Acid Stress on DEGs’ Translational Efficiency
3.4. Effects of Acid Stress on the Dynamic Profiles of Translational Efficiency and Transcription
3.5. Effects of Acid Stress on Ribosome Accumulation in the ORF Regions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Micr. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, S.; Soltani, H.; Reid, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.Q. Growth, survival, and metabolic activities of probiotics Lactobacillus rhamnosus GG and Saccharomyces cerevisiae var. boulardii CNCM-I745 in fermented coffee brews. Int. J. Food Microbiol. 2021, 350, 109229. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Luo, J.; Wu, K.; Chen, Q.; Hao, L.; Zhou, X.; Wang, X.; Liu, C.; Zhou, H. Dendrobium candidum extract on the bioactive and fermentation properties of Lactobacillus rhamnosus GG in fermented milk. Food Biosci. 2021, 41, 100987. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, K.; Gao, L.; Cai, D.; Wang, B.; Zhao, G.; Liu, J.; Hao, J. Improvement of l-lactic acid fermentation by Lactobacillus rhamnosus immobilized in polyvinyl alcohol/Fe3O4 composite using sweet sorghum juice. Ind. Crop. Prod. 2022, 182, 114922. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Kirmizigul, A.; Atlama, K.; Yilmaz, B. The viability of Lactobacillus rhamnosus in orange juice fortified with nettle (Urtica dioica L.) and bioactive properties of the juice during storage. LWT-Food Sci. Technol. 2020, 118, 108707. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Ji, X.M.; Wu, Z.M.; Zhang, J.; Du, G.C. Improved acid-stress tolerance of Lactococcus lactis NZ9000 and Escherichia coli bl21 by overexpression of the anti-acid component rect. J. Ind. Microbiol. Biotechnol. 2018, 45, 1091–1101. [Google Scholar] [CrossRef]
- Yanez, R.; Marques, S.; Girio, F.M.; Roseiro, J.C. The effect of acid stress on lactate production and growth kinetics in Lactobacillus rhamnosus cultures. Process. Biochem. 2008, 43, 356–361. [Google Scholar] [CrossRef]
- Wu, C.D.; Huang, J.; Zhou, R.Q. Progress in engineering acid stress resistance of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 1055–1063. [Google Scholar] [CrossRef]
- Koponen, J.; Laakso, K.; Koskenniemi, K.; Kankainen, M.; Savijoki, K.; Nyman, T.A.; de Vos, W.M.; Tynkkynen, S.; Kalkkinen, N.; Varmanen, P. Effect of acid stress on protein expression and phosphorylation in Lactobacillus rhamnosus GG. J. Proteom. 2012, 75, 1357–1374. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Yang, J.H.; Yang, P.S.; Wu, Z.M.; Zhang, J.; Du, G.C. Enhanced acid-stress tolerance in lactococcus lactis nz9000 by overexpression of abc transporters. Microb. Cell Factories 2019, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.R.; Larsen, R.L.; Deibel, V.; Steele, J.L. Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J. Bacteriol. 2010, 192, 2445–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.X.; Gao, J.; Liu, D.B.; Li, Y.; Liu, W.L. Adaptational changes in physiological and transcriptional responses of Bifidobacterium longum involved in acid stress resistance after successive batch cultures. Microb. Cell. Fact. 2019, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Oh, E.; Weissman, J.S. The anti-shine-dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 2012, 484, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.S.; Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Ingolia, N.T. Ribosome footprint profiling of translation throughout the genome. Cell 2016, 165, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mrna fragments. Nat. Protoc. 2012, 7, 1534–1550. [Google Scholar] [CrossRef]
- Waudby, C.A.; Dobson, C.M.; Christodoulou, J. Nature and regulation of protein folding on the ribosome. Trends Biochem. Sci. 2019, 44, 914–926. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.C.; Zinshteyn, B.; Wehner, K.A.; Green, R. High-resolution ribosome profiling defines discrete ribosome elongation states and translational regulation during cellular stress. Mol. Cell 2019, 73, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gerashchenko, M.V.; Lobanov, A.V.; Gladyshev, V.N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2012, 109, 17394–17399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Xiao, Z.T.; Zou, Q.; Fang, J.H.; Wang, Q.F.; Yang, X.R.; Gao, N. Ribosome profiling reveals genome-wide cellular translational regulation upon heat stress in Escherichia coli. Genom. Proteom. Bioinf. 2017, 15, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.W.; Yang, J.Q.; Dai, A.M.; Wang, Y.M.; Li, W.; Xie, Z. Ribosome profiling reveals translational regulation of mammalian cells in response to hypoxic stress. BMC Genom. 2017, 18, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Shin, J.; Jin, S.; Lee, J.K.; Kim, D.R.; Kim, S.C.; Cho, S.; Cho, B.K. Genome-scale analysis of syngas fermenting acetogenic bacteria reveals the translational regulation for its autotrophic growth. BMC Genom. 2018, 19, 837. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bao, T.; Yi, H.; Zhang, Z.; Zhang, K.; Liu, X.; Lin, X.; Zhang, Z.; Feng, Z. Ribosome profiling and RNA sequencing reveal genome-wide cellular translation and transcription regulation under osmotic stress in Lactobacillus rhamnosus ATCC 53103. Front. Microbiol. 2021, 12, 781454. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Karaletsos, T.; Drewe, P.; Sreedharan, V.T.; Kuo, D.; Singh, K.; Wendel, H.G.; Ratsch, G. Ribodiff: Detecting changes of mRNA translation efficiency from ribosome footprints. Bioinformatics 2017, 33, 139–141. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.Y.; Wang, S.S.; Zhang, Y.; Wang, S.; Zhao, J.; Feng, H.; Sun, P.P.; Fang, C.B.; Xie, X.B. Genome-wide characterization and expression analysis of ATP-binding cassette (abc) transporters in strawberry reveal the role of fvabcc11 in cadmium tolerance. Sci. Hortic. 2020, 271, 109464. [Google Scholar] [CrossRef]
- Qin, J.Y.; Wang, X.W.; Wang, L.D.; Zhu, B.B.; Zhang, X.H.; Yao, Q.S.; Xu, P. Comparative transcriptome analysis reveals different molecular mechanisms of bacillus coagulans 2–6 response to sodium lactate and calcium lactate during lactic acid production. PLoS ONE 2015, 10, e0124316. [Google Scholar] [CrossRef] [Green Version]
- Guan, N.Z.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, J.G.; Miao, S.; Zhao, Y.; Zhu, H.J.; Qiao, M.Q.; Saris, P.E.J.; Qiao, J.J. Contribution of YthA, a PspC family transcriptional regulator of Lactococcus lactis F44 acid tolerance and nisin yield: A transcriptomic approach. Appl. Environ. Microb. 2018, 84, e02483-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piersimoni, L.; Giangrossi, M.; Marchi, P.; Brandi, A.; Gualerzi, C.O.; Pon, C.L. De novo synthesis and assembly of rRNA into ribosomal subunits during cold acclimation in Escherichia coli. J. Mol. Biol. 2016, 428, 1558–1573. [Google Scholar] [CrossRef] [PubMed]
- Goncheva, M.I.; Flannagan, R.S.; Sterling, B.E.; Laakso, H.A.; Friedrich, N.C.; Kaiser, J.C.; Watson, D.W.; Wilson, C.H.; Sheldon, J.R.; McGavin, M.J.; et al. Stress-induced inactivation of the Staphylococcus aureus purine biosynthesis repressor leads to hypervirulence. Nat. Commun. 2019, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Shi, J.P.; Chen, J.; Zhang, M.; Sun, S.L.; Xie, S.J.; Li, X.J.; Zeng, B.A.; Peng, L.Z.; Hauck, A.; et al. Ribosome profiling reveals dynamic translational landscape in maize seedlings under drought stress. Plant J. 2015, 84, 1206–1218. [Google Scholar] [CrossRef]
- Ingolia, N.T. Ribosome profiling: New views of translation, from single codons to genome scale applications of next-generation sequencing-innovation. Nat. Rev. Genet. 2014, 15, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Cao, Y.F.; Zhou, D.G.; Hu, S.F.; Tan, W.J.; Xiao, X.L.; Yu, Y.G.; Li, X.F. Global transcriptomic analysis of Cronobacter Sakazakii CICC 21544 by RNA-seq under inorganic acid and organic acid stresses. Food Res. Int. 2020, 130, 108963. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Ding, Y.; Niemczyk, M.; Kudla, G.; Plotkin, J.B. Rate-limiting steps in yeast protein translation. Cell 2013, 153, 1589–1601. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.K.; Chen, F.C. Selective constraint on the upstream open reading frames that overlap with coding sequences in animals. PLoS ONE 2012, 7, e48413. [Google Scholar] [CrossRef] [Green Version]
- Juntawong, P.; Girke, T.; Bazin, J.; Bailey-Serres, J. Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, E203–E212. [Google Scholar] [CrossRef] [Green Version]
| Category | Gene_ID | Gene_Name | TE_CG | TE_AS | log2FC | FDR |
|---|---|---|---|---|---|---|
| Upregulated | LGG_RS00110 | -- | 6.75 | 43.02 | 2.67 | 0.0396827 |
| LGG_RS00155 | -- | 0.67 | 4.93 | 2.88 | 0.0153195 | |
| LGG_RS00490 | trpF | 0.28 | 13.15 | 5.55 | 0.0303543 | |
| LGG_RS00500 | trpD | 0.61 | 41.48 | 6.09 | 2.95 × 10−6 | |
| LGG_RS00715 | TTE1650 | 0.01 | 0.07 | 2.81 | 0.0409188 | |
| LGG_RS02170 | -- | 0.01 | 1.7 | 7.41 | 1.666 × 10−9 | |
| LGG_RS02790 | TTE1650 | 0.01 | 0.07 | 2.81 | 0.0409188 | |
| LGG_RS03170 | HSP17.6C | 0.01 | 0.35 | 5.13 | 2.827 × 10−8 | |
| LGG_RS04550 | TTE1650 | 0.01 | 0.07 | 2.81 | 0.0409188 | |
| LGG_RS04690 | -- | 0.1 | 0.79 | 2.98 | 0.0112087 | |
| LGG_RS04695 | nodI | 0.09 | 0.67 | 2.9 | 0.0327759 | |
| LGG_RS06620 | fmnP | 0.24 | 1.47 | 2.61 | 0.0184817 | |
| LGG_RS06985 | carA | 4.43 | 25.52 | 2.53 | 0.0251229 | |
| LGG_RS06990 | pyrC | 1.76 | 12.29 | 2.8 | 0.0081897 | |
| LGG_RS06995 | pyrB | 0.48 | 10.29 | 4.42 | 1.185 × 10−7 | |
| LGG_RS07000 | pyrP | 0.38 | 11.49 | 4.92 | 2.265 × 10−9 | |
| LGG_RS07005 | pyrR1 | 0.15 | 4.68 | 4.96 | 2.265 × 10−9 | |
| LGG_RS08210 | TTE1650 | 0.01 | 0.07 | 2.81 | 0.0409188 | |
| LGG_RS08915 | -- | 0.86 | 4.82 | 2.49 | 0.0409188 | |
| LGG_RS09795 | clpC | 1.15 | 19.98 | 4.12 | 3.804 × 10−6 | |
| LGG_RS10200 | acpP | 5.56 | 33.81 | 2.6 | 0.0251229 | |
| LGG_RS10665 | -- | 1.94 | 10.39 | 2.42 | 0.0396827 | |
| LGG_RS13400 | Cndp1 | 1.92 | 15.49 | 3.01 | 0.0219037 | |
| LGG_RS13405 | oppA | 1.72 | 73.85 | 5.42 | 2.659E-06 | |
| LGG_RS13420 | hsp18 | 0.78 | 9.47 | 3.6 | 0.0001423 | |
| LGG_RS13465 | glnP | 0.02 | 0.12 | 2.58 | 0.0327759 | |
| LGG_RS13475 | argH | 0.24 | 1.6 | 2.74 | 0.0278452 | |
| Downregulated | LGG_RS05995 | -- | 903 | 2.64 | −8.42 | 0.0251229 |
| LGG_RS08685 | purD | 15.22 | 0.44 | −5.11 | 5.176 × 10−10 | |
| LGG_RS08690 | purH | 22.29 | 1.58 | −3.82 | 5.257 × 10−6 | |
| LGG_RS09365 | oppA | 3.01 | 0.56 | −2.43 | 0.0219037 | |
| LGG_RS13815 | -- | 40.17 | 4.72 | −3.09 | 0.002218 |
| Category | Pathway ID | Pathway Name | p-Value | Genes | Genes |
|---|---|---|---|---|---|
| Upregulated | ko00250 | Alanine, aspartate, and glutamate metabolism | 0.002 | LGG_RS06985, LGG_RS06995, LGG_RS13475 | carA, pyrB, argH |
| ko00240 | Pyrimidine metabolism | 0.002 | LGG_RS06985, LGG_RS06990, LGG_RS06995, LGG_RS07005 | carA, pyrC, pyrB, pyrR1 | |
| ko00400 | Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.008 | LGG_RS00490, LGG_RS00500 | trpF, trpD | |
| ko00999 | Biosynthesis of secondary metabolites—unclassified | 0.029 | LGG_RS10200 | acpP | |
| ko01110 | Biosynthesis of secondary metabolites | 0.057 | LGG_RS00490, LGG_RS00500, LGG_RS13475 | trpF, trpD, argH | |
| ko01100 | Metabolic pathways | 0.068 | LGG_RS00490, LGG_RS00500, LGG_RS06985, LGG_RS06990, LGG_RS06995, LGG_RS07005, LGG_RS13475 | trpF, trpD, carA, pyrC, pyrB, pyrR1, argH | |
| ko00220 | Arginine biosynthesis | 0.071 | LGG_RS13475 | argH | |
| ko01230 | Biosynthesis of amino acids | 0.108 | LGG_RS00490, LGG_RS00500, LGG_RS13475 | trpF, trpD, argH | |
| Downregulated | ko02024 | Quorum sensing | 0.151 | LGG_RS09365 | oppA |
| ko00230 | Purine metabolism | 0.187 | LGG_RS08685 LGG_RS08690 | purD, purH | |
| ko02010 | ABC transporters | 0.432 | LGG_RS09365 | oppA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, K.; Zhang, Z.; Zhang, Z.; Lin, X.; Liu, X.; Feng, Z.; Yi, H. Ribosome Profiling Reveals Genome-Wide Cellular Translational Regulation in Lacticaseibacillus rhamnosus ATCC 53103 under Acid Stress. Foods 2022, 11, 1411. https://doi.org/10.3390/foods11101411
Fan X, Zhang K, Zhang Z, Zhang Z, Lin X, Liu X, Feng Z, Yi H. Ribosome Profiling Reveals Genome-Wide Cellular Translational Regulation in Lacticaseibacillus rhamnosus ATCC 53103 under Acid Stress. Foods. 2022; 11(10):1411. https://doi.org/10.3390/foods11101411
Chicago/Turabian StyleFan, Xuejing, Kenan Zhang, Zongcai Zhang, Zhen Zhang, Xue Lin, Xin Liu, Zhen Feng, and Huaxi Yi. 2022. "Ribosome Profiling Reveals Genome-Wide Cellular Translational Regulation in Lacticaseibacillus rhamnosus ATCC 53103 under Acid Stress" Foods 11, no. 10: 1411. https://doi.org/10.3390/foods11101411
APA StyleFan, X., Zhang, K., Zhang, Z., Zhang, Z., Lin, X., Liu, X., Feng, Z., & Yi, H. (2022). Ribosome Profiling Reveals Genome-Wide Cellular Translational Regulation in Lacticaseibacillus rhamnosus ATCC 53103 under Acid Stress. Foods, 11(10), 1411. https://doi.org/10.3390/foods11101411







