Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure
Abstract
:1. Introduction
2. Materials and Methods
3. Review of the Mediterranean Studies on the Link between Deep-Sea VMEs and Fishery Resources
3.1. Open Slope, Soft Bottoms
3.2. Open Slope, Hard Bottoms
3.3. Canyons
3.4. Seamounts
4. Results
4.1. Data from FAO Official Statistics
4.2. Data from MEDITS Trawl Surveys (NorthWestern Ionian Sea, GSA 19)
5. Discussion
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Mediterranean and Black Sea Fisheries 2020; FAO, General Fisheries Commission for the Mediterranean: Rome, Italy, 2020; ISBN 978-92-5-133724-0. [Google Scholar]
- Gordon, J.D.M. Deep-Water Fisheries at the Atlantic Frontier. Cont. Shelf Res. 2001, 21, 987–1003. [Google Scholar] [CrossRef]
- Morato, T.; Watson, R.; Pitcher, T.J.; Pauly, D. Fishing down the Deep. Fish Fish. 2006, 7, 24–34. [Google Scholar] [CrossRef]
- Villasante, S.; Morato, T.; Rodriguez-Gonzalez, D.; Antelo, M.; Österblom, H.; Watling, L.; Nouvian, C.; Gianni, M.; Macho, G. Sustainability of Deep-Sea Fish Species under the European Union Common Fisheries Policy. Ocean. Coast. Manag. 2012, 70, 31–37. [Google Scholar] [CrossRef]
- Caddy, J.F. Toward a Comparative Evaluation of Human Impacts on Fishery Ecosystems of Enclosed and Semi-enclosed Seas. Rev. Fish. Sci. 1993, 1, 57–95. [Google Scholar] [CrossRef]
- Rogers, A.D. The Biology of Seamounts. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 30, pp. 305–350. ISBN 978-0-12-026130-7. [Google Scholar]
- Koslow, J.A. Seamounts and the Ecology of Deep-Sea Fisheries. Am. Sci. 1997, 85, 168–176. [Google Scholar]
- De Leo, F.C.; Smith, C.R.; Rowden, A.A.; Bowden, D.A.; Clark, M.R. Submarine Canyons: Hotspots of Benthic Biomass and Productivity in the Deep Sea. Proc. Biol. Sci. 2010, 277, 2783–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosså, J.H.; Mortensen, P.B.; Furevik, D.M. The Deep-Water Coral Lophelia Pertusa in Norwegian Waters: Distribution and Fishery Impacts. Hydrobiologia 2002, 471, 1–12. [Google Scholar] [CrossRef]
- Husebø, Å.; Nøttestad, L.; Fosså, J.H.; Furevik, D.M.; Jørgensen, S.B. Distribution and Abundance of Fish in Deep-Sea Coral Habitats. Hydrobiologia 2002, 471, 91–99. [Google Scholar] [CrossRef]
- Cartes, J.E.; Maynou, F.; Sardà, F.; Lloris, D.; Tudela, S. The Mediterranean Deep-Sea Ecosystems: An Overview of Their Diversity, Structure, Functioning and Anthropogenic Impacts; WWF, IUCN, Eds.; WWF Mediterranean Program; IUCN Centre for Mediterranean Cooperation: Málaga, Spain, 2004; 64p. [Google Scholar]
- D’Onghia, G.; Maiorano, P.; Sion, L.; Giove, A.; Capezzuto, F.; Carlucci, R.; Tursi, A. Effects of Deep-Water Coral Banks on the Abundance and Size Structure of the Megafauna in the Mediterranean Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 397–411. [Google Scholar] [CrossRef]
- Muñoz, P.D.; Murillo, F.J.; Sayago-Gil, M.; Serrano, A.; Laporta, M.; Otero, I.; Gómez, C. Effects of Deep-Sea Bottom Longlining on the Hatton Bank Fish Communities and Benthic Ecosystem, North-East Atlantic. J. Mar. Biol. Assoc. 2011, 91, 939–952. [Google Scholar] [CrossRef]
- Company, J.B.; Ramirez-Llodra, E.; Sardà, F.; Aguzzi, J.; Puig, P.; Canals, M.; Calafat, A.; Palanques, A.; Solé, M.; Sanchez-Vidal, A.; et al. Submarine canyons in the Catalan Sea (NW Mediterranean): Megafaunal biodiversity patterns and anthropogenicthreats. In Mediterranean Submarine Canyons: Ecology and Governance; Würtz, M., Ed.; IUCN: Gland, Switzerland; Málaga, Spain, 2012; pp. 133–144. [Google Scholar]
- Farrugio, H. A Refugium for the Spawners of Exploited Mediterranean Marine Species: The Canyons of the Continental Slope of the Gulf of Lion. In Mediterranean Submarine Canyons: Ecology and Gorvernance; Wurtz, M., Ed.; IUCN: Gland, Switzerland; Malaga, Spain, 2012; pp. 45–49. [Google Scholar]
- Henry, L.-A.; Moreno Navas, J.; Roberts, J.M. Multi-Scale Interactions between Local Hydrography, Seabed Topography, and Community Assembly on Cold-Water Coral Reefs. Biogeosciences 2013, 10, 2737–2746. [Google Scholar] [CrossRef] [Green Version]
- Fabri, M.-C.; Pedel, L.; Beuck, L.; Galgani, F.; Hebbeln, D.; Freiwald, A. Megafauna of Vulnerable Marine Ecosystems in French Mediterranean Submarine Canyons: Spatial Distribution and Anthropogenic Impacts. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2014, 104, 184–207. [Google Scholar] [CrossRef] [Green Version]
- Kutti, T.; Bergstad, O.A.; Fosså, J.H.; Helle, K. Cold-Water Coral Mounds and Sponge-Beds as Habitats for Demersal Fish on the Norwegian Shelf. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 122–133. [Google Scholar] [CrossRef]
- Hinz, H. Impact of Bottom Fishing on Animal Forests: Science, Conservation, and Fisheries Management. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1041–1059. [Google Scholar]
- Bo, M. Unveiling the Deep Biodiversity of the Janua Seamount (Ligurian Sea): First Mediterranean Sighting of the Rare Atlantic Bamboo Coral Chelidonisis Aurantiaca Studer, 1890. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 156, 103186. [Google Scholar] [CrossRef]
- Lack, M.; Traffic Oceania; Short, K.; Willock, A. Managing Risk and Uncertainty in Deep-Sea Fisheries: Lessons from Orange Roughy; WWF Traffic Oceania: Sydney, Australia, 2003; 73p. [Google Scholar]
- Jennings, S.; Greenstreet, S.P.; Reynolds, J.D. Structural Change in an Exploited Fish Community: A Consequence of Differential Fishing Effects on Species with Contrasting Life Histories. J. Anim. Ecol. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Koslow, J. Continental Slope and Deep-Sea Fisheries: Implications for a Fragile Ecosystem. ICES J. Mar. Sci. 2000, 57, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Clark, M. Are Deepwater Fisheries Sustainable?—The Example of Orange Roughy (Hoplostethus Atlanticus) in New Zealand. Fish. Res. 2001, 51, 123–135. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction Vulnerability in Marine Populations. Fish Fish. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Ellis, J.R.; Goodwin, N.B.; Grant, A.; Reynolds, J.D.; Jennings, S. Methods of Assessing Extinction Risk in Marine Fishes. Fish Fish. 2004, 5, 255–276. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.W.L.; Pitcher, T.J.; Pauly, D. A Fuzzy Logic Expert System to Estimate Intrinsic Extinction Vulnerabilities of Marine Fishes to Fishing. Biol. Conserv. 2005, 124, 97–111. [Google Scholar] [CrossRef]
- International Council for the Exploration of the Sea (ICES). Report of the ICES Advisory Committee on Fishery Management ICES Cooperative Research Report No. 246; ICES: Copenhagen, Denmark, 2001. [Google Scholar]
- Watson, R.; Morato, T. Exploitation Patterns in Seamount Fisheries: A Prelimary Analysis. In Fisheries Centre Research Reports; Appendix 1; Morato, T., Pauly, D., Eds.; University of British Columbia: Vancouver, BC, Canada; Volume 12, pp. 61–66.
- Devine, J.A.; Baker, K.D.; Haedrich, R.L. Deep-Sea Fishes Qualify as Endangered. Nature 2006, 439, 29. [Google Scholar] [CrossRef] [PubMed]
- International Council for the Exploration of the Sea (ICES). Report of the ICES Advisory Committee on Fishery Management, Advisory Committee on the Marine Evironment and Advisory Committee on Ecosystems; Book 8; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2007; ISBN 978-87-7482-000-0. [Google Scholar]
- Bailey, D.M.; Collins, M.A.; Gordon, J.D.; Zuur, A.F.; Priede, I.G. Long-Term Changes in Deep-Water Fish Populations in the Northeast Atlantic: A Deeper Reaching Effect of Fisheries? Proc. R. Soc. B Biol. Sci. 2009, 276, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Sadovy, Y.; Cheung, W.L. Near Extinction of a Highly Fecund Fish: The One That Nearly Got Away. Fish Fish. 2003, 4, 86–99. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the Council and the European Parliament on Review of the Management of Deep-Sea Fish Stocks; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Norse, E.A.; Brooke, S.; Cheung, W.W.; Clark, M.R.; Ekeland, I.; Froese, R.; Gjerde, K.M.; Haedrich, R.L.; Heppell, S.S.; Morato, T. Sustainability of Deep-Sea Fisheries. Mar. Policy 2012, 36, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 201–352. ISBN 978-0-12-026134-5. [Google Scholar]
- Auster, P.J.; Langton, R.W. The Effects of Fishing on Fish Habitat. Am. Fish. Soc. Symp. 1999, 22, 150–187. [Google Scholar]
- Caddy, J.F. Marine Habitat and Cover: Their Importance for Productive Coastal Fishery Resources. In Monographs on Oceanographic Methodlogy; UNESCO Publishing: Paris, France, 2007; Volume 11. [Google Scholar]
- Caddy, J.F. The Importance Of “Cover” In The Life Histories Of Demersal And Benthic Marine Resources: A Neglected Issue in Fisheries Assessment And Management. Bull. Mar. Sci. 2008, 83, 46. [Google Scholar]
- Juanes, F. Role of Habitat in Mediating Mortality during the Post-Settlement Transition Phase of Temperate Marine Fishes. J. Fish Biol. 2007, 70, 661–677. [Google Scholar] [CrossRef]
- Roberts, C.M. Deep Impact: The Rising Toll of Fishing in the Deep Sea. Trends Ecol. Evol. 2002, 17, 242–245. [Google Scholar] [CrossRef]
- Koenig, C.C.; Coleman, F.C.; Grimes, C.B.; Fitzhugh, G.R.; Scanlon, K.M.; Gledhill, C.T.; Grace, M. Protection of Fish Spawning Habitat for the Conservation of Warm-Temperate Reef-Fish Fisheries of Shelf-Edge Reefs of Florida. Bull. Mar. Sci. 2000, 66, 24. [Google Scholar]
- Reed, J.K.; Shepard, A.N.; Koenig, C.C.; Scanlon, K.M.; Gilmore, R.G. Mapping, Habitat Characterization, and Fish Surveys of the Deep-Water Oculina Coral Reef Marine Protected Area: A Review of Historical and Current Research. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 443–465. ISBN 978-3-540-24136-2. [Google Scholar]
- Hall–Spencer, J.; Allain, V.; Fosså, J.H. Trawling Damage to Northeast Atlantic Ancient Coral Reefs. Proc. R. Soc. Lond. B 2002, 269, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Du Preez, C.; Tunnicliffe, V. Shortspine Thornyhead and Rockfish (Scorpaenidae) Distribution in Response to Substratum, Biogenic Structures and Trawling. Mar. Ecol. Prog. Ser. 2011, 425, 217–231. [Google Scholar] [CrossRef] [Green Version]
- FAO. Report of the Technical Consultation on International Guidelines for the Management of Deep-Sea Fisheries in the High Seas: Rome, 4–8 February and 25–29 August 2008; FAO Fisheries and Aquaculture Report; Organisation des Nations Unies pour L’Alimentation et L’Agriculture, Ed.; FAO: Rome, Italy, 2009; ISBN 978-92-5-006190-0. [Google Scholar]
- General Fisheries Commission for the Mediterranean. Criteria for the Identification of Sensitive Habitats of Relevance for the Management of Priority Species; General Fisheries Commission for the Mediterranean: Malaga, Spain, 2009; Volume 3. [Google Scholar]
- General Fisheries Commission for the Mediterranean. Report of the First Meeting of the Working Group on Vulnerable Marine Ecosystems (WGVME); General Fisheries Commission for the Mediterranean: Malaga, Spain, 2017; Available online: http://www.fao.org/gfcm/technical-meetings/detail/en/c/885358/ (accessed on 8 March 2022).
- General Fisheries Commission for the Mediterranean. Report of the Second Meeting of the Working Group on Vulnerable Marine Ecosystems (WGVME); FAO Headquarters: Rome, Italy, 2018; Available online: http://www.fao.org/gfcm/technical-meetings/detail/en/c/1142043 (accessed on 8 March 2022).
- Otero, M.D.M.; Numa, C.; Bo, M.; Orejas, C.; Garrabou, J.; Cerrano, C.; Kružic, P.; Antoniadou, C.; Aguilar, R.; Kipson, S. Overview of the Conservation Status of Mediterranean Anthozoa; International Union for Conservation of Nature: Malaga, Spain, 2017; ISBN 2-8317-1845-7. [Google Scholar]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing Impact on Deep Mediterranean Rocky Habitats as Revealed by ROV Investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Bo, M.; Bavestrello, G.; Angiolillo, M.; Calcagnile, L.; Canese, S.; Cannas, R.; Cau, A.; D’Elia, M.; D’Oriano, F.; Follesa, M.C.; et al. Persistence of Pristine Deep-Sea Coral Gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 2015, 10, e0119393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimienti, G.; Bo, M.; Taviani, M.; Mastrototaro, F. Occurrence and Biogeography of Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 213–243. [Google Scholar]
- Chimienti, G.; Mastrototaro, F.; D’Onghia, G. Mesophotic and Deep-Sea Vulnerable Coral Habitats of the Mediterranean Sea: Overview and Conservation Perspectives. In Advances in the Studies of the Benthic Zone; Soto, L.A., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar] [CrossRef] [Green Version]
- Chimienti, G.; De Padova, D.; Adamo, M.; Mossa, M.; Bottalico, A.; Lisco, A.; Ungaro, N.; Mastrototaro, F. Effects of Global Warming on Mediterranean Coral Forests. Sci. Rep. 2021, 11, 20703. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.; Bigford, T.E.; Leathery, S.; Hill, R.L.; Bickers, K. Ecosystem Approaches to Fishery Management through Essential Fish Habitat. Bull. Mar. Sci. 2000, 66, 8. [Google Scholar]
- Capezzuto, F.; Sion, L.; Ancona, F.; Carlucci, R.; Carluccio, A.; Cornacchia, L.; Maiorano, P.; Ricci, P.; Tursi, A.; D’Onghia, G. Cold-Water Coral Habitats and Canyons as Essential Fish Habitats in the Southern Adriatic and Northern Ionian Sea (Central Mediterranean). Ecol. Quest. 2018, 29, 9–23. [Google Scholar] [CrossRef]
- D’Onghia, G. Cold-Water Corals as Shelter, Feeding and Life-History Critical Habitats for Fish Species: Ecological Interactions and Fishing Impact. In Mediterranean Cold-Water Corals: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 335–356. [Google Scholar]
- Garcia, S.M. Ecosystem Approach to Fisheries: Issue, Terminology, Principles, Institutional Foundations, Implementation and Outlook. FAO Fish Tech. Pap. 2003, 443, 1–71. [Google Scholar]
- de Juan, S.; Lleonart, J. A Conceptual Framework for the Protection of Vulnerable Habitats Impacted by Fishing Activities in the Mediterranean High Seas. Ocean. Coast. Manag. 2010, 53, 717–723. [Google Scholar] [CrossRef]
- de Juan, S.; Moranta, J.; Hinz, H.; Barberá, C.; Ojeda-Martinez, C.; Oro, D.; Ordines, F.; Ólafsson, E.; Demestre, M.; Massutí, E. A Regional Network of Sustainable Managed Areas as the Way Forward for the Implementation of an Ecosystem-Based Fisheries Management in the Mediterranean. Ocean. Coast. Manag. 2012, 65, 51–58. [Google Scholar] [CrossRef]
- Tursi, A.; Maiorano, P.; Sion, L.; D’Onghia, G. Fishery Resources: Between Ecology and Economy. Rend. Fis. Acc. Lincei 2015, 26, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Grehan, A.J.; Arnaud-Haond, S.; D’Onghia, G.; Savini, A.; Yesson, C. Towards Ecosystem Based Management and Monitoring of the Deep Mediterranean, North-East Atlantic and Beyond. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 1–7. [Google Scholar] [CrossRef]
- Otero, M.D.M.; Marin, P. 46 Conservation of Cold-Water Corals in the Mediterranean: Current Status and Future Prospects for Improvement. In Mediterranean Cold-Water Corals: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 535–545. [Google Scholar]
- Relini, G.; Orsi Relini, L. The Decline of Red Shrimps Stocks in the Gulf of Genoa. Investig. Pesq. 1987, 51, 254–260. [Google Scholar]
- Relini, G. La Pesca Batiale in Liguria. Biol. Mar. Mediterr. 2007, 14, 190–244. [Google Scholar]
- Sardà, F.; D’Onghia, G.; Politou, C.Y.; Company, J.B.; Maiorano, P.; Kapiris, K. Deep-Sea Distribution, Biological and Ecological Aspects of Aristeus Antennatus (Risso, 1816) in the Western and Central Mediterranean Sea. Sci. Mar. 2004, 68, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Würtz, M.; Rovere, M. Atlas of the Mediterranean Seamounts and Seamount-like Structures; IUCN Gland: Gland, Switzerland, 2015; ISBN 2-8317-1750-7. [Google Scholar]
- Fernandez-Arcaya, U.; Ramirez-Llodra, E.; Aguzzi, J.; Allcock, A.L.; Davies, J.S.; Dissanayake, A.; Harris, P.; Howell, K.; Huvenne, V.A.I.; Macmillan-Lawler, M.; et al. Ecological Role of Submarine Canyons and Need for Canyon Conservation: A Review. Front. Mar. Sci. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Rueda, J.L.; Urra, J.; Aguilar, R.; Angeletti, L.; Bo, M.; García-Ruiz, C.; González-Duarte, M.M.; López, E.; Madurell, T.; Maldonado, M. 29 Cold-Water Coral Associated Fauna in the Mediterranean Sea and Adjacent Areas. In Mediterranean Cold-Water Corals: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 295–333. [Google Scholar]
- Sion, L.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Cornacchia, L.; Maiorano, P.; Pollice, A.; Ricci, P.; Tursi, A.; et al. Does the Bari Canyon (Central Mediterranean) Influence the Fish Distribution and Abundance? Prog. Oceanogr. 2019, 170, 81–92. [Google Scholar] [CrossRef]
- Armstrong, C.W.; Falk-Petersen, J. Habitat–Fisheries Interactions: A Missing Link? ICES J. Mar. Sci./J. Cons. 2008, 65, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Komyakova, V.; Munday, P.L.; Jones, G.P. Comparative Analysis of Habitat Use and Ontogenetic Habitat-Shifts among Coral Reef Damselfishes. Environ. Biol. Fishes 2019, 102, 1201–1218. [Google Scholar] [CrossRef]
- Würtz, M. Submarine Canyons and Their Role in the Mediterranean Ecosystem. In Mediterranean Submarine Canyons; Würtz, M., Ed.; IUCN: Gland, Switzerland; Málaga, Spain; Mediterranean Submarine Canyons: Ecology and Governance: Gland, Switzerland; Málaga, Spain, 2012; pp. 11–26. [Google Scholar]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 3-319-21012-2. [Google Scholar]
- IUCN. Thematic Report—Conservation Overview of Mediterranean Deep-Sea Biodiversity: A Strategic Assessment; IUCN: Gland, Switzerland; Malaga, Spain, 2019; 122p. [Google Scholar]
- Orejas, C.; Jiménez, C. 1 An Introduction to the Research on Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jiménez, C., Eds.; Coral Reefs of the World; Springer International Publishing: Cham, Switzerland, 2019; Volume 9, pp. 3–12. ISBN 978-3-319-91607-1. [Google Scholar]
- D’Onghia, G.; Maiorano, P.; Carlucci, R.; Capezzuto, F.; Carluccio, A.; Tursi, A.; Sion, L. Comparing Deep-Sea Fish Fauna between Coral and Non-Coral “Megahabitats” in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea). PLoS ONE 2012, 7, e44509. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Cardone, F.; Carlucci, R.; Carluccio, A.; Chimienti, G.; Corriero, G.; Longo, C.; Maiorano, P.; Mastrototaro, F.; et al. Macro- and Megafauna Recorded in the Submarine Bari Canyon (Southern Adriatic, Mediterranean Sea) Using Different Tools. Medit. Mar. Sci. 2015, 16, 180. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Carluccio, A.; Carlucci, R.; Giove, A.; Mastrototaro, F.; Panza, M.; Sion, L.; Tursi, A.; Maiorano, P. Exploring Composition and Behaviour of Fish Fauna by in Situ Observations in the Bari Canyon (Southern Adriatic Sea, Central Mediterranean). Mar. Ecol. 2015, 36, 541–556. [Google Scholar] [CrossRef]
- D’Onghia, G.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Pollice, A.; Ricci, P.; Sion, L.; Tursi, A. New Records of Cold-Water Coral Sites and Fish Fauna Characterization of a Potential Network Existing in the Mediterranean Sea. Mar. Ecol. 2016, 37, 1398–1422. [Google Scholar] [CrossRef]
- D’Onghia, G.; Calculli, C.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Grehan, A.; Indennidate, A.; Maiorano, P.; Mastrototaro, F.; Pollice, A. Anthropogenic Impact in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea): Observations and Conservation Straits. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 87–101. [Google Scholar] [CrossRef]
- D’Onghia, G.; Sion, L.; Capezzuto, F. Cold-Water Coral Habitats Benefit Adjacent Fisheries along the Apulian Margin (Central Mediterranean). Fish. Res. 2019, 213, 172–179. [Google Scholar] [CrossRef]
- Capezzuto, F.; Calculli, C.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Pollice, A.; Sion, L.; Tursi, A.; D’Onghia, G. Revealing the Coral Habitat Effect on Benthopelagic Fauna Diversity in the Santa Maria Di Leuca Cold-water Coral Province Using Different Devices and Bayesian Hierarchical Modelling. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1608–1622. [Google Scholar] [CrossRef]
- Capezzuto, F.; Ancona, F.; Calculli, C.; Sion, L.; Maiorano, P.; D’Onghia, G. Feeding of the Deep-Water Fish Helicolenus dactylopterus (Delaroche, 1809) in Different Habitats: From Muddy Bottoms to Cold-Water Coral Habitats. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 159, 103252. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS Trawl Survey Specifications in an Ecosystem Approach to Fishery Management. Sci. Mar. 2019, 83, 9. [Google Scholar] [CrossRef]
- Capezzuto, F.; Carlucci, R.; Maiorano, P.; Sion, L.; Battista, D.; Giove, A.; Indennidate, A.; Tursi, A.; D’Onghia, G. The Bathyal Benthopelagic Fauna in the North-Western Ionian Sea: Structure, Patterns and Interactions. Chem. Ecol. 2010, 26, 199–217. [Google Scholar] [CrossRef]
- Maiorano, P.; Sion, L.; Carlucci, R.; Capezzuto, F.; Giove, A.; Costantino, G.; Panza, M.; D’Onghia, G.; Tursi, A. The Demersal Faunal Assemblage of the North-Western Ionian Sea (Central Mediterranean): Current Knowledge and Perspectives. Chem. Ecol. 2010, 26, 219–240. [Google Scholar] [CrossRef]
- Maiorano, P.; Sabatella, R.F.; Marzocchi, B.M. (Eds.) Annuario sullo Stato delle Risorse e sulle Strutture Produttive dei Mari Italiani; SIBM: Genova, Italy, 2015; 432p. [Google Scholar]
- Tursi, A.; Mastrototaro, F.; Matarrese, A.; Maiorano, P.; D’Onghia, G. Biodiversity of the White Coral Reefs in the Ionian Sea (Central Mediterranean). Chem. Ecol. 2004, 20, 107–116. [Google Scholar] [CrossRef]
- Taviani, M.; Remia, A.; Corselli, C.; Freiwald, A.; Malinverno, E.; Mastrototaro, F.; Savini, A.; Tursi, A. First Geo-Marine Survey of Living Cold-Water Lophelia Reefs in the Ionian Sea (Mediterranean Basin). Facies 2005, 50, 409–417. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Beuck, L.; Campiani, E.; Canese, S.; Foglini, F.; Freiwald, A.; Montagna, P.; Trincardi, F. Reprint of ‘On and off the Beaten Track: Megafaunal Sessile Life and Adriatic Cascading Processes’. Mar. Geol. 2016, 375, 146–160. [Google Scholar] [CrossRef]
- Morelli, D. La Cartografia Marina: Ricerche Ed Applicazioni Orientate Ai Rischi Geologico-Ambientali in Aree Campione. Ph.D. Thesis, University of Genoa, Genoa, Italy, 2008. [Google Scholar]
- Freiwald, A.; Beuck, L.; Rüggeberg, A.; Taviani, M.; Hebbeln, D.; R/V Meteor Cruise M70-1 Participants. The White Coral Community in the Central Mediterranean Sea Revealed by ROV Surveys. Oceanography 2009, 22, 58–74. [Google Scholar] [CrossRef] [Green Version]
- Mastrototaro, F.; D’Onghia, G.; Corriero, G.; Matarrese, A.; Maiorano, P.; Panetta, P.; Gherardi, M.; Longo, C.; Rosso, A.; Sciuto, F.; et al. Biodiversity of the White Coral Bank off Cape Santa Maria Di Leuca (Mediterranean Sea): An Update. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 412–430. [Google Scholar] [CrossRef]
- Mastrototaro, F.; Maiorano, P.; Vertino, A.; Battista, D.; Indennidate, A.; Savini, A.; Tursi, A.; D’Onghia, G. A Facies of Kophobelemnon (C Nidaria, O Ctocorallia) from S Anta M Aria Di L Euca Coral Province (Mediterranean Sea). Mar. Ecol. 2013, 34, 313–320. [Google Scholar] [CrossRef]
- Angeletti, L.; Taviani, M.; Canese, S.; Foglini, F.; Mastrototaro, F.; Argnani, A.; Trincardi, F.; Bakran-Petricioli, T.; Ceregato, A.; Chimienti, G. New Deep-Water Cnidarian Sites in the Southern Adriatic Sea. Mediterr. Mar. Sci. 2014, 15, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Carbonara, P.; Zupa, W.; Follesa, M.C.; Cau, A.; Capezzuto, F.; Chimienti, G.; D’Onghia, G.; Lembo, G.; Pesci, P.; Porcu, C.; et al. Exploring a Deep-Sea Vulnerable Marine Ecosystem: Isidella elongata (Esper, 1788) Species Assemblages in the Western and Central Mediterranean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 166, 103406. [Google Scholar] [CrossRef]
- Pérès, J.-M.; Picard, J. Nouveau Manuel de Bionomie Benthique de La Mer Méditerranée. Recueil des Travaux de la Station marine d’Endoume 1964, 31, 1–137. [Google Scholar]
- Azouz, A. Les Fonds Chalutables de La Région Nord de La Tunisie: 1. Cadre Physique et Biocoénoses Benthiques. Bull. Inst. Océanogr. Pêche. Salammbô 1973, 2, 473–559. [Google Scholar]
- Maynou, F.; Sardà, F.; Tudela, S.; Demestre, M. Management Strategies for Red Shrimp (Aristeus Antennatus) Fisheries in the Catalan Sea (NW Mediterranean) Based on Bioeconomic Simulation Analysis. Aquat. Living Resour. 2006, 19, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Kapiris, K. Feeding Habits of Both Deep-Water Red Shrimps, Aristaeomorpha foliacea and Aristeus antennatus (Decapoda, Aristeidae) in the Ionian Sea (E. Mediterranean). In Food Quality; Kapiris, K., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0560-2. [Google Scholar]
- Maynou, F.; Cartes, J.E. Effects of Trawling on Fish and Invertebrates from Deep-Sea Coral Facies of Isidella Elongata in the Western Mediterranean. J. Mar. Biol. Ass. 2012, 92, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Cartes, J.E.; LoIacono, C.; Mamouridis, V.; López-Pérez, C.; Rodríguez, P. Geomorphological, Trophic and Human Influences on the Bamboo Coral Isidella Elongata Assemblages in the Deep Mediterranean: To What Extent Does Isidella Form Habitat for Fish and Invertebrates? Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 76, 52–65. [Google Scholar] [CrossRef]
- Smith, C.J.; Mytilineou, C.; Papadopoulou, K.N.; Pancucci-Papadopoulou, M.A.; Salomidi, M. ROV Observations on Fish and Megafauna in Deep Coral Areas of the Eastern Ionian. Rapp. Comm. Int. L’exploration Sci. Mer Méditerranée 2010, 39, 669. [Google Scholar]
- Lauria, V.; Garofalo, G.; Fiorentino, F.; Massi, D.; Milisenda, G.; Piraino, S.; Russo, T.; Gristina, M. Species Distribution Models of Two Critically Endangered Deep-Sea Octocorals Reveal Fishing Impacts on Vulnerable Marine Ecosystems in Central Mediterranean Sea. Sci. Rep. 2017, 7, 8049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrototaro, F.; Chimienti, G.; Acosta, J.; Blanco, J.; Garcia, S.; Rivera, J.; Aguilar, R. Isidella Elongata (Cnidaria: Alcyonacea) Facies in the Western Mediterranean Sea: Visual Surveys and Descriptions of Its Ecological Role. Eur. Zool. J. 2017, 84, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Mytilineou, C.h.; Smith, C.J.; Anastasopoulou, A.; Papadopoulou, K.N.; Christidis, G.; Bekas, P.; Kavadas, S.; Dokos, J. New Cold-Water Coral Occurrences in the Eastern Ionian Sea: Results from Experimental Long Line Fishing. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 146–157. [Google Scholar] [CrossRef]
- Eumofa. The EU Fish Market, 107. 2019. Available online: https://doi.org/10.2771/168390 (accessed on 6 March 2022).
- Chimienti, G.; De Padova, D.; Mossa, M.; Mastrototaro, F. A Mesophotic Black Coral Forest in the Adriatic Sea. Sci. Rep. 2020, 10, 8504. [Google Scholar] [CrossRef]
- Cau, A.; Follesa, M.C.; Moccia, D.; Bellodi, A.; Mulas, A.; Bo, M.; Canese, S.; Angiolillo, M.; Cannas, R. Leiopathes glaberrima Millennial Forest from SW Sardinia as Nursery Ground for the Small Spotted Catshark Scyliorhinus Canicula: Catshark Nursery Ground in a Millennial Black Coral Forest. Aquatic Conserv. Mar. Freshw. Ecosyst. 2017, 27, 731–735. [Google Scholar] [CrossRef]
- Hebbeln, D. Report and Preliminary Results of RV POSEIDON Cruise POS 385 “Cold-Water Corals of the Alboran Sea (Western Mediterranean Sea)”, Faro-Toulon, 29 May–16 June 2009. Ber. Fachbereich Geowiss. Univ. Brem. 2009, 273, 1–79. [Google Scholar]
- Angeletti, L.; Mecho, A.; Doya, C.; Micallef, A.; Huvenne, V.; Georgiopoulou, A.; Taviani, M. First Report of Live Deep-Water Cnidarian Assemblages from the Malta Escarpment. Ital. J. Zool. 2015, 82, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.; Sanders, J.; Tandstad, M.; Carocci, F.; Fuller, J. Vulnerable Marine Ecosystems: Processes and Practices in the High Seas. In FAO Fisheries and Aquaculture Technical Paper; FAO: Roma, Italy, 2016. [Google Scholar]
- Freiwald, A.; Fossa, J.H.; Grehan, A.; Koslow, T.; Roberts, J.M. Cold-Water Coral Reefs: Out of Sight-No Longer out of Mind; UNEP-WCMC: Cambridge, UK, 2004; ISBN 92-807-2453-3. [Google Scholar]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the Deep: The Biology and Geology of Cold-Water Coral Ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebbeln, D.; Van Rooij, D.; Wienberg, C. Good Neighbours Shaped by Vigorous Currents: Cold-Water Coral Mounds and Contourites in the North Atlantic. Mar. Geol. 2016, 378, 171–185. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Vanreusel, A.; Gooday, A.J.; Levin, L.A.; Priede, I.G.; Buhl-Mortensen, P.; Gheerardyn, H.; King, N.J.; Raes, M. Biological Structures as a Source of Habitat Heterogeneity and Biodiversity on the Deep Ocean Margins: Biological Structures and Biodiversity. Mar. Ecol. 2010, 31, 21–50. [Google Scholar] [CrossRef]
- Linley, T.D.; Lavaleye, M.; Maiorano, P.; Bergman, M.; Capezzuto, F.; Cousins, N.J.; D’Onghia, G.; Duineveld, G.; Shields, M.A.; Sion, L. Effects of Cold-water Corals on Fish Diversity and Density (European Continental Margin: Arctic, NE Atlantic and Mediterranean Sea): Data from Three Baited Lander Systems. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 8–21. [Google Scholar] [CrossRef]
- Orsi Relini, L.; Relini, G. The Red Shrimps Fishery in the Ligurian Sea: Mismanagement or Not? FAO Fish. Rep. 1985, 336, 99–106. [Google Scholar]
- Tobar, R.; Sardà, F. Análisis de La Evolución de Las Capturas de Gamba Rosada, Aristeus antennatus (Risso, 1816), En Los Últimos Decenios En Cataluña. Inf. Técn. Inv. Pesq. 1987, 142, 3–20. [Google Scholar]
- Sardà, F.; Cartes, J.E. Spatio-Temporal Variations in Megabenthos Abundance in Three Different Habitats of the Catalan Deep-Sea (Western Mediterranean). Mar. Biol. 1994, 120, 211–219. [Google Scholar] [CrossRef]
- Sardà, F.; Company, J.B.; Bahamón, N.; Rotllant, G.; Flexas, M.M.; Sánchez, J.D.; Zúñiga, D.; Coenjaerts, J.; Orellana, D.; Jordà, G.; et al. Relationship between Environment and the Occurrence of the Deep-Water Rose Shrimp Aristeus antennatus (Risso, 1816) in the Blanes Submarine Canyon (NW Mediterranean). Prog. Oceanogr. 2009, 82, 227–238. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Company, J.B.; Sardà, F.; Rotllant, G. Megabenthic Diversity Patterns and Community Structure of the Blanes Submarine Canyon and Adjacent Slope in the Northwestern Mediterranean: A Human Overprint?: Megabenthic Diversity in a Mediterranean Canyon and Slope. Mar. Ecol. 2010, 31, 167–182. [Google Scholar] [CrossRef]
- Puig, P.; Company, J.B.; Sardà, F.; Palanques, A. Responses of Deep-Water Shrimp Populations to Intermediate Nepheloid Layer Detachments on the Northwestern Mediterranean Continental Margin. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 2195–2207. [Google Scholar] [CrossRef]
- Fernandez-Arcaya, U.; Rotllant, G.; Ramirez-Llodra, E.; Recasens, L.; Aguzzi, J.; Flexas, M.M.; Sanchez-Vidal, A.; López-Fernández, P.; García, J.A.; Company, J.B. Reproductive Biology and Recruitment of the Deep-Sea Fish Community from the NW Mediterranean Continental Margin. Prog. Oceanogr. 2013, 118, 222–234. [Google Scholar] [CrossRef]
- Orejas, C.; Gori, A.; Iacono, C.L.; Puig, P.; Gili, J.-M.; Dale, M.R. Cold-Water Corals in the Cap de Creus Canyon, Northwestern Mediterranean: Spatial Distribution, Density and Anthropogenic Impact. Mar. Ecol. Prog. Ser. 2009, 397, 37–51. [Google Scholar] [CrossRef]
- Cau, A.; Alvito, A.; Moccia, D.; Canese, S.; Pusceddu, A.; Rita, C.; Angiolillo, M.; Follesa, M.C. Submarine Canyons along the Upper Sardinian Slope (Central Western Mediterranean) as Repositories for Derelict Fishing Gears. Mar. Pollut. Bull. 2017, 123, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Taviani, M.; Angeletti, L.; Canese, S.; Cannas, R.; Cardone, F.; Cau, A.; Cau, A.B.; Follesa, M.C.; Marchese, F.; Montagna, P. The “Sardinian Cold-Water Coral Province” in the Context of the Mediterranean Coral Ecosystems. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2017, 145, 61–78. [Google Scholar] [CrossRef]
- Sabatini, A.; Follesa, M.C.; Locci, I.; Pendugiu, A.A.; Pesci, P.; Cau, A. Assemblages in a Submarine Canyon: Influence of Depth and Time. In Biodiversity in Enclosed Seas and Artificial Marine Habitats; Springer: Berlin/Heidelberg, Germany, 2007; pp. 265–271. [Google Scholar]
- Yoklavich, M.M.; Greene, H.G.; Cailliet, G.M.; Sullivan, D.E.; Lea, R.N.; Love, M.S. Habitat Associations of Deep-Water Rockfishes in a Submarine Canyon: An Example of a Natural Refuge. Fish. Bull. 2000, 98, 625–641. [Google Scholar]
- Tudela, S.; Simard, F.; Skinner, J.; Guglielmi, P. The Mediterranean Deep-Sea Ecosystems: A Proposal for Their Conservation. In The Mediterranean Deep-Sea Ecosystems: An Overview of Their Diversity, Structure, Functioning and Anthropogenic Impacts, with a Proposal for Conservation; IUCN Publications Services: Málaga, Spain; Rome, Italy, 2004; pp. 39–47. [Google Scholar]
- Angeletti, L.; D’Onghia, G.; Otero, M.D.M.; Settanni, A.; Spedicato, M.T.; Taviani, M. A Perspective for Best Governance of the Bari Canyon Deep-Sea Ecosystems. Water 2021, 13, 1646. [Google Scholar] [CrossRef]
- Zibrowius, H.; Taviani, M. Remarkable Sessile Fauna Associated with Deep Coral and Other Calcareous Substrates in the Strait of Sicily, Mediterranean Sea. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Erlangen Earth Conference Series; Springer: Berlin/Heidelberg, Germany, 2005; pp. 807–819. ISBN 978-3-540-24136-2. [Google Scholar]
- Bo, M.; Bertolino, M.; Borghini, M.; Castellano, M.; Covazzi Harriague, A.; Di Camillo, C.G.; Gasparini, G.; Misic, C.; Povero, P.; Pusceddu, A.; et al. Characteristics of the Mesophotic Megabenthic Assemblages of the Vercelli Seamount (North Tyrrhenian Sea). PLoS ONE 2011, 6, e16357. [Google Scholar] [CrossRef] [Green Version]
- Orsi Relini, L.; Relini, G. Gaidropsarus Granti from a Ligurian Seamount: A Mediterranean Native Species? Mar. Ecol. 2014, 35, 35–40. [Google Scholar] [CrossRef]
- Sabatini, A.; Follesa, M.C.; Locci, I.; Matta, G.; Palmas, F.; Pendugiu, A.A.; Pesci, P.; Cau, A. Demersal Assemblages in Two Trawl Fishing Lanes Located on the Baronie Seamount (Central Western Mediterranean). J. Mar. Biol. Ass. 2011, 91, 65–75. [Google Scholar] [CrossRef]
- Galil, B.; Zibrowius, H. First Benthos Samples from Eratosthenes Seamount, Eastern Mediterranean. Senckenbergiana Marit. 1998, 28, 111–121. [Google Scholar] [CrossRef]
- Öztürk, B.; Topcu, E.N.; Topaloglu, B. A Preliminary Study On Two Seamounts In The Eastern Mediterranean Sea. Rapp. Comm. Int. Mer Médit. 2010, 39, 620. [Google Scholar]
- Sperone, E.; Parise, G.; Leone, A.; Milazzo, C.; Circosta, V.; Santoro, G.; Paolillo, G.; Micarelli, P.; Tripepi, S. Spatiotemporal Patterns of Distribution of Large Predatory Sharks in Calabria (Central Mediterranean, Southern Italy). Acta Adriat. 2012, 53, 13–24. [Google Scholar]
- De la Torriente Diez, A.; González-Irusta, J.M.; Serrano, A.; Aguilar, R.; Sánchez, F.; Blanco, M.; Punzón, A. Spatial Assessment of Benthic Habitats Vulnerability to Bottom Fishing in a Mediterranean Seamount. Mar. Policy 2022, 135, 104850. [Google Scholar] [CrossRef]
- Trincardi, F.; Foglini, F.; Verdicchio, G.; Asioli, A.; Correggiari, A.; Minisini, D.; Piva, A.; Remia, A.; Ridente, D.; Taviani, M. The Impact of Cascading Currents on the Bari Canyon System, SW-Adriatic Margin (Central Mediterranean). Mar. Geol. 2007, 246, 208–230. [Google Scholar] [CrossRef]
- Budillon, G.; Bue, N.L.; Siena, G.; Spezie, G. Hydrographic Characteristics of Water Masses and Circulation in the Northern Ionian Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 441–457. [Google Scholar] [CrossRef]
- Gacic, M.; Schroeder, K.; Civitarese, G.; Vetrano, A.; Borzelli, G.E. On the Relationship among the Adriatic–Ionian Bimodal Oscillating System (BiOS), the Eastern Mediterranean Salinity Variations and the Western Mediterranean Thermohaline Cell. BiOS 2012, 9, 2561–2580. [Google Scholar]
- D’Onghia, G.; Indennidate, A.; Giove, A.; Savini, A.; Capezzuto, F.; Sion, L.; Vertino, A.; Maiorano, P. Distribution and Behaviour of Deep-Sea Benthopelagic Fauna Observed Using Towed Cameras in the Santa Maria Di Leuca Cold-Water Coral Province. Mar. Ecol. Prog. Ser. 2011, 443, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, M.; Zupa, W.; Ligas, A.; Capezzuto, F.; Chatzispyrou, A.; Follesa, M.C.; Gancitano, V.; Guijarro, B.; Isajlovic, I.; Jadaud, A. Spatiotemporal Abundance Pattern of Deep-Water Rose Shrimp, Parapenaeus Longirostris, and Norway Lobster, Nephrops Norvegicus, in European Mediterranean Waters. Sci. Mar. 2019, 83, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Cartes, J.E.; Maynou, F.; Fanelli, E.; Papiol, V.; Lloris, D. Long-Term Changes in the Composition and Diversity of Deep-Slope Megabenthos and Trophic Webs off Catalonia (Western Mediterranean): Are Trends Related to Climatic Oscillations? Prog. Oceanogr. 2009, 82, 32–46. [Google Scholar] [CrossRef]
- Carpentieri, P.; Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G.D. Feeding Habits of European Hake (Merluccius merluccius) in the Central Mediterranean Sea. Fish. Bull. 2005, 103, 411–416. [Google Scholar]
- Bartolino, V.; Colloca, F.; Sartor, P.; Ardizzone, G. Modelling Recruitment Dynamics of Hake, Merluccius merluccius, in the Central Mediterranean in Relation to Key Environmental Variables. Fish. Res. 2008, 92, 277–288. [Google Scholar] [CrossRef]
- Guijarro, B.; Bitetto, I.; D’Onghia, G.; Follesa, M.C.; Kapiris, K.; Mannini, A.; Marković, O.; Micallef, R.; Ragonese, S.; Skarvelis, K. Spatial and Temporal Patterns in the Mediterranean Populations of Aristaeomorpha foliacea and Aristeus antennatus (Crustacea: Decapoda: Aristeidae) Based on the MEDITS Surveys. Sci. Mar. 2019, 83, 57–70. [Google Scholar] [CrossRef]
- Sion, L.; Zupa, W.; Calculli, C.; Garofalo, G.; Hidalgo, M.; Jadaud, A.; Lefkaditou, E.; Ligas, A.; Peristeraki, P.; Bitetto, I. Spatial Distribution Pattern of European Hake, Merluccius merluccius (Pisces: Merlucciidae), in the Mediterranean Sea. Sci. Mar. 2019, 83, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, R.; Lembo, G.; Maiorano, P.; Capezzuto, F.; Mariano, C.A.; Sion, L.; Spedicato, M.T.; Ungaro, N.; Tursi, A.; D’Onghia, G. Nursery Areas of Red Mullet (Mullus Barbatus), Hake (Merluccius merluccius) and Deep-Water Rose Shrimp (Parapenaeus Longirostris) in the Eastern-Central Mediterranean Sea. Estuar. Coast. Shelf Sci. 2009, 83, 529–538. [Google Scholar] [CrossRef]
- Murenu, M.; Cau, A.; Colloca, F.; Sartor, P.; Fiorentino, F.; Garofalo, G.; Piccinetti, C.; Manfredi, C.; D′Onghia, G.; Carlucci, R. Mapping the Potential Locations of European Hake (Merluccius merluccius) Nurseries in the Italian Waters. In GIS/Spatial Analyses in Fishery and Aquatic Sciences; Nishida, T., Kailola, P.J., Hollingworth, C.E., Eds.; FAO Fishery Gis Research Group: Rome, Italy, 2010; Volume 4. [Google Scholar]
- Druon, J.-N.; Fiorentino, F.; Murenu, M.; Knittweis, L.; Colloca, F.; Osio, C.; Mérigot, B.; Garofalo, G.; Mannini, A.; Jadaud, A.; et al. Modelling of European Hake Nurseries in the Mediterranean Sea: An Ecological Niche Approach. Prog. Oceanogr. 2015, 130, 188–204. [Google Scholar] [CrossRef]
- Hidalgo, M.; Ligas, A.; Bellido, J.M.; Bitetto, I.; Carbonara, P.; Carlucci, R.; Guijarro, B.; Jadaud, A.; Lembo, G.; Manfredi, C. Size-Dependent Survival of European Hake Juveniles in the Mediterranean Sea. Sci. Mar. 2019, 83, 207–221. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Zupa, W.; Carbonara, P.; Casciaro, L.; Bitetto, I.; Facchini, M.T.; Gaudio, P.; Palmisano, M.; Lembo, G. Lo Stato Delle Risorse Biologiche e Della Pesca Nel Basso Adriatico e Nello Ionio Nord Occidentale. In Atti del Convegno il Mare Adriatico e le sue Risorse; Marini, M., Bombace, G., Iacobone, G., Eds.; Carlo Saladino Editore: Palermo, Italy, 2017; pp. 177–208. [Google Scholar]
- Carlucci, R.; Bandelj, V.; Ricci, P.; Capezzuto, F.; Sion, L.; Maiorano, P.; Tursi, A.; Solidoro, C.; Libralato, S. Exploring Spatio-Temporal Changes in the Demersal and Benthopelagic Assemblages of the North-Western Ionian Sea (Central Mediterranean Sea). Mar. Ecol. Prog. Ser. 2018, 598, 1–19. [Google Scholar] [CrossRef]
- Carlucci, R.; Capezzuto, F.; Cipriano, G.; D’Onghia, G.; Fanizza, C.; Libralato, S.; Maglietta, R.; Maiorano, P.; Sion, L.; Tursi, A.; et al. Assessment of Cetacean–Fishery Interactions in the Marine Food Web of the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). Rev. Fish Biol. Fish. 2021, 31, 135–156. [Google Scholar] [CrossRef]
- Carlucci, R.; Manea, E.; Ricci, P.; Cipriano, G.; Fanizza, C.; Maglietta, R.; Gissi, E. Managing Multiple Pressures for Cetaceans’ Conservation with an Ecosystem-Based Marine Spatial Planning Approach. J. Environ. Manag. 2021, 287, 112240. [Google Scholar] [CrossRef]
- Cipriano, G.; Carlucci, R.; Bellomo, S.; Santacesaria, F.C.; Fanizza, C.; Ricci, P.; Maglietta, R. Behavioral Pattern of Risso’s Dolphin (Grampus griseus) in the Gulf of Taranto (Northern Ionian Sea, Central-Eastern Mediterranean Sea). JMSE 2022, 10, 175. [Google Scholar] [CrossRef]
- Foskolos, I.; Koutouzi, N.; Polychronidis, L.; Alexiadou, P.; Frantzis, A. A Taste for Squid: The Diet of Sperm Whales Stranded in Greece, Eastern Mediterranean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 155, 103164. [Google Scholar] [CrossRef]
- Luna, A.; Sánchez, P.; Chicote, C.; Gazo, M. Cephalopods in the Diet of Risso’s Dolphin (Grampus griseus) from the Mediterranean Sea: A Review. Mar. Mammal. Sci. 2022, 38, 725–741. [Google Scholar] [CrossRef]
- Ricci, P.; Ingrosso, M.; Cipriano, G.; Fanizza, C.; Maglietta, R.; Renò, V.; Tursi, A.; Carlucci, R. Top-down Cascading Effects Driven by the Odontocetes in the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). In Proceedings of the IMEKO Metrology for the Sea, Naples, Italy, 5–7 October 2020. [Google Scholar]
- Ricci, P.; Manea, E.; Cipriano, G.; Cascione, D.; D’Onghia, G.; Ingrosso, M.; Fanizza, C.; Maiorano, P.; Tursi, A.; Carlucci, R. Addressing Cetacean–Fishery Interactions to Inform a Deep-Sea Ecosystem-Based Management in the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). J. Mar. Sci. Eng. 2021, 9, 872. [Google Scholar] [CrossRef]
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.B.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as Marine Ecosystem Engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Llodra, E.; Tyler, P.A.; Baker, M.C.; Bergstad, O.A.; Clark, M.R.; Escobar, E.; Levin, L.A.; Menot, L.; Rowden, A.A.; Smith, C.R.; et al. Man and the Last Great Wilderness: Human Impact on the Deep Sea. PLoS ONE 2011, 6, e22588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, L.A.; Le Bris, N. The Deep Ocean under Climate Change. Science 2015, 350, 766–768. [Google Scholar] [CrossRef] [Green Version]
- Paulus, E. Shedding Light on Deep-Sea Biodiversity—A Highly Vulnerable Habitat in the Face of Anthropogenic Change. Front. Mar. Sci. 2021, 8, 281. [Google Scholar] [CrossRef]
- Díaz, S.; Fargione, J.; Chapin, F.S., III; Tilman, D. Biodiversity Loss Threatens Human Well-Being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef] [Green Version]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R.; et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Armstrong, C.W. Cold Water Coral Reef Management from an Ecosystem Service Perspective. Mar. Policy 2014, 50, 126–134. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J. The Value of the World’s Ecosystem Services and Natural Capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Millenniumeco System Assessment. Ecosystems and Human Well-Being: Wetlands and Water; World Resources Institute: Washington, DC, USA, 2005; ISBN 1-56973-597-2. [Google Scholar]
- Fonds Mondial pour la Nature. Living Planet Report 2010: Biodiversity, Biocapacity and Development; World Wide Fund for Nature: Gland, Switzerland, 2010; ISBN 978-2-940443-08-6. [Google Scholar]
- Armstrong, C.W.; Grehan, A.J.; Kahui, V.; Mikkelsen, E.; Reithe, S.; van den Hove, S. Bioeconomic Modeling and the Management of Cold-Water Coral Resources. Oceanography 2009, 22, 86–91. [Google Scholar] [CrossRef]
- Foley, N.S. The Ecological and Economic Value of Cold-Water Coral Ecosystems. Ocean Coast. Manag. 2010, 53, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.I.; Clarke, K.R. Effects of Demersal Trawling on Ecosystem Functioning in the North Sea: A Modelling Study. Mar. Ecol. Prog. Ser. 2007, 336, 63–75. [Google Scholar] [CrossRef]
- Armstrong, C.W.; Foley, N.S.; Tinch, R.; van den Hove, S. Services from the Deep: Steps towards Valuation of Deep Sea Goods and Services. Ecosyst. Serv. 2012, 2, 2–13. [Google Scholar] [CrossRef]
- Pikitch Habitat Use and Demographic Population Structure of Elasmobranchs at a Caribbean Atoll (Glover’s Reef, Belize). Mar. Ecol. Prog. Ser. 2005, 302, 187–197. [CrossRef]
- UNEP. Report of the Conference of the Parties to the Convention on Biological Diversity on the Work of Its Ninth Meeting, UNEP/CBD/COP/9/29. In Proceedings of the Conference of the Parties to the Convention on Biological Diversity Ninth Meeting, Bonn, Germany, 19–30 May 2008. [Google Scholar]
- Secretariat of the Convention on Biological Diversity. Global Biodiversity Outlook 4; Secretariat of the Convention on Biological Diversity: Montréal, QC, Canada, 2014; 155p. [Google Scholar]
- UN. Sustainable Fisheries, Including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and Related Instruments; UNGA A/RES/59/25. In Proceedings of the The 59th Session of the United Nations General Assembly, New York, NY, USA, 14 September 2004. [Google Scholar]
- UN. Sustainannuable fisheries, including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish. Stocks and Highly Migratory Fish Stocks, and related instruments. UNGA A/RES/61/105. In Proceedings of the The 61th Session of the United Nations General Assembly, New York, NY, USA, 12 September 2006. [Google Scholar]
- UN. Sustainable fisheries, including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and related instruments UNGA A/RES/64/72. In Proceedings of the The 64th Session of the United Nations General Assembly, New York, NY, USA, 15 September 2009. [Google Scholar]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Marine Spatial Plan Directive 2014/89/EU. Available online: http://www.marsplan.ro/en/common-msp/directive-2014-89-eu.html (accessed on 8 March 2022).
- Oceana. Oceana MedNet, MPA Proposal for the Mediterranean Sea. 100 Reasons to Reach 10%; Oceana: Madrid, Spain, 2011. [Google Scholar]
- Micheli, F.; Levin, N.; Giakoumi, S.; Katsanevakis, S.; Abdulla, A.; Coll, M.; Fraschetti, S.; Kark, S.; Koutsoubas, D.; Mackelworth, P.; et al. Setting Priorities for Regional Conservation Planning in the Mediterranean Sea. PLoS ONE 2013, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Bastari, A.; Pica, D.; Ferretti, F.; Micheli, F.; Cerrano, C. Sea Pens in the Mediterranean Sea: Habitat Suitability and Opportunities for Ecosystem Recovery. ICES J. Mar. Sci. 2018, 75, 1722–1732. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- European Union. Communication From The Commission To The European Parliament, The Council, The European Economic And Social Committee And The Committee Of The Regions Eu. In Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Taviani, M.; Angeletti, L.; Antolini, B.; Ceregato, A.; Froglia, C.; Lopez Correa, M.; Montagna, P.; Remia, A.; Trincardi, F.; Vertino, A. Geo-Biology of Mediterranean Deep-Water Coral Ecosystems. Mar. Res. CNR 2011, 6, 705–719. [Google Scholar]
- Lembo, G.; Spedicato, M.T. Lo Stato Delle Risorse Demersali Nei Mari Italiani. GSA 18–Adriatico Meridionale. State Ital. Mar. Fish. Aquac. 2011, 1, 79–87. [Google Scholar]
- FAO. Report of the FAO Workshop on Deep-Sea Fisheries and Vulnerable Marine Ecosystems of the Mediterranean; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- European Union. Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions. In Blue Growth Opportunities for Marine and Maritime Sustainable Growth; COM/2012/0494 final; European Union: Brussels, Belgium, 2012. [Google Scholar]
- Danovaro, R.; Fanelli, E.; Aguzzi, J.; Billett, D.; Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Gjerde, K.; Jamieson, A.J.; Kark, S. Ecological Variables for Developing a Global Deep-Ocean Monitoring and Conservation Strategy. Nat. Ecol. Evol. 2020, 4, 181–192. [Google Scholar] [CrossRef] [PubMed]










| Landing Tonnes | ||||
|---|---|---|---|---|
| Mean ± SE | % | Spearman’s ρ | p | |
| MED | 57,774 ± 1857 | - | −0.059 | n.s |
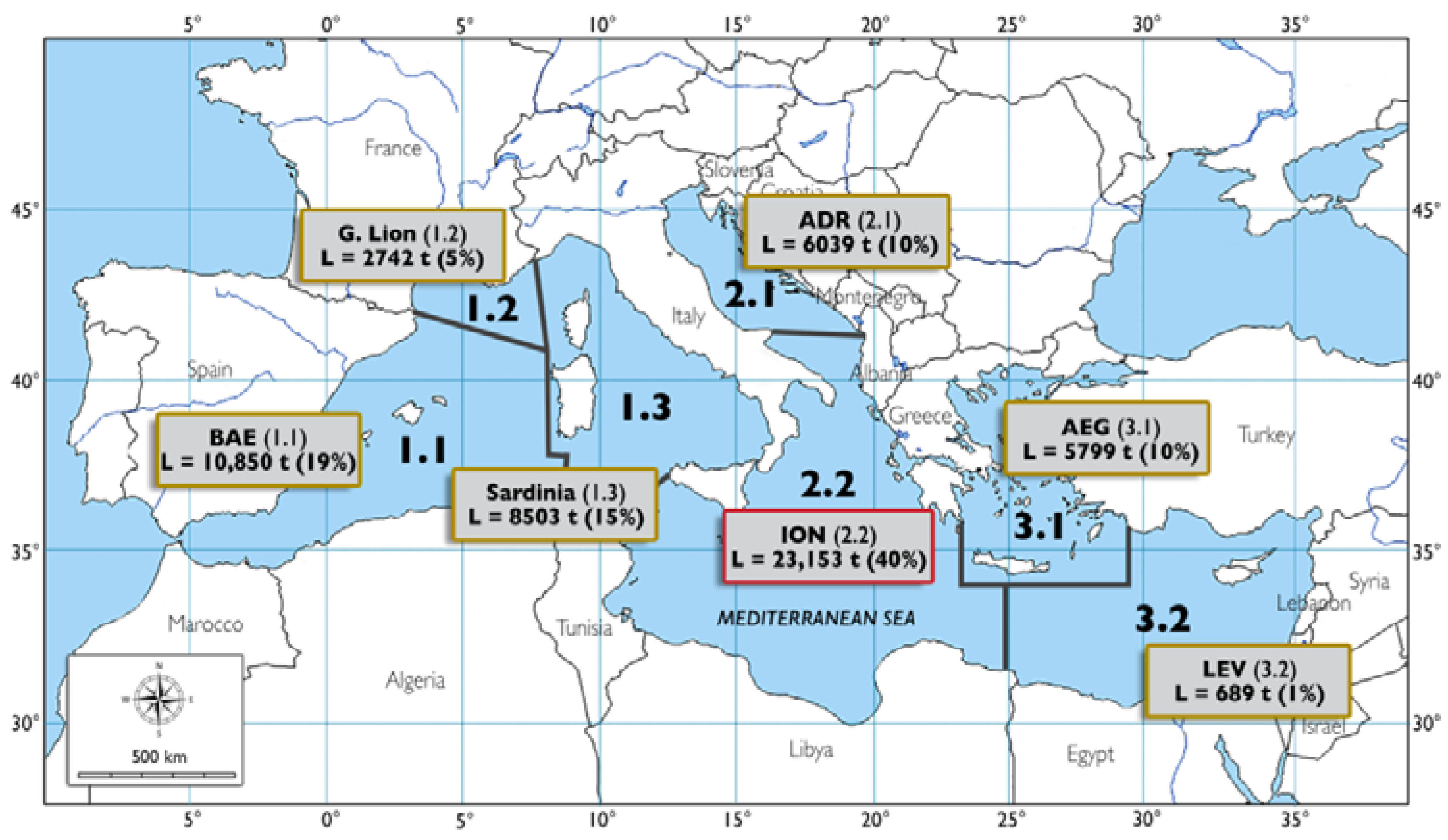
| ION | 23,153 ± 1754 | 40% | −0.210 | n.s |
| M. merluccius | 9865 ± 1445 | 43% | −0.637 | <0.001 |
| P. longirostris | 7597 ± 521 | 33% | 0.426 | <0.05 |
| A. foliacea and A. antennatus | 1921 ± 177 | 8% | 0.446 | <0.05 |
| N. norvegicus | 1673 ± 117 | 7% | −0.588 | <0.01 |
| Lophius spp. | 1330 ± 207 | 6% | −0.462 | <0.05 |
| C. conger | 296 ± 41 | 1% | 0.079 | n.s |
| G. melastomus | 373 ± 101 | 2% | 0.853 | <0.001 |
| H. dactylopterus | 52 ± 26 | >0.2% | 0.584 | <0.01 |
| P. blennoides | 17 ± 7 | >0.2% | 0.542 | <0.01 |
| P. bogaraveo | 9 ± 3 | >0.2% | 0.681 | <0.001 |
| P. americanus | 20 ± 4 | >0.2% | −0.060 | n.s |
| Landing (Tonnes) | ||||
|---|---|---|---|---|
| Country | Mean ± SE | % | Spearman ρ | p |
| Italy | 19,504 ± 1792 | 84% | −0.353 | n.s. |
| Greece | 1062 ± 67 | 5% | −0.332 | n.s. |
| Malta | 48 ± 7 | <0.2% | 0.760 | <0.001 |
| Tunisia | 1418 ± 192 | 6% | 0.902 | <0.001 |
| Albania | 1109 ± 215 | 5% | 0.856 | <0.001 |
| kg/km2 | N/km2 | |||||
|---|---|---|---|---|---|---|
| Mean ± s.d. | Spearman ρ | p | Mean ± s.d. | Spearman ρ | p | |
| Merluccius merluccius | 19.98 ± 7.40 | 0.338 | n.s. | 585 ± 385 | 0.336 | n.s. |
| Parapenaeus longirostris | 8.87 ± 4.50 | 0.737 | <0.001 | 1697 ± 1086 | 0.804 | <0.001 |
| Aristaeomorpha foliacea | 4.34 ± 3.63 | 0.692 | <0.001 | 362 ± 284 | 0.599 | <0.001 |
| Aristeus antennatus | 7.77 ± 2.67 | −0.391 | <0.05 | 427 ± 173 | −0.034 | n.s. |
| Nephrops norvegicus | 1.59 ± 0.90 | −0.766 | <0.001 | 61 ± 65 | −0.785 | <0.001 |
| Phycis blennoides | 6.59 ± 3.47 | 0.313 | n.s. | 272 ± 211 | 0.052 | n.s. |
| Lophius spp. | 7.77 ± 2.67 | 0.023 | n.s. | 427 ± 173 | −0.326 | n.s. |
| Pagellus bogaraveo | 2.06 ± 2.44 | 0.538 | <0.01 | 145 ± 333 | 0.634 | <0.001 |
| Galeus melastomus | 16.96 ± 10.54 | 0.344 | n.s. | 125 ± 67 | −0.085 | n.s. |
| Effect | Estimate | SE | t | p | ||
|---|---|---|---|---|---|---|
| P. longirostris | Biomass (kg/km2) | Intercept | 4.33 | 0.42 | 10.29 | <0.001 |
| Fishing effort | −0.01 | 0.01 | −5.30 | <0.001 | ||
| Intercept | −3.36 | 2.16 | −1.56 | 0.136 | ||
| Temperature | 0.36 | 0.14 | 2.55 | 0.02 | ||
| Density (N/km2) | Intercept | 9.9 | 0.52 | 19.10 | <0.001 | |
| Fishing effort | −0.01 | 0.01 | −4.97 | <0.001 | ||
| Intercept | 0.97 | 2.57 | 0.38 | 0.712 | ||
| Temperature | 0.42 | 0.17 | 2.49 | 0.022 | ||
| A. foliacea | Biomass (kg/km2) | Intercept | 5.04 | 0.72 | 6.97 | <0.001 |
| Fishing effort | −0.02 | 0.01 | −5.14 | <0.001 | ||
| Intercept | −9.48 | 3.39 | 2.80 | 0.011 | ||
| Temperature | 0.72 | 0.23 | 3.21 | 0.005 | ||
| Density (N/km2) | Intercept | 8.39 | 0.81 | 10.32 | <0.001 | |
| Fishing effort | −0.01 | 0.003 | −3.18 | 0.005 | ||
| Intercept | −1.61 | 3.38 | −0.48 | 0.639 | ||
| Temperature | 0.5 | 0.22 | 2.21 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorano, P.; Capezzuto, F.; Carluccio, A.; Calculli, C.; Cipriano, G.; Carlucci, R.; Ricci, P.; Sion, L.; Tursi, A.; D’Onghia, G. Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure. Foods 2022, 11, 1420. https://doi.org/10.3390/foods11101420
Maiorano P, Capezzuto F, Carluccio A, Calculli C, Cipriano G, Carlucci R, Ricci P, Sion L, Tursi A, D’Onghia G. Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure. Foods. 2022; 11(10):1420. https://doi.org/10.3390/foods11101420
Chicago/Turabian StyleMaiorano, Porzia, Francesca Capezzuto, Angela Carluccio, Crescenza Calculli, Giulia Cipriano, Roberto Carlucci, Pasquale Ricci, Letizia Sion, Angelo Tursi, and Gianfranco D’Onghia. 2022. "Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure" Foods 11, no. 10: 1420. https://doi.org/10.3390/foods11101420
APA StyleMaiorano, P., Capezzuto, F., Carluccio, A., Calculli, C., Cipriano, G., Carlucci, R., Ricci, P., Sion, L., Tursi, A., & D’Onghia, G. (2022). Food from the Depths of the Mediterranean: The Role of Habitats, Changes in the Sea-Bottom Temperature and Fishing Pressure. Foods, 11(10), 1420. https://doi.org/10.3390/foods11101420








